Antifungal therapeutic drug monitoring and azole dose modification















- Slides: 45

Anti-fungal therapeutic drug monitoring and azole dose modification Tim Felton Advances Against Aspergillosis Istanbul, 2012

Contents • Triazoles – an overview • Therapeutic drug monitoring (TDM) • Specific drugs Advances Against Aspergillosis Istanbul, 2012

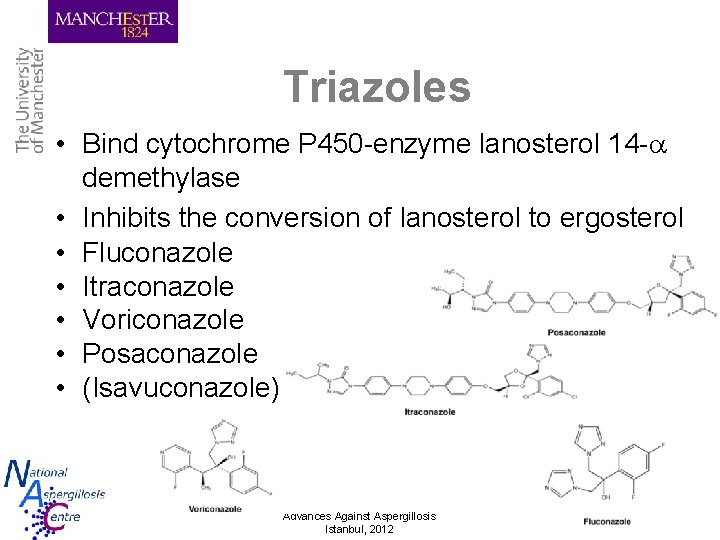
Triazoles • Bind cytochrome P 450 -enzyme lanosterol 14 - demethylase • Inhibits the conversion of lanosterol to ergosterol • Fluconazole • Itraconazole • Voriconazole • Posaconazole • (Isavuconazole) Advances Against Aspergillosis Istanbul, 2012

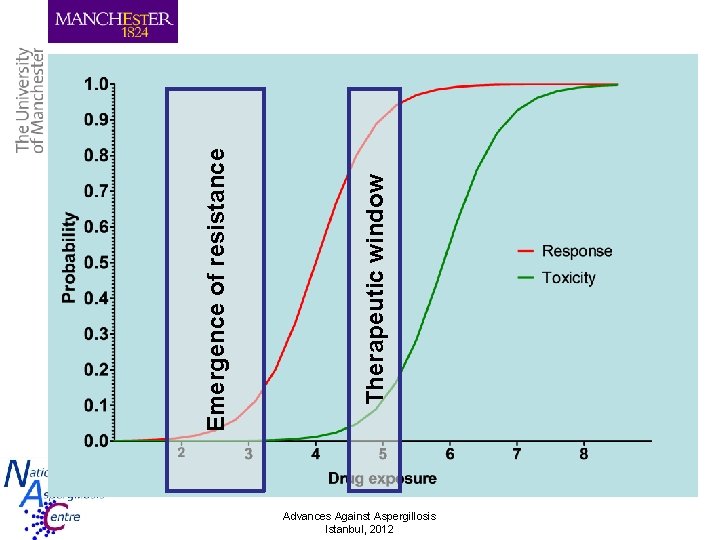
Emergence of resistance Therapeutic window Advances Against Aspergillosis Istanbul, 2012

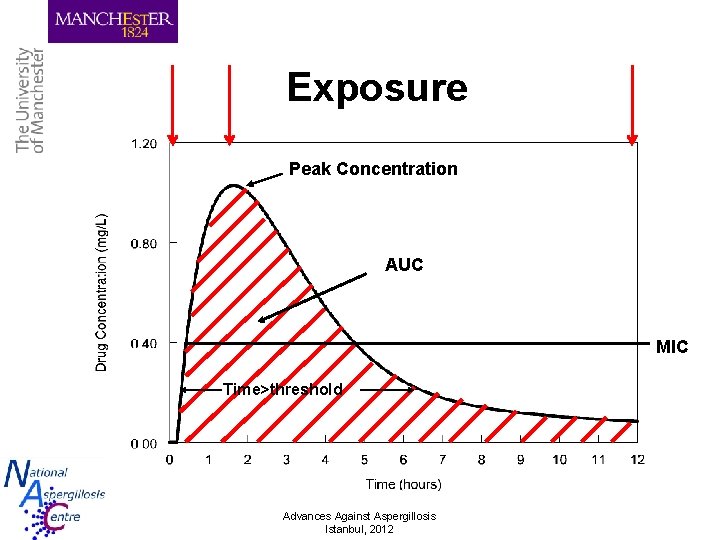
Exposure Peak Concentration AUC MIC Time>threshold Advances Against Aspergillosis Istanbul, 2012

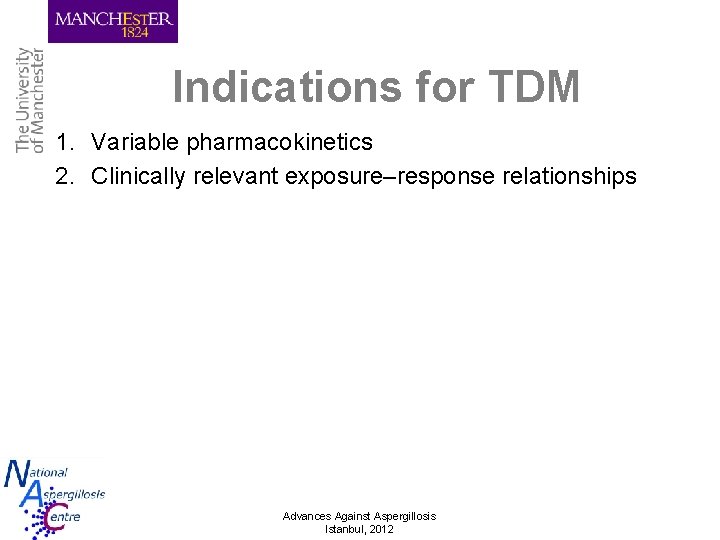
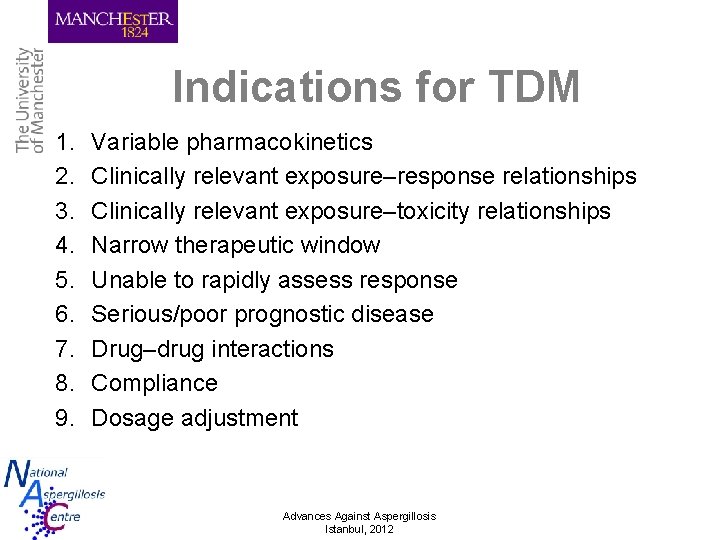
Indications for TDM 1. Variable pharmacokinetics Advances Against Aspergillosis Istanbul, 2012

DOSE PK-PD: the black arts BIOMARKER • Quantifiable • Linked to pathogenesis • Linked to an outcome of clinical interest OUTCOME OF CLINICAL INTEREST/IMPORTANCE • Survival • Resolution of clinical syndrome Conc Dose PHARMACOKINETICS PHARMACODYNAMICS Advances Against Aspergillosis Istanbul, 2012

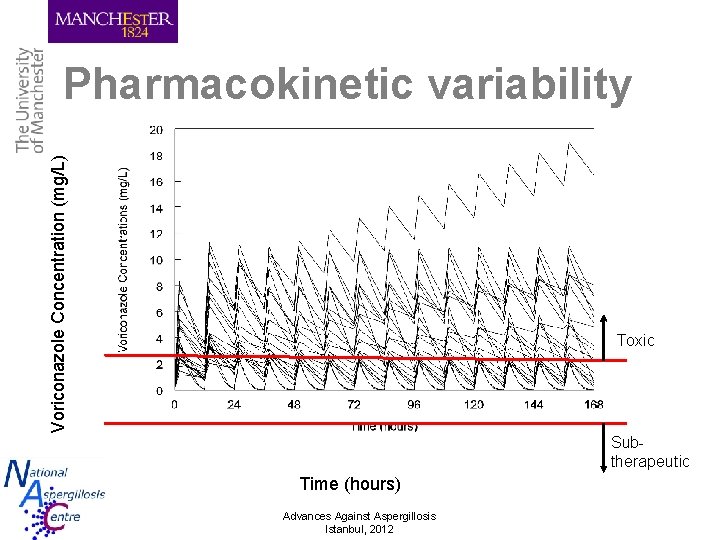
Voriconazole Concentration (mg/L) Pharmacokinetic variability Toxic Subtherapeutic Time (hours) Advances Against Aspergillosis Istanbul, 2012

Pharmacokinetic variability Advances Against Aspergillosis Istanbul, 2012 Hope WW. AAC. 2012. In press

Pharmacokinetic variability Advances Against Aspergillosis Istanbul, 2012 Howard S. TDM. 2012. 34: 72 -76

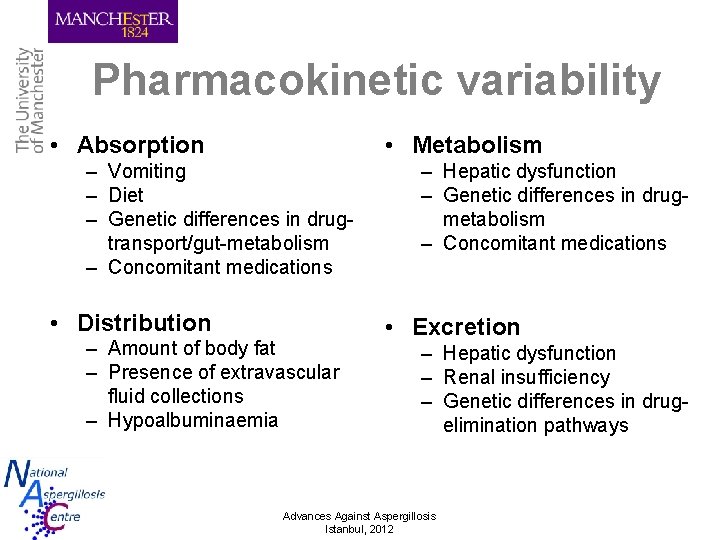
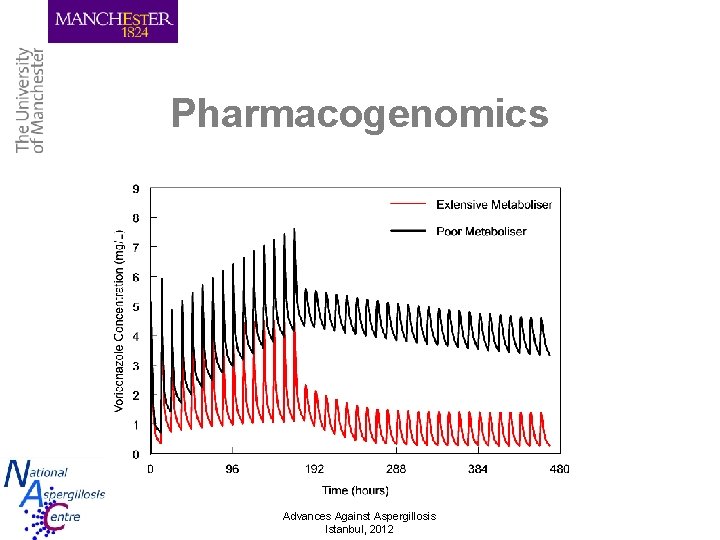
Pharmacokinetic variability • Absorption • Metabolism – Vomiting – Diet – Genetic differences in drugtransport/gut-metabolism – Concomitant medications • Distribution – Amount of body fat – Presence of extravascular fluid collections – Hypoalbuminaemia – Hepatic dysfunction – Genetic differences in drugmetabolism – Concomitant medications • Excretion – Hepatic dysfunction – Renal insufficiency – Genetic differences in drugelimination pathways Advances Against Aspergillosis Istanbul, 2012

Pharmacogenomics Advances Against Aspergillosis Istanbul, 2012

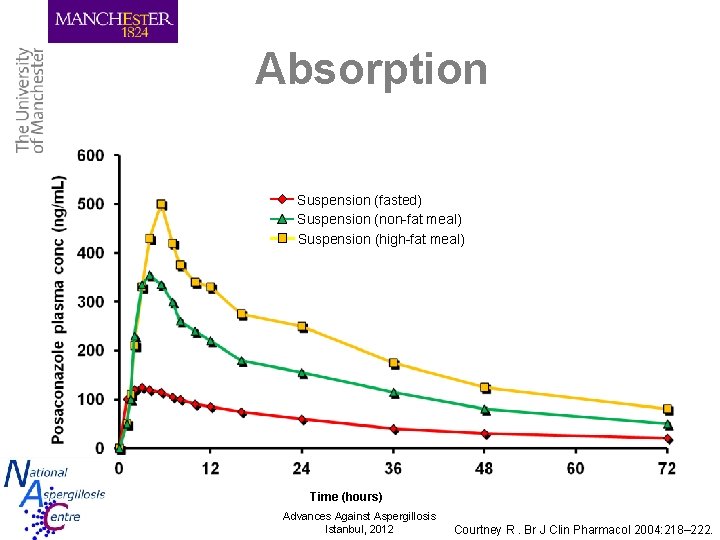
Absorption Suspension (fasted) Suspension (non-fat meal) Suspension (high-fat meal) Time (hours) Advances Against Aspergillosis Istanbul, 2012 Courtney R. Br J Clin Pharmacol 2004: 218– 222.

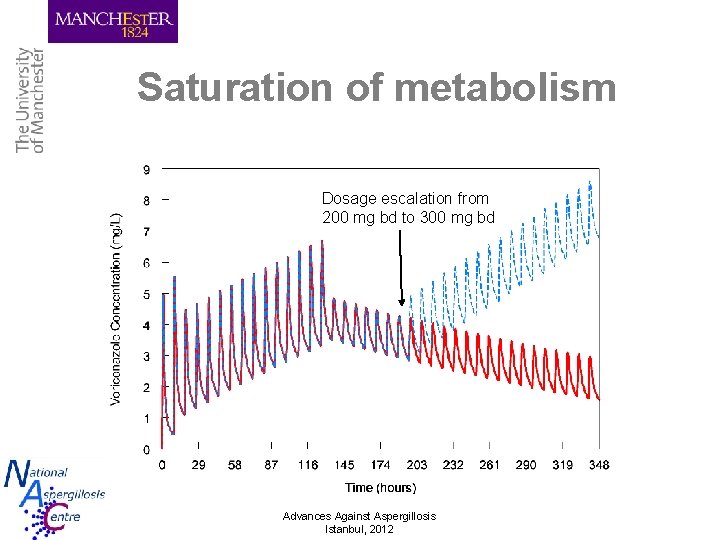
Saturation of metabolism Dosage escalation from 200 mg bd to 300 mg bd Advances Against Aspergillosis Istanbul, 2012

Indications for TDM 1. Variable pharmacokinetics 2. Clinically relevant exposure–response relationships Advances Against Aspergillosis Istanbul, 2012

Indications for TDM 1. Variable pharmacokinetics 2. Clinically relevant exposure–response relationships Advances Against Aspergillosis Istanbul, 2012

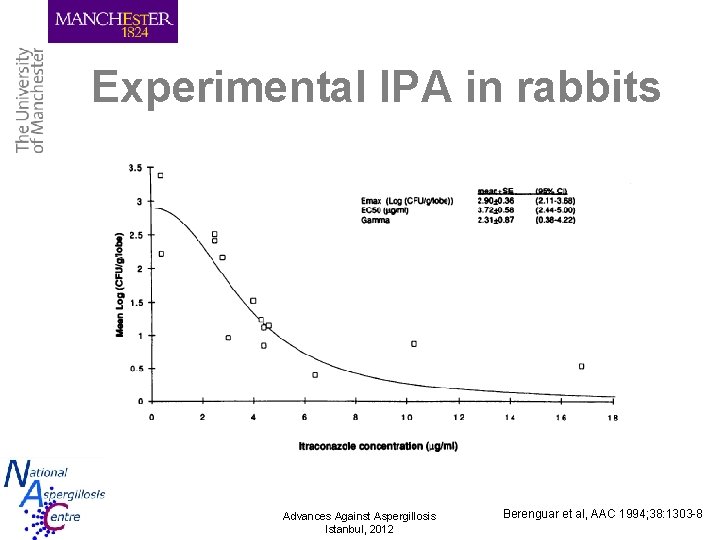
Experimental IPA in rabbits Advances Against Aspergillosis Istanbul, 2012 Berenguar et al, AAC 1994; 38: 1303 -8

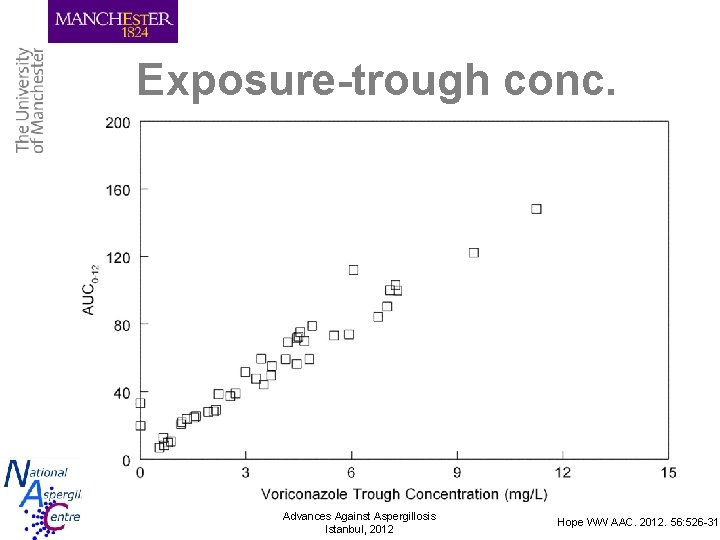
Exposure-trough conc. Advances Against Aspergillosis Istanbul, 2012 Hope WW AAC. 2012. 56: 526 -31

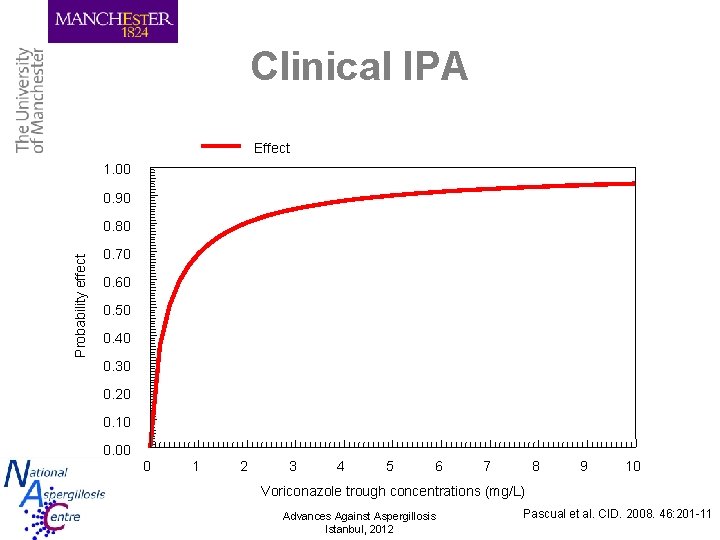
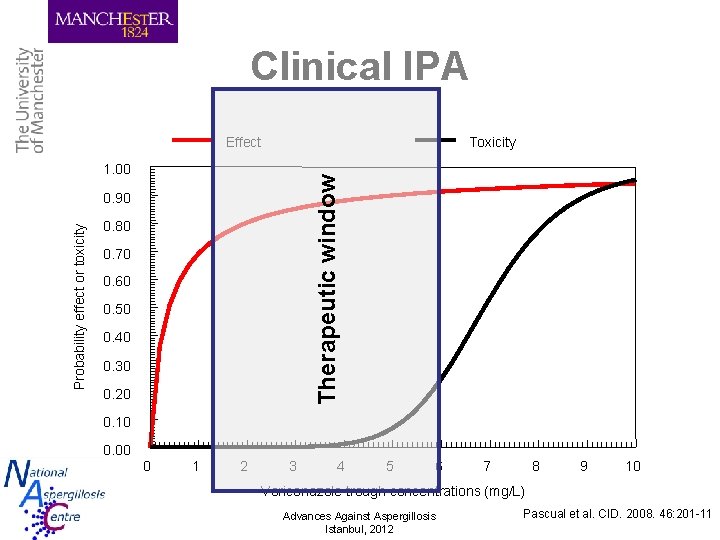
Clinical IPA Effect 1. 00 0. 90 Probability effect 0. 80 0. 70 0. 60 0. 50 0. 40 0. 30 0. 20 0. 10 0. 00 0 1 2 3 4 5 6 7 8 9 10 Voriconazole trough concentrations (mg/L) Advances Against Aspergillosis Istanbul, 2012 Pascual et al. CID. 2008. 46: 201 -11

Indications for TDM 1. Variable pharmacokinetics 2. Clinically relevant exposure–response relationships 3. Clinically relevant exposure–toxicity relationships Advances Against Aspergillosis Istanbul, 2012

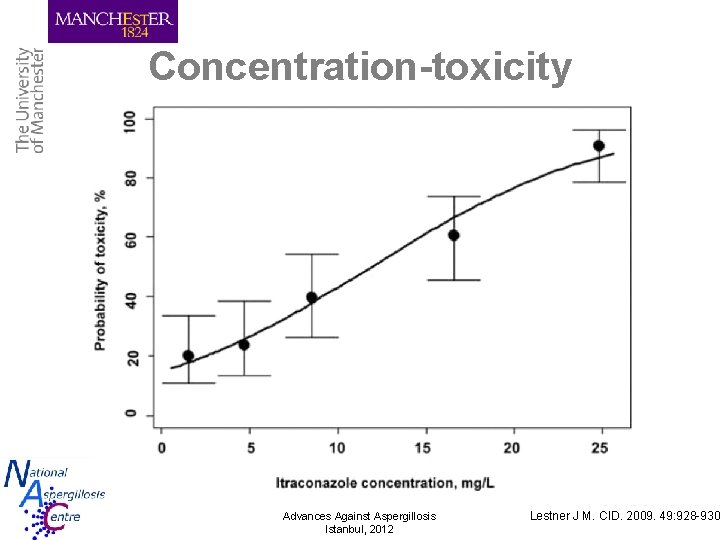
Concentration-toxicity Advances Against Aspergillosis Istanbul, 2012 Lestner J M. CID. 2009. 49: 928 -930

Clinical IPA Effect Toxicity 1. 00 Probability effect or toxicity 0. 90 0. 80 0. 70 0. 60 0. 50 0. 40 0. 30 0. 20 0. 10 0. 00 0 1 2 3 4 5 6 7 8 9 10 Voriconazole trough concentrations (mg/L) Advances Against Aspergillosis Istanbul, 2012 Pascual et al. CID. 2008. 46: 201 -11

Indications for TDM 1. 2. 3. 4. Variable pharmacokinetics Clinically relevant exposure–response relationships Clinically relevant exposure–toxicity relationships Narrow therapeutic window Advances Against Aspergillosis Istanbul, 2012

Clinical IPA Effect Toxicity Therapeutic window 1. 00 Probability effect or toxicity 0. 90 0. 80 0. 70 0. 60 0. 50 0. 40 0. 30 0. 20 0. 10 0. 00 0 1 2 3 4 5 6 7 8 9 10 Voriconazole trough concentrations (mg/L) Advances Against Aspergillosis Istanbul, 2012 Pascual et al. CID. 2008. 46: 201 -11

Indications for TDM 1. 2. 3. 4. 5. 6. 7. 8. 9. Variable pharmacokinetics Clinically relevant exposure–response relationships Clinically relevant exposure–toxicity relationships Narrow therapeutic window Unable to rapidly assess response Serious/poor prognostic disease Drug–drug interactions Compliance Dosage adjustment Advances Against Aspergillosis Istanbul, 2012

Specific dugs • • Fluconazole Itraconazole Voriconazole Posaconazole • • • Indication Pharmacology/pharmacokinetics Exposure–response relationship Exposure–toxicity relationship TDM target Advances Against Aspergillosis Istanbul, 2012

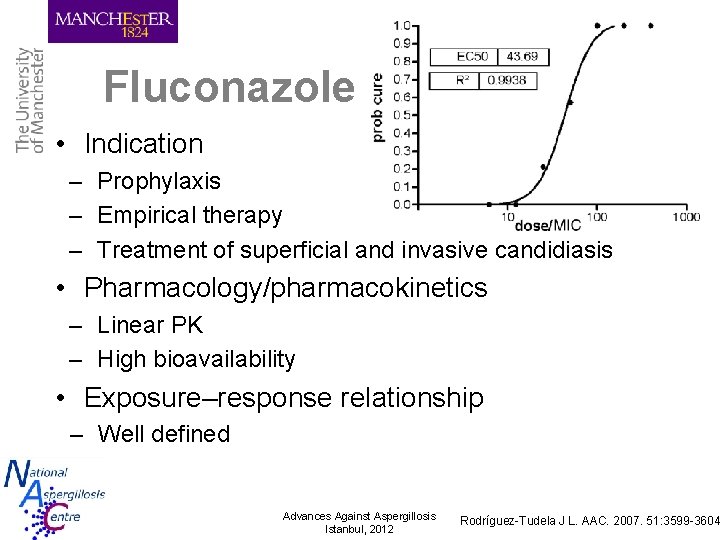
Fluconazole • Indication – Prophylaxis – Empirical therapy – Treatment of superficial and invasive candidiasis • Pharmacology/pharmacokinetics – Linear PK – High bioavailability • Exposure–response relationship – Well defined Advances Against Aspergillosis Istanbul, 2012 Rodríguez-Tudela J L. AAC. 2007. 51: 3599 -3604

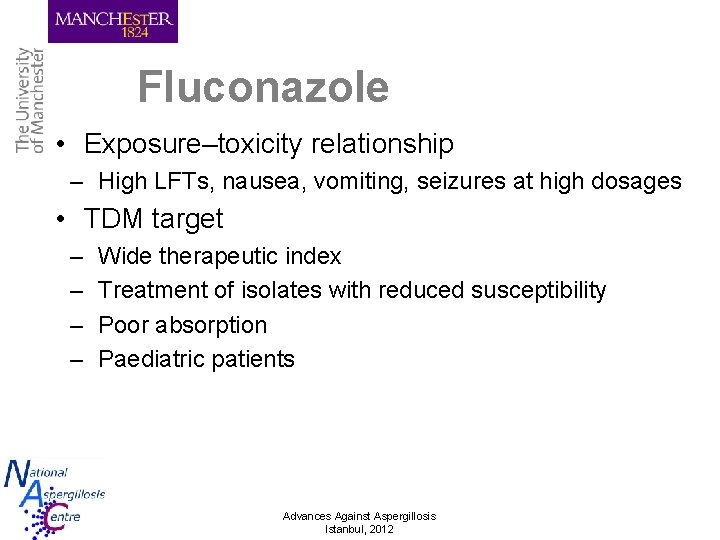
Fluconazole • Exposure–toxicity relationship – High LFTs, nausea, vomiting, seizures at high dosages • TDM target – – Wide therapeutic index Treatment of isolates with reduced susceptibility Poor absorption Paediatric patients Advances Against Aspergillosis Istanbul, 2012

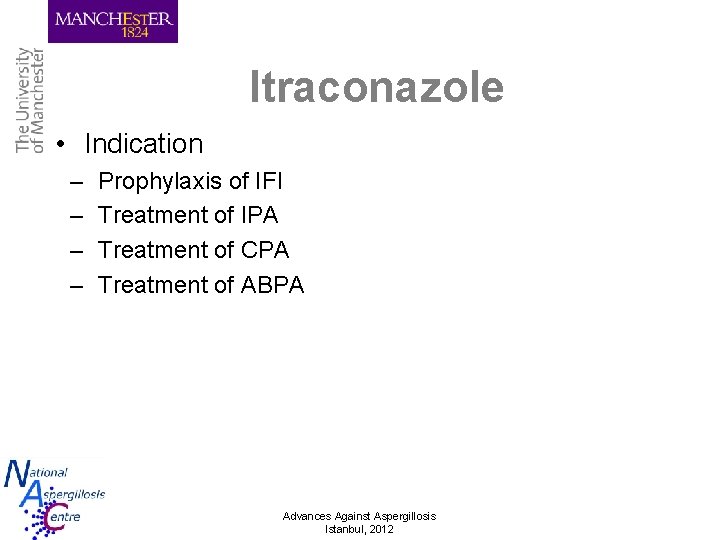
Itraconazole • Indication – – Prophylaxis of IFI Treatment of IPA Treatment of CPA Treatment of ABPA Advances Against Aspergillosis Istanbul, 2012

Itraconazole • Pharmacology/pharmacokinetics – – – – – Highly lipophilic and protein bound capsule solubility in acidic environment Different manufactures' capsules behave differently absorption with PPI and H 2 -antagonists Suspension (cyclodextrin) 20 -50% higher bioavailability Non-linear (probably) Extensive variability Active metabolite (OH-itraconazole) (bioassay/HPLC) CYP 3 A 4 Advances Against Aspergillosis Istanbul, 2012

Itraconazole • Exposure–response relationship – Peak itraconazole levels and successful outcome of mucosal candidiasis in patients with AIDS – In vivo data • Exposure–toxicity relationship – Gastrointestinal intolerance, hypokalaemia, fatigue, ankle oedema, cardiac failure and deranged LFTs – Nausea more common with suspension (cyclodextrin) Advances Against Aspergillosis Istanbul, 2012

Itraconazole • TDM target – 200 mg b. i. d. – Trough concentration – Lower level • Prophylaxis in neutropenia & treatment of oesophageal candidasis in HIV • 0. 5 mg/L HPLC or 5 mg/L bioassay – Upper level • 17 mg/L (bioassay)due to high probability of toxicity Advances Against Aspergillosis Istanbul, 2012

Itraconazole • Tips to improve low levels – 1. 2. 3. 4. 5. 6. Usually poor absorption Capsule with food or acid drink? Stop PPI or H 2 -antagonist (if possible) Compliance Consistently prescribe the same preparation Check for drug interactions Increase to capsule 300 mg twice daily or change to suspension 200 mg twice daily 7. Check serum levels again! Advances Against Aspergillosis Istanbul, 2012

Itraconazole • Tips to reduce high levels +/- toxicity – 1. 2. 3. Usually saturated clearance Stop drug for 1 -2 weeks Re-start at a lower dose Check serum levels again! Advances Against Aspergillosis Istanbul, 2012

Voriconazole • Indication – Disseminated candidasis – IPA and CPA • Pharmacology/pharmacokinetics – – – Excellent bioavailabilty (96%) IV preparation – cyclodextrin (potentially nephrotoxic) Marked PK variability (100 -fold) Sex, age and CYP 2 C 19 genotype only partially explain Weight important in paediatric patients CYP 2 C 19, 3 A 4 and 2 C 9 substrate Advances Against Aspergillosis Istanbul, 2012 Herbrecht R. NEJM. 2002. 347: 408 -15

Voriconazole • Exposure–response relationship – In-vivo, in-vitro and clinical data • Exposure–toxicity relationship – Gradual increase in probability with increasing concentration – Abnormal LFTs, visual disturbance, photosensitivity, confusion etc Advances Against Aspergillosis Istanbul, 2012 Pascual et al. CID. 2008: 46; 201 -11

Voriconazole • TDM target – 200 mg b. i. d. (i. v. loading dose) – Trough concentration – Lower level • Trough 1 mg/L associated with 70% probability of success – Upper level • Less well established • >6 mg/L associated with high probability of CNS toxicity and hepatitis Advances Against Aspergillosis Istanbul, 2012

Voriconazole • Tips on use – Loading dose – Switch IV to oral • Tips to improve low levels – Dosage escalation carefully by 50 mg daily – Check levels every 1 -2/52 • Tips to reduce high levels +/- toxicity – Stop for 1 week or by TDM then reduce dosage – Stop omeprazole – Check levels Advances Against Aspergillosis Istanbul, 2012

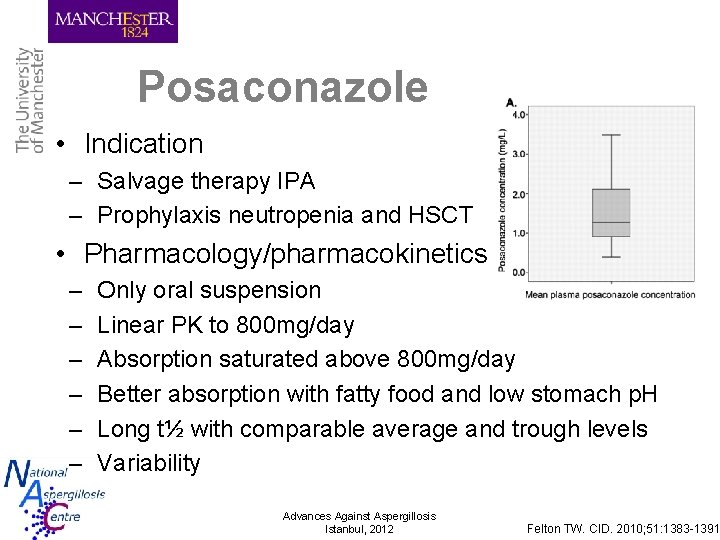
Posaconazole • Indication – Salvage therapy IPA – Prophylaxis neutropenia and HSCT • Pharmacology/pharmacokinetics – – – Only oral suspension Linear PK to 800 mg/day Absorption saturated above 800 mg/day Better absorption with fatty food and low stomach p. H Long t½ with comparable average and trough levels Variability Advances Against Aspergillosis Istanbul, 2012 Felton TW. CID. 2010; 51: 1383 -1391

Posaconazole • Exposure–response relationship – In-vivo (mouse IC and rabbit IPA) – Increased clinical response with increasing average and trough concentration • Exposure–toxicity relationship – GI intolerance, abnormal LFT – No dose dependent Advances Against Aspergillosis Istanbul, 2012 Walsh T. CID. 2007. 44. 2 -12

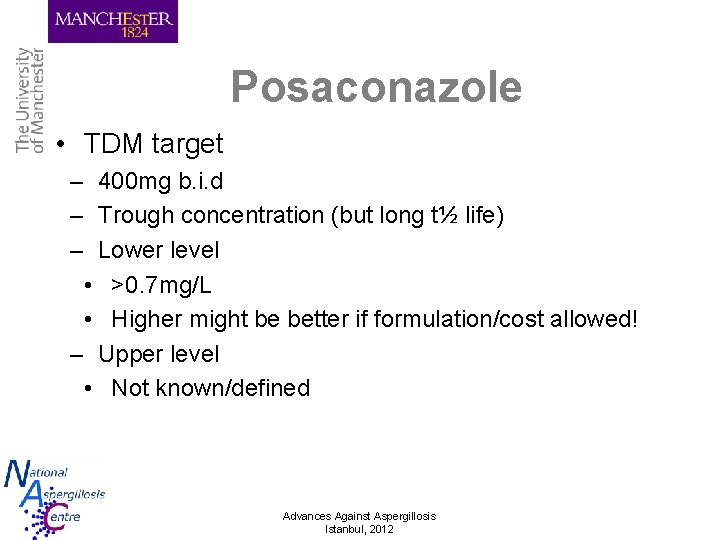
Posaconazole • TDM target – 400 mg b. i. d – Trough concentration (but long t½ life) – Lower level • >0. 7 mg/L • Higher might be better if formulation/cost allowed! – Upper level • Not known/defined Advances Against Aspergillosis Istanbul, 2012

Posaconazole • Tips to improve low levels – – – Fatty foods, milk or fatty food supplements Stop enzyme inducers Stop PPIs Can try fractionating the regimen Dosage escalation unhelpful above 800 mg/day Advances Against Aspergillosis Istanbul, 2012

Indications for TDM 1. 2. 3. 4. 5. 6. 7. 8. 9. Variable pharmacokinetics Clinically relevant exposure–response relationships Clinically relevant exposure–toxicity relationships Narrow therapeutic window Unable to rapidly assess response Serious/poor prognostic disease Drug–drug interactions Compliance Dosage adjustment Advances Against Aspergillosis Istanbul, 2012

Emergence of resistance Therapeutic window Advances Against Aspergillosis Istanbul, 2012

Conclusions • TDM required for itraconazole and voriconazole • Probably for posaconazole • TDM should – Improve outcomes – Reduce emergence of resistance – BUT there is an associated cost Advances Against Aspergillosis Istanbul, 2012
 Modifikasi perilaku self management
Modifikasi perilaku self management Young rule
Young rule How to determine drip rate
How to determine drip rate Pediatric dose calculation formula
Pediatric dose calculation formula Parenteral dosage calculations
Parenteral dosage calculations Dosage calculation formula
Dosage calculation formula Controlled substance log example
Controlled substance log example Envelope method of drug distribution is suitable for
Envelope method of drug distribution is suitable for Complete floor stock system
Complete floor stock system How many ml of amoxicillin for child
How many ml of amoxicillin for child Classification of antifungal drugs
Classification of antifungal drugs Mechanism of action of antifungal drugs
Mechanism of action of antifungal drugs Capsofungin
Capsofungin Dr.saeed ahmed
Dr.saeed ahmed Antifungal sensitivity test
Antifungal sensitivity test Thrush tablet boots
Thrush tablet boots Define adulteration of crude drugs
Define adulteration of crude drugs Who program for international drug monitoring
Who program for international drug monitoring Alabama prescription drug monitoring program
Alabama prescription drug monitoring program Who collaborating centre for international drug monitoring
Who collaborating centre for international drug monitoring Texaspmp
Texaspmp Who programme for international drug monitoring
Who programme for international drug monitoring Nurses role in therapeutic community
Nurses role in therapeutic community 7 rights of medication administration in order
7 rights of medication administration in order Association for applied and therapeutic humor
Association for applied and therapeutic humor Stately motility of clostridium
Stately motility of clostridium Interception threat
Interception threat Pre and post modification
Pre and post modification Stem and root modifications
Stem and root modifications Research on the pros and cons of genetic engineering.
Research on the pros and cons of genetic engineering. Biotechnology selective breeding
Biotechnology selective breeding Morphology of inflorescence
Morphology of inflorescence Therapeutic orientations
Therapeutic orientations Penyakit masyarakat sasaran bintibluh
Penyakit masyarakat sasaran bintibluh Therapeutic exercise chapter 1 mcqs
Therapeutic exercise chapter 1 mcqs Therapeutic equipments
Therapeutic equipments Therapeutic drift
Therapeutic drift Therapeutic communication introduction
Therapeutic communication introduction Traingle of care
Traingle of care Therapeutic story writing
Therapeutic story writing Therapeutic communication principles
Therapeutic communication principles Principles of therapeutic exercise
Principles of therapeutic exercise Therapeutic index
Therapeutic index High therapeutic index
High therapeutic index High therapeutic index
High therapeutic index Classification of therapeutic exercise
Classification of therapeutic exercise