Ichaemic stroke from cardiologist point of view Petr



- Slides: 37

Ichaemic stroke from cardiologist point of view Petr Jansky University Hospital Motol Prague 5. 9. 2014

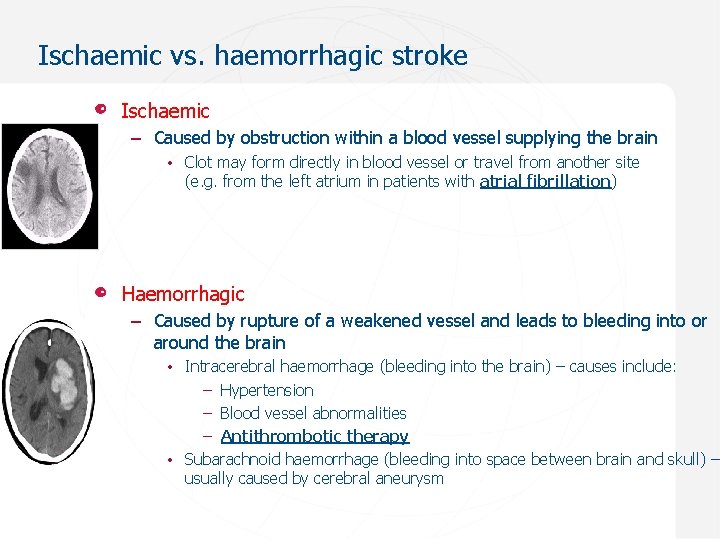
Ischaemic vs. haemorrhagic stroke Ischaemic – Caused by obstruction within a blood vessel supplying the brain • Clot may form directly in blood vessel or travel from another site (e. g. from the left atrium in patients with atrial fibrillation) Haemorrhagic – Caused by rupture of a weakened vessel and leads to bleeding into or around the brain • Intracerebral haemorrhage (bleeding into the brain) – causes include: – Hypertension – Blood vessel abnormalities – Antithrombotic therapy • Subarachnoid haemorrhage (bleeding into space between brain and skull) – usually caused by cerebral aneurysm

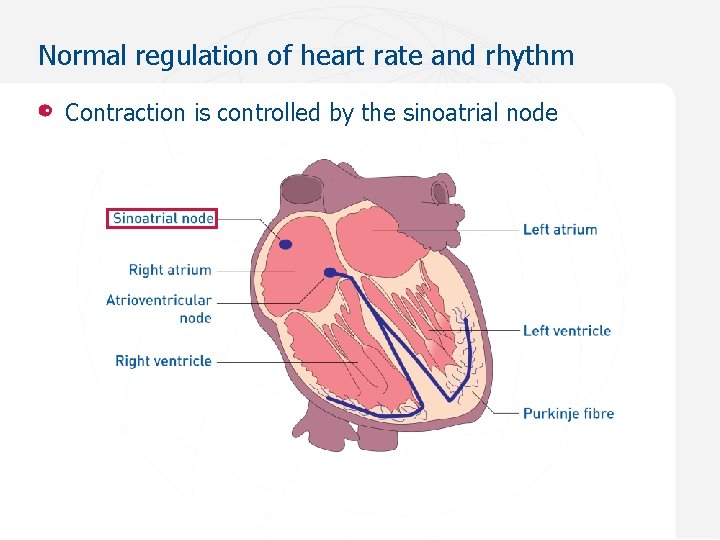
Normal regulation of heart rate and rhythm Contraction is controlled by the sinoatrial node

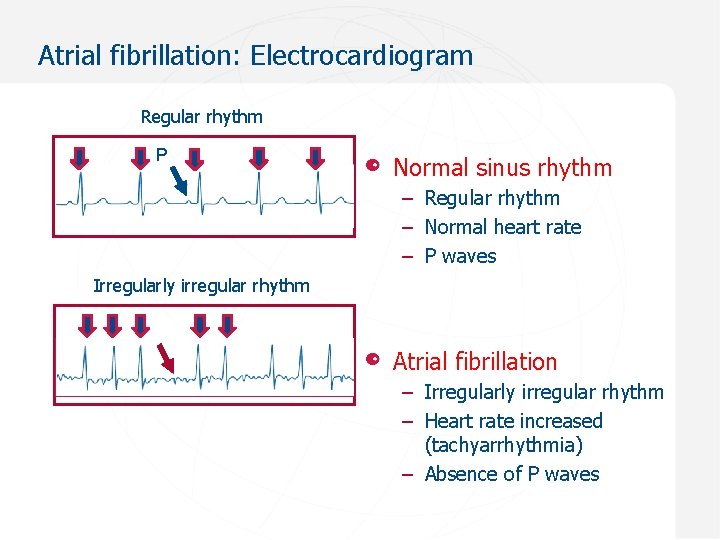
Atrial fibrillation: Electrocardiogram Regular rhythm P Normal sinus rhythm – Regular rhythm – Normal heart rate – P waves Irregularly irregular rhythm Atrial fibrillation – Irregularly irregular rhythm – Heart rate increased (tachyarrhythmia) – Absence of P waves

Consequences of atrial fibrillation Reduction in cardiac output can precipitate heart failure, leading to distinctive symptoms such as: – – – Peripheral oedema Dyspnoea Pulmonary oedema Fatigue Chest pain Formation of blood clots (thrombosis) on the atrial walls that can dislodge (embolize), leading to stroke and systemic embolism

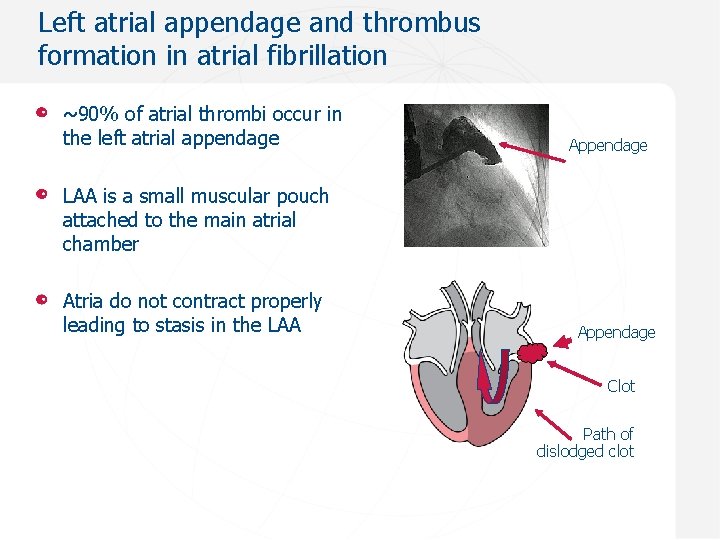
Left atrial appendage and thrombus formation in atrial fibrillation ~90% of atrial thrombi occur in the left atrial appendage Appendage LAA is a small muscular pouch attached to the main atrial chamber Atria do not contract properly leading to stasis in the LAA Appendage Clot Path of dislodged clot

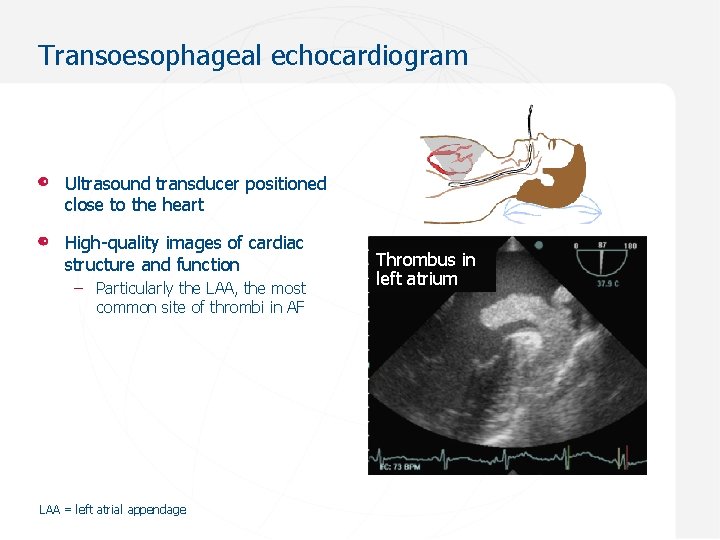
Transoesophageal echocardiogram Ultrasound transducer positioned close to the heart High-quality images of cardiac structure and function – Particularly the LAA, the most common site of thrombi in AF LAA = left atrial appendage Thrombus in left atrium

Atrial fibrillation is a common disorder Responsible for a third of all hospitalizations for cardiac rhythm disturbances 1 Estimated prevalence: – Europe: 4. 5 million 1 – USA: 5. 1 million 2 Affects approximately 2. 0% of the US population 2 1. ACC/AHA/ESC guidelines: Fuster V et al. Circulation 2006; 114: e 257– 354 & Eur Heart J 2006; 27: 1979– 2030; 2. Miyasaka Y et al. Circulation 2006; 114: 119– 25; 3. Heeringa J et al. Eur Heart J 2006; 27: 949– 53

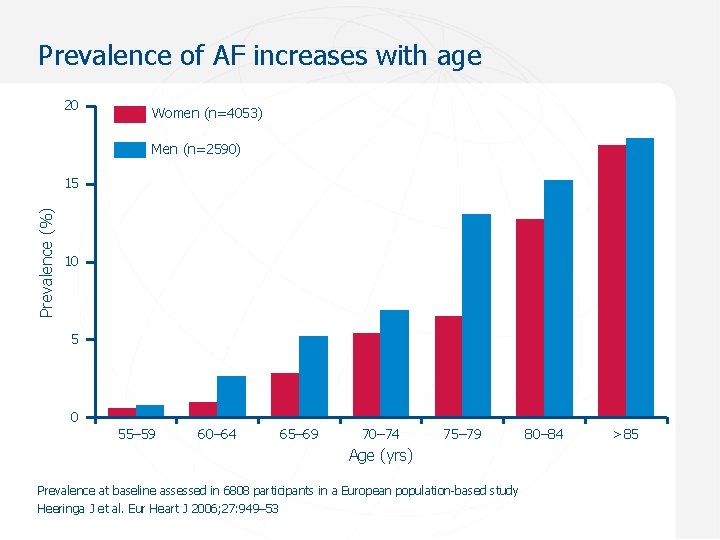
Prevalence of AF increases with age 20 Women (n=4053) Men (n=2590) Prevalence (%) 15 10 5 0 55– 59 60– 64 65– 69 70– 74 75– 79 Age (yrs) Prevalence at baseline assessed in 6808 participants in a European population-based study Heeringa J et al. Eur Heart J 2006; 27: 949– 53 80– 84 >85

AF is an increasingly common disorder 60% increase in hospital admissions for AF over the past 20 years 20% increase in prevalence expected over next decade Increasing prevalence driven by: – Increased longevity of populations worldwide – Rising prevalence of chronic heart disease – Rising prevalence of AF risk factors, e. g. diabetes mellitus Benyoucef S et al. Atrial fibrillation. 2008; Friberg J et al. Epidemiology 2003; 14: 666– 72

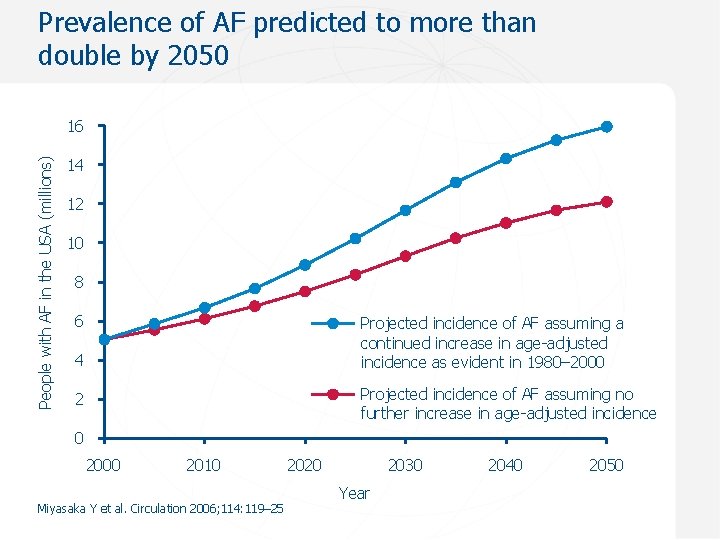
Prevalence of AF predicted to more than double by 2050 People with AF in the USA (millions) 16 14 12 10 8 6 Projected incidence of AF assuming a continued increase in age-adjusted incidence as evident in 1980– 2000 4 Projected incidence of AF assuming no further increase in age-adjusted incidence 2 0 2000 2010 Miyasaka Y et al. Circulation 2006; 114: 119– 25 2020 2030 Year 2040 2050

Stroke is the leading complication of AF AF increases the risk of all types of stroke 5 -fold 1 Without prevention, approximately 1 in 20 patients will have a stroke each year 2 AF is responsible for nearly one-third of all strokes 3 1. Savelieva I et al. Ann Med 2007; 39: 371– 91; 2. Atrial Fibrillation Investigators. Arch Intern Med 1994; 154: 1449– 57; 3. Hannon N et al. Cerebrovasc Dis 2010; 29: 43– 9

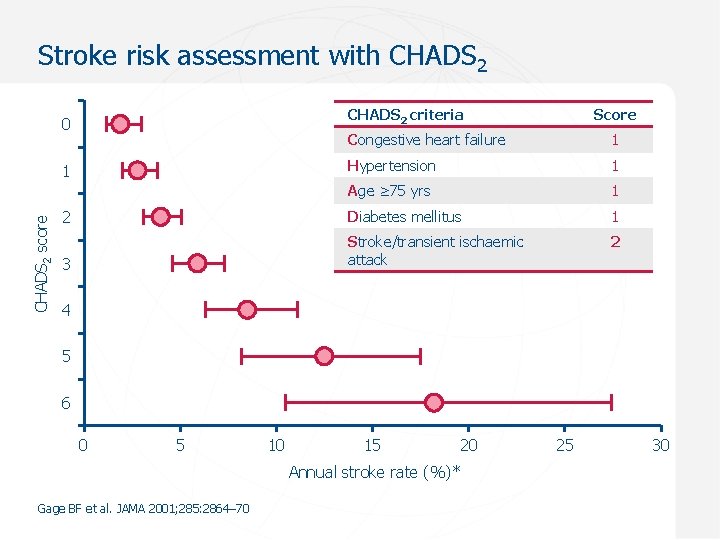
Stroke risk assessment with CHADS 2 criteria 0 Congestive heart failure 1 Hypertension 1 Age ≥ 75 yrs 1 2 Diabetes mellitus 1 2 3 Stroke/transient ischaemic attack 1 CHADS 2 score Score 4 5 6 0 5 10 15 20 Annual stroke rate (%)* Gage BF et al. JAMA 2001; 285: 2864– 70 25 30

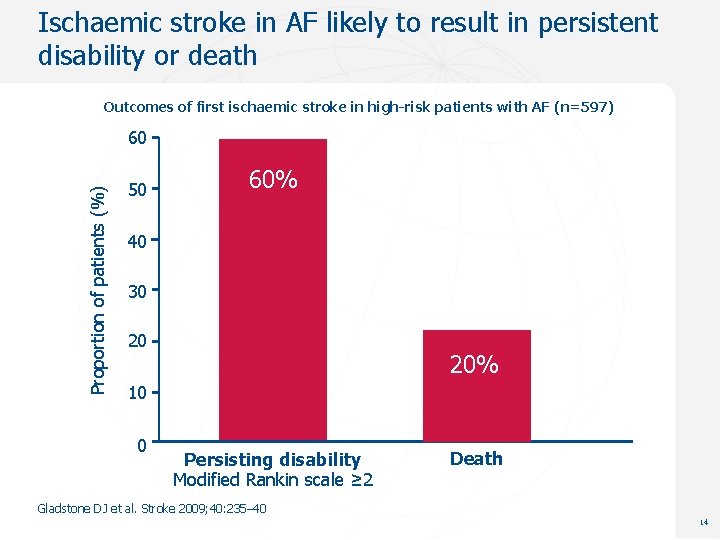
Ischaemic stroke in AF likely to result in persistent disability or death Outcomes of first ischaemic stroke in high-risk patients with AF (n=597) Proportion of patients (%) 60 50 60% 40 30 20 20% 10 0 Persisting disability Modified Rankin scale ≥ 2 Death Gladstone DJ et al. Stroke 2009; 40: 235– 40 14

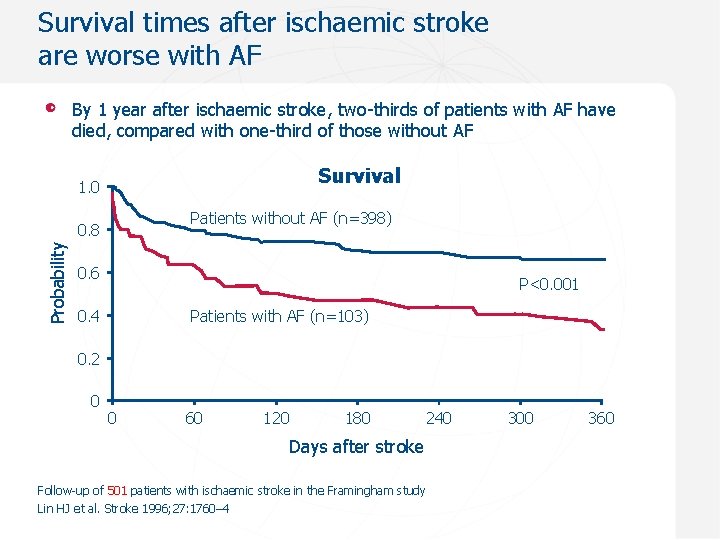
Survival times after ischaemic stroke are worse with AF By 1 year after ischaemic stroke, two-thirds of patients with AF have died, compared with one-third of those without AF 1. 00 Survival 1. 0 Patients without AF (n=398) Probability 0. 8 0. 6 P<0. 001 0. 4 Patients with AF (n=103) 0. 2 0 0 60 120 180 240 Days after stroke Follow-up of 501 patients with ischaemic stroke in the Framingham study Lin HJ et al. Stroke 1996; 27: 1760– 4 300 360

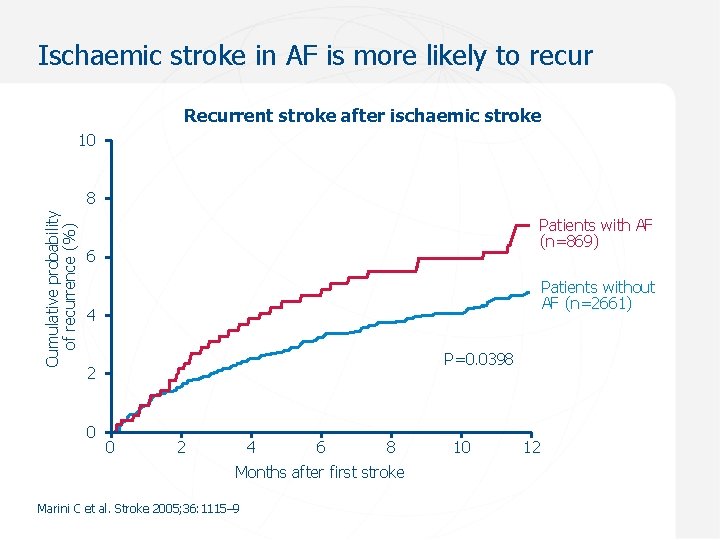
Ischaemic stroke in AF is more likely to recur Recurrent stroke after ischaemic stroke 10 Cumulative probability of recurrence (%) 8 Patients with AF (n=869) 6 Patients without AF (n=2661) 4 P=0. 0398 2 0 0 2 4 6 8 Months after first stroke Marini C et al. Stroke 2005; 36: 1115– 9 10 12

Management of AF has two broad objectives Prevention of complications, including thromboembolism (particularly ischaemic stroke) and heart failure Relief of symptoms Choice of antithrombotic therapy should be tailored to the patient based on: Risk of thromboembolism Risk of bleeding ESC guidelines: Camm J et al. Eur Heart J 2010; 31: 2369– 429; ACCF/AHA/HRS Focused Update Guidelines: Fuster V et al. J Am Coll Cardiol 2011; 57: e 101– 98 Sept 2012

Goals for anticoagulation therapy in AF Prevent ischaemic stroke Minimize haemorrhagic stroke (minimize risk of intracranial bleeding)

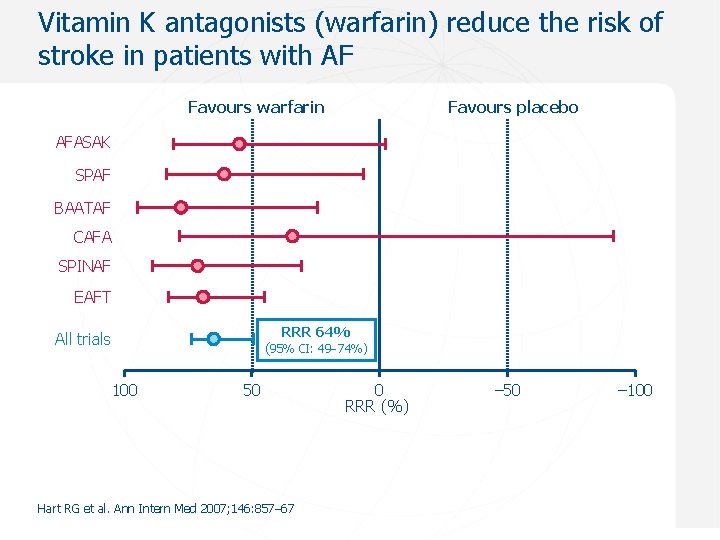
Vitamin K antagonists (warfarin) reduce the risk of stroke in patients with AF Favours warfarin Favours placebo AFASAK SPAF BAATAF CAFA SPINAF EAFT RRR 64% All trials (95% CI: 49 74%) 100 50 Hart RG et al. Ann Intern Med 2007; 146: 857– 67 0 RRR (%) – 50 – 100

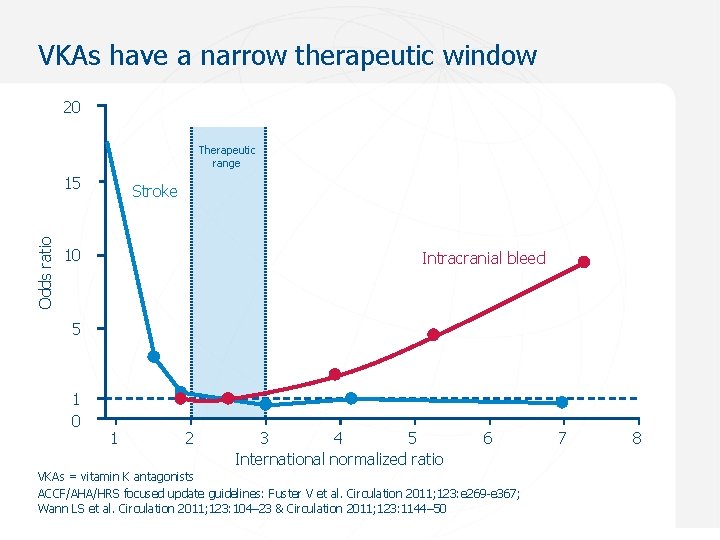
VKAs have a narrow therapeutic window 20 Therapeutic range Odds ratio 15 Stroke 10 Intracranial bleed 5 1 0 1 2 3 4 5 International normalized ratio 6 7 8 VKAs = vitamin K antagonists ACCF/AHA/HRS focused update guidelines: Fuster V et al. Circulation 2011; 123: e 269 -e 367; Wann LS et al. Circulation 2011; 123: 104– 23 & Circulation 2011; 123: 1144– 50 Sept 2012

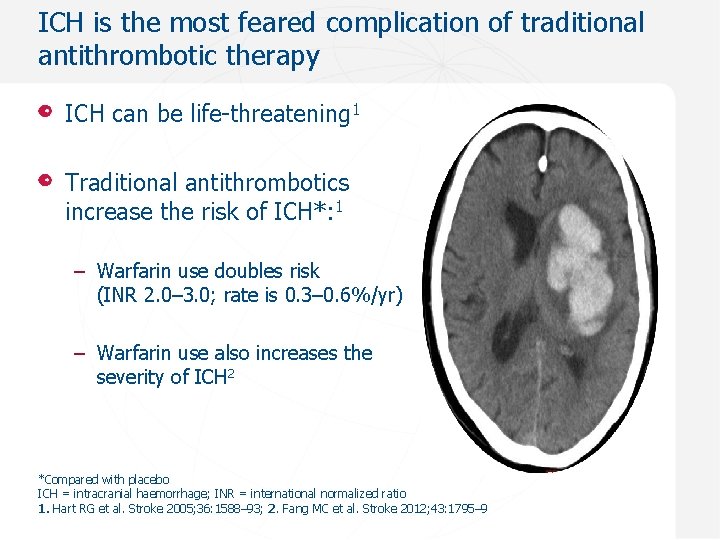
ICH is the most feared complication of traditional antithrombotic therapy ICH can be life-threatening 1 Traditional antithrombotics increase the risk of ICH*: 1 – Warfarin use doubles risk (INR 2. 0– 3. 0; rate is 0. 3– 0. 6%/yr) – Warfarin use also increases the severity of ICH 2 *Compared with placebo ICH = intracranial haemorrhage; INR = international normalized ratio 1. Hart RG et al. Stroke 2005; 36: 1588– 93; 2. Fang MC et al. Stroke 2012; 43: 1795– 9

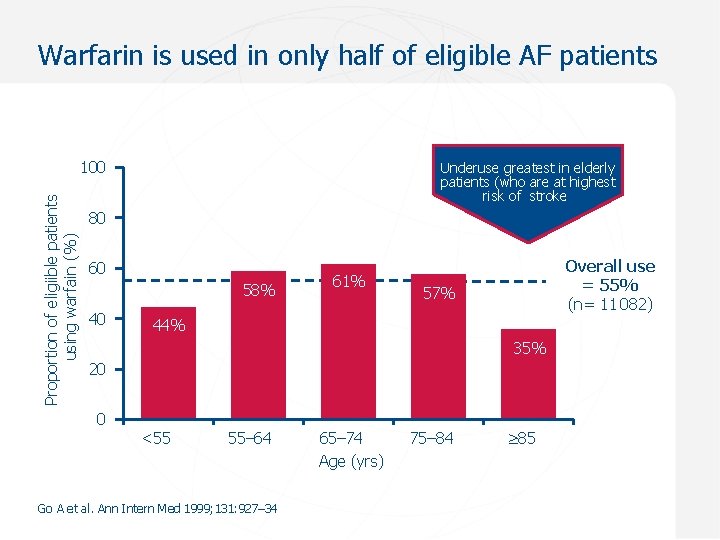
Warfarin is used in only half of eligible AF patients Proportion of eligiible patients using warfain (%) 100 Underuse greatest in elderly patients (who are at highest risk of stroke) 80 60 58% 40 61% Overall use = 55% (n= 11 082) 57% 44% 35% 20 0 <55 55– 64 Go A et al. Ann Intern Med 1999; 131: 927– 34 65– 74 Age (yrs) 75– 84 85

Global AF registry Funded by a grant from Boehringer Ingelheim Aim: to compare regional differences in predisposing conditions for AF and its treatment – Focus on BP management and anticoagulation Prospective registry across all continents – Patients enrolled between January 2008 and April 2011 – 47 countries, 163 sites, 15 174 patients Includes patients presenting to an emergency department BP = blood pressure Healey J et al. ESC 2011

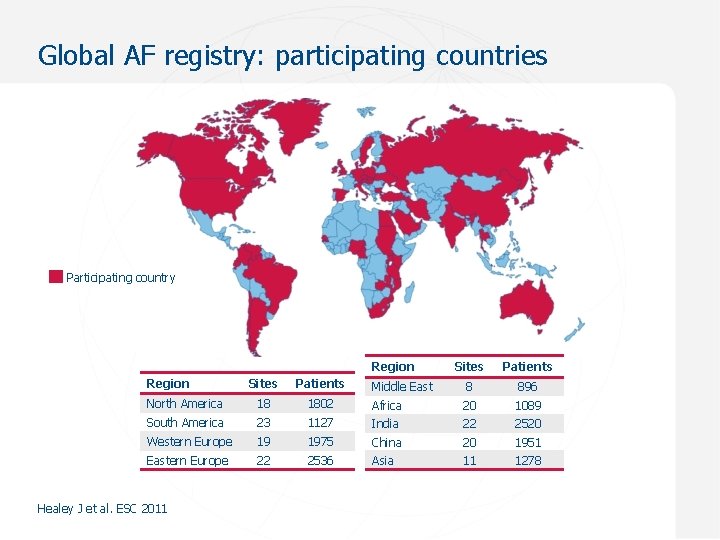
Global AF registry: participating countries Participating country Region Sites Patients Middle East 8 896 Sites Patients North America 18 1802 Africa 20 1089 South America 23 1127 India 22 2520 Western Europe 19 1975 China 20 1951 Eastern Europe 22 2536 Asia 11 1278 Healey J et al. ESC 2011

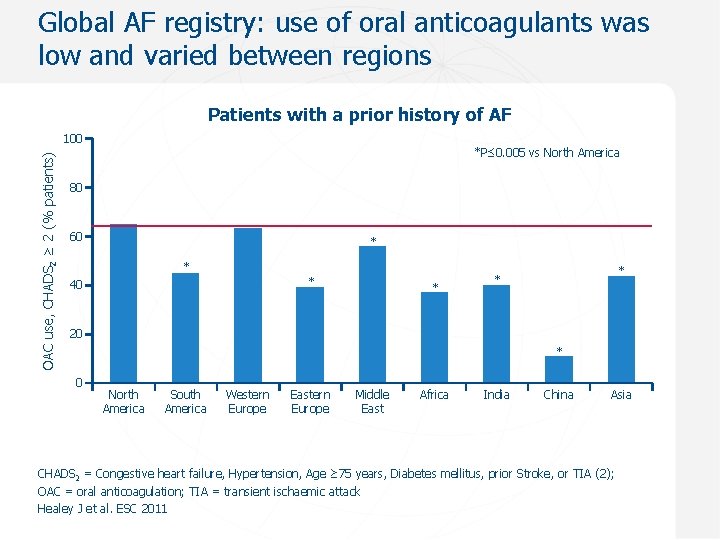
Global AF registry: use of oral anticoagulants was low and varied between regions Patients with a prior history of AF OAC use, CHADS 2 ≥ 2 (% patients) 100 *P≤ 0. 005 vs North America 80 60 * * * 40 * * * 20 * 0 North America South America Western Europe Eastern Europe Middle East Africa India China Asia CHADS 2 = Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, prior Stroke, or TIA (2); OAC = oral anticoagulation; TIA = transient ischaemic attack Healey J et al. ESC 2011

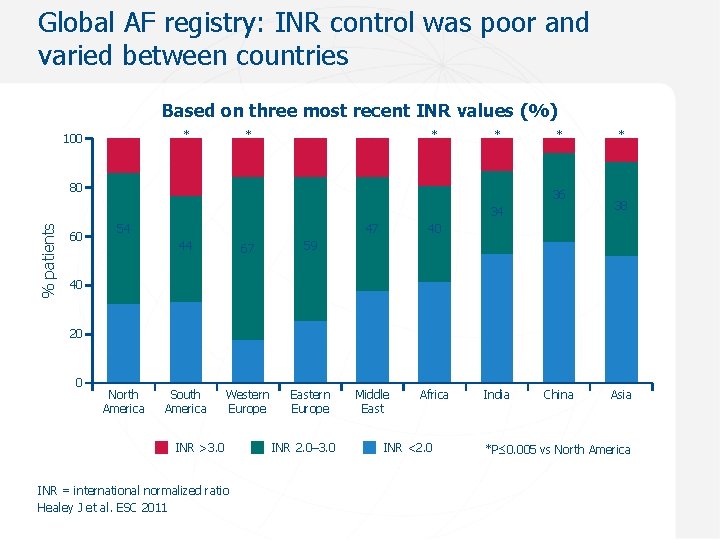
Global AF registry: INR control was poor and varied between countries Based on three most recent INR values (%) * 100 * * * 80 * 36 % patients 34 60 54 44 67 59 South America Western Europe Eastern Europe 47 40 Middle East Africa * 38 40 20 0 North America INR >3. 0 INR = international normalized ratio Healey J et al. ESC 2011 INR 2. 0– 3. 0 INR <2. 0 India China Asia *P≤ 0. 005 vs North America

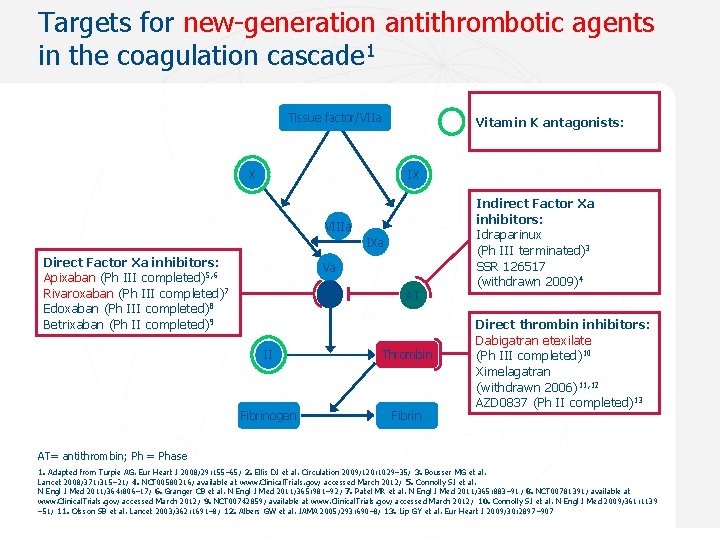
Targets for new-generation antithrombotic agents in the coagulation cascade 1 Tissue factor/VIIa X Vitamin K antagonists: IX VIIIa IXa Direct Factor Xa inhibitors: Apixaban (Ph III completed)5, 6 Rivaroxaban (Ph III completed)7 Edoxaban (Ph III completed)8 Betrixaban (Ph II completed)9 Va Xa AT II Thrombin Fibrinogen Fibrin Indirect Factor Xa inhibitors: Idraparinux (Ph III terminated)3 SSR 126517 (withdrawn 2009)4 Direct thrombin inhibitors: Dabigatran etexilate (Ph III completed)10 Ximelagatran (withdrawn 2006)11, 12 AZD 0837 (Ph II completed)13 AT= antithrombin; Ph = Phase 1. Adapted from Turpie AG. Eur Heart J 2008; 29: 155– 65; 2. Ellis DJ et al. Circulation 2009; 120: 1029– 35; 3. Bousser MG et al. Lancet 2008; 371: 315– 21; 4. NCT 00580216; available at www. Clinical. Trials. gov; accessed March 2012; 5. Connolly SJ et al. N Engl J Med 2011; 364: 806– 17; 6. Granger CB et al. N Engl J Med 2011; 365: 981– 92; 7. Patel MR et al. N Engl J Med 2011; 365: 883– 91; 8. NCT 00781391; available at www. Clinical. Trials. gov; accessed March 2012; 9. NCT 00742859; available at www. Clinical. Trials. gov; accessed March 2012; 10. Connolly SJ et al. N Engl J Med 2009; 361: 1139 – 51; 11. Olsson SB et al. Lancet 2003; 362: 1691– 8; 12. Albers GW et al. JAMA 2005; 293: 690– 8; 13. Lip GY et al. Eur Heart J 2009; 30: 2897– 907 Sept 2012

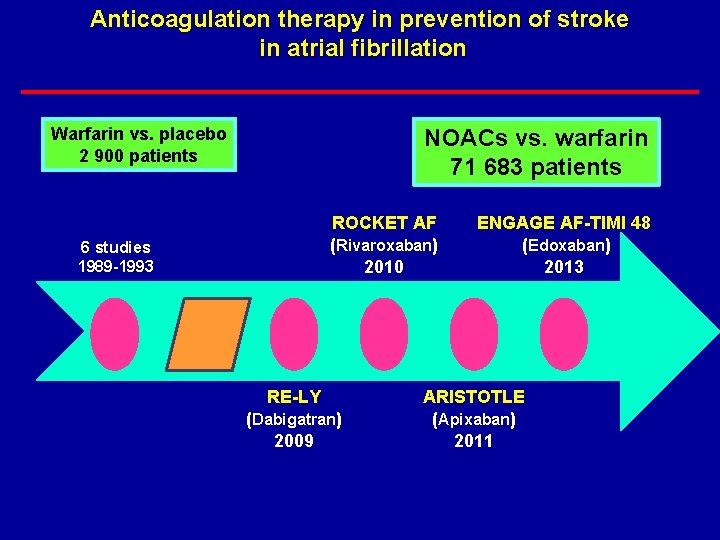
Anticoagulation therapy in prevention of stroke in atrial fibrillation Warfarin vs. placebo 2 900 patients NOACs vs. warfarin 71 683 patients ROCKET AF (Rivaroxaban) 2010 6 studies 1989 -1993 RE-LY (Dabigatran) 2009 ENGAGE AF-TIMI 48 (Edoxaban) 2013 ARISTOTLE (Apixaban) 2011

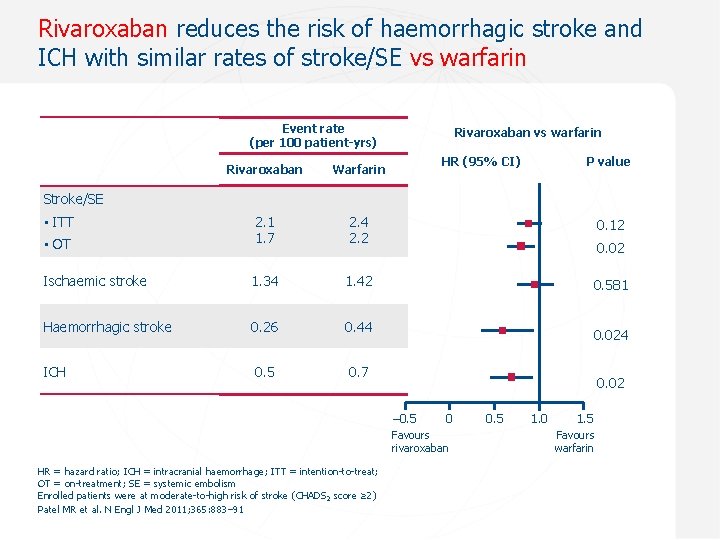
Rivaroxaban reduces the risk of haemorrhagic stroke and ICH with similar rates of stroke/SE vs warfarin Event rate (per 100 patient-yrs) Rivaroxaban vs warfarin HR (95% CI) P value Rivaroxaban Warfarin 2. 4 2. 2 0. 12 • OT 2. 1 1. 7 Ischaemic stroke 1. 34 1. 42 0. 581 Haemorrhagic stroke 0. 26 0. 44 ICH 0. 5 0. 7 Stroke/SE • ITT 0. 024 0. 02 – 0. 5 0 Favours 0. 1 rivaroxaban HR = hazard ratio; ICH = intracranial haemorrhage; ITT = intention-to-treat; OT = on-treatment; SE = systemic embolism Enrolled patients were at moderate-to-high risk of stroke (CHADS 2 score ≥ 2) Patel MR et al. N Engl J Med 2011; 365: 883– 91 0. 5 1. 0 1. 5 Favours warfarin

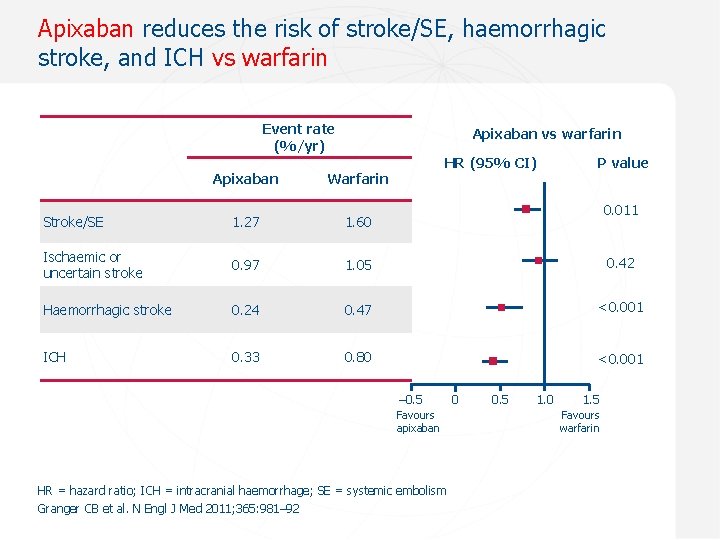
Apixaban reduces the risk of stroke/SE, haemorrhagic stroke, and ICH vs warfarin Event rate (%/yr) Apixaban vs warfarin HR (95% CI) P value Apixaban Warfarin Stroke/SE 1. 27 1. 60 Ischaemic or uncertain stroke 0. 97 1. 05 0. 42 Haemorrhagic stroke 0. 24 0. 47 <0. 001 ICH 0. 33 0. 80 <0. 001 0. 011 – 0. 5 0. 1 Favours apixaban HR = hazard ratio; ICH = intracranial haemorrhage; SE = systemic embolism Granger CB et al. N Engl J Med 2011; 365: 981– 92 0 0. 5 1. 0 1. 5 Favours warfarin

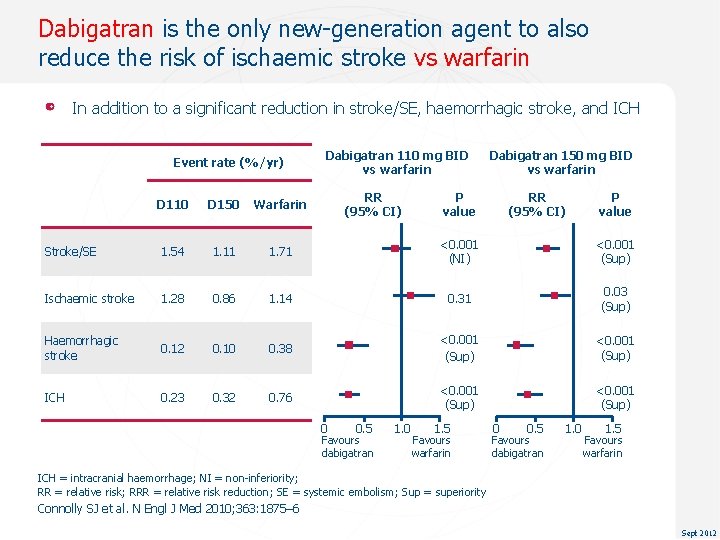
Dabigatran is the only new-generation agent to also reduce the risk of ischaemic stroke vs warfarin In addition to a significant reduction in stroke/SE, haemorrhagic stroke, and ICH Event rate (%/yr) Dabigatran 110 mg BID vs warfarin RR (95% CI) Dabigatran 150 mg BID vs warfarin P value RR (95% CI) P value D 110 D 150 Warfarin Stroke/SE 1. 54 1. 11 1. 71 <0. 001 (NI) <0. 001 (Sup) Ischaemic stroke 1. 28 0. 86 1. 14 0. 31 0. 03 (Sup) Haemorrhagic stroke 0. 12 0. 10 0. 38 <0. 001 (Sup) ICH 0. 23 0. 32 0. 76 <0. 001 (Sup) 0 0. 5 Favours dabigatran 1. 0 1. 5 Favours warfarin ICH = intracranial haemorrhage; NI = non-inferiority; RR = relative risk; RRR = relative risk reduction; SE = systemic embolism; Sup = superiority Connolly SJ et al. N Engl J Med 2010; 363: 1875– 6 Sept 2012

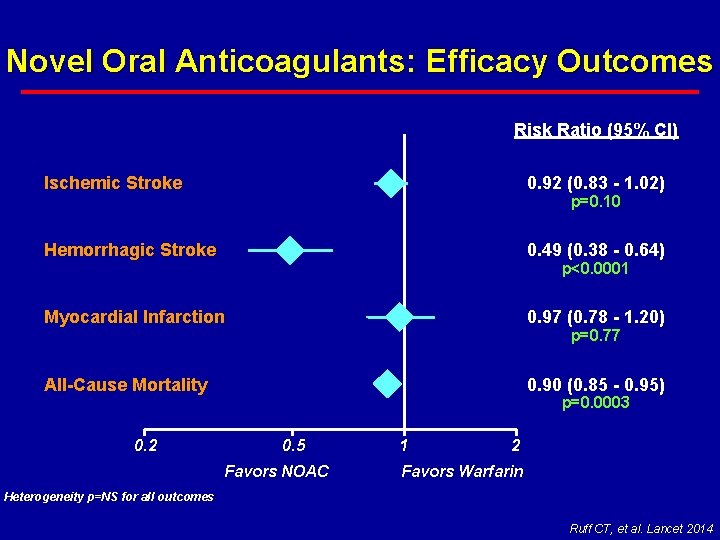
Novel Oral Anticoagulants: Efficacy Outcomes Risk Ratio (95% CI) Ischemic Stroke 0. 92 (0. 83 - 1. 02) Hemorrhagic Stroke 0. 49 (0. 38 - 0. 64) Myocardial Infarction 0. 97 (0. 78 - 1. 20) All-Cause Mortality 0. 90 (0. 85 - 0. 95) 0. 2 p=0. 10 p<0. 0001 p=0. 77 p=0. 0003 0. 5 Favors NOAC 1 2 Favors Warfarin Heterogeneity p=NS for all outcomes Ruff CT, et al. Lancet 2014

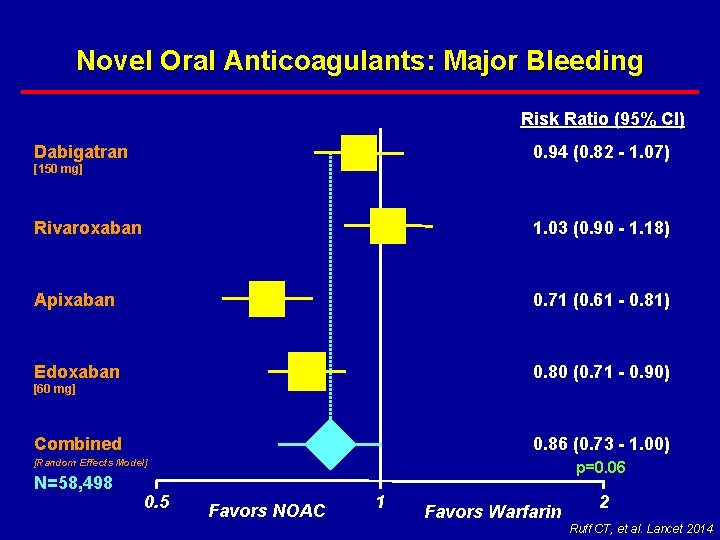
Novel Oral Anticoagulants: Major Bleeding Risk Ratio (95% CI) 0. 94 (0. 82 - 1. 07) Dabigatran [150 mg] Rivaroxaban 1. 03 (0. 90 - 1. 18) Apixaban 0. 71 (0. 61 - 0. 81) Edoxaban 0. 80 (0. 71 - 0. 90) Combined 0. 86 (0. 73 - 1. 00) [60 mg] [Random Effects Model] N=58, 498 0. 5 p=0. 06 Favors NOAC 1 Favors Warfarin 2 Ruff CT, et al. Lancet 2014

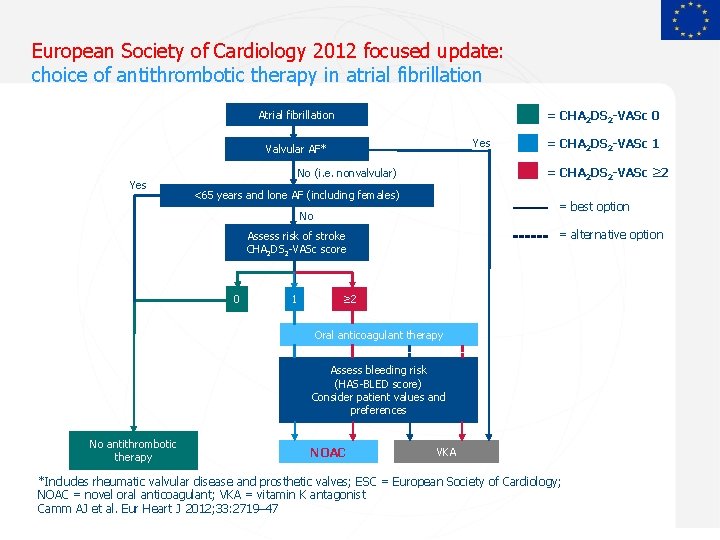
European Society of Cardiology 2012 focused update: choice of antithrombotic therapy in atrial fibrillation Atrial fibrillation = CHA 2 DS 2 -VASc 0 Yes Valvular AF* Yes = CHA 2 DS 2 -VASc ≥ 2 No (i. e. nonvalvular) <65 years and lone AF (including females) = best option No = alternative option Assess risk of stroke CHA 2 DS 2 -VASc score 0 1 = CHA 2 DS 2 -VASc 1 ≥ 2 Oral anticoagulant therapy Assess bleeding risk (HAS-BLED score) Consider patient values and preferences No antithrombotic therapy NOAC VKA *Includes rheumatic valvular disease and prosthetic valves; ESC = European Society of Cardiology; NOAC = novel oral anticoagulant; VKA = vitamin K antagonist Camm AJ et al. Eur Heart J 2012; 33: 2719– 47 Sept 2012

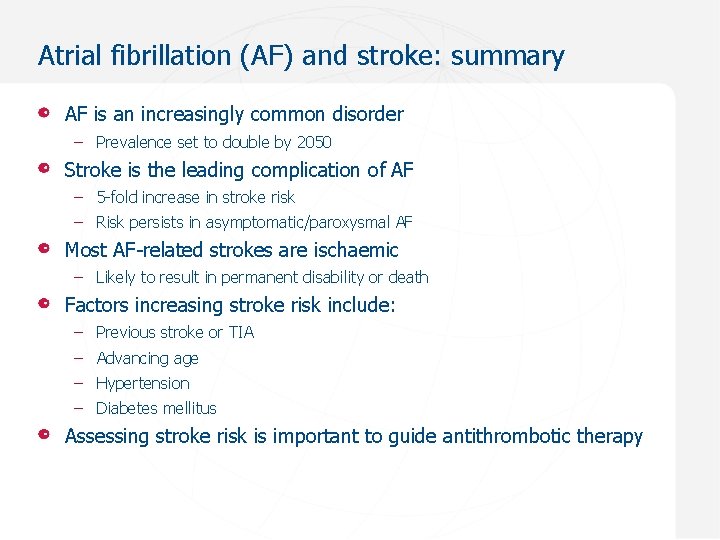
Atrial fibrillation (AF) and stroke: summary AF is an increasingly common disorder – Prevalence set to double by 2050 Stroke is the leading complication of AF – 5 -fold increase in stroke risk – Risk persists in asymptomatic/paroxysmal AF Most AF-related strokes are ischaemic – Likely to result in permanent disability or death Factors increasing stroke risk include: – Previous stroke or TIA – Advancing age – Hypertension – Diabetes mellitus Assessing stroke risk is important to guide antithrombotic therapy

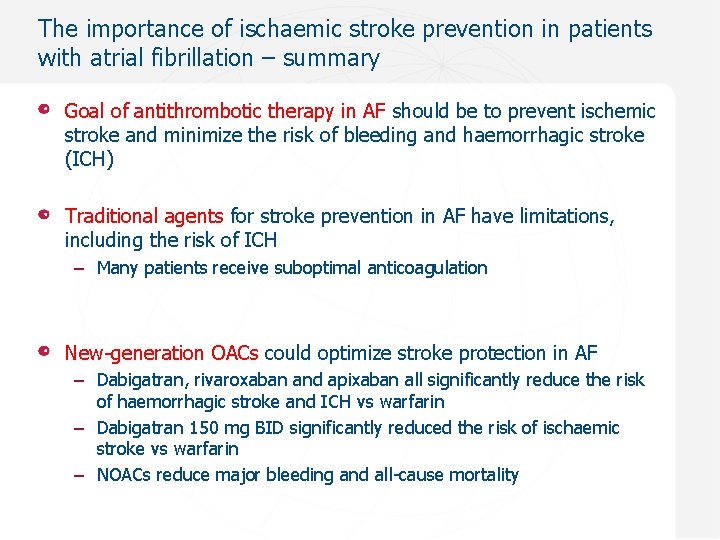
The importance of ischaemic stroke prevention in patients with atrial fibrillation – summary Goal of antithrombotic therapy in AF should be to prevent ischemic stroke and minimize the risk of bleeding and haemorrhagic stroke (ICH) Traditional agents for stroke prevention in AF have limitations, including the risk of ICH – Many patients receive suboptimal anticoagulation New-generation OACs could optimize stroke protection in AF – Dabigatran, rivaroxaban and apixaban all significantly reduce the risk of haemorrhagic stroke and ICH vs warfarin – Dabigatran 150 mg BID significantly reduced the risk of ischaemic stroke vs warfarin – NOACs reduce major bleeding and all-cause mortality

Thank you !
 Ichaemic
Ichaemic Anterior stroke vs posterior stroke
Anterior stroke vs posterior stroke Dr mazhar cardiologist
Dr mazhar cardiologist Pseudofusion beat
Pseudofusion beat Endocardial cushion defects
Endocardial cushion defects Dr slezka cardiologist
Dr slezka cardiologist Cardiologist in kodaikanal
Cardiologist in kodaikanal Dr susan nolan
Dr susan nolan Dr amit shah cardiologist
Dr amit shah cardiologist Gpwsi cardiology
Gpwsi cardiology Beaumont cardiologist near me
Beaumont cardiologist near me Modified duke criteria 2020
Modified duke criteria 2020 Dr siegfried cardiologist
Dr siegfried cardiologist Petr lucie a tma
Petr lucie a tma Stavitel petr
Stavitel petr Petr měl obdélník šířky 2 cm a neznámé délky
Petr měl obdélník šířky 2 cm a neznámé délky Petr a lucie rozbor
Petr a lucie rozbor Petr a lucie hlavní myšlenka
Petr a lucie hlavní myšlenka Petr skryja
Petr skryja Petr iljič čajkovskij prezentace
Petr iljič čajkovskij prezentace Petr dejmek
Petr dejmek Petr svoboda lab
Petr svoboda lab Petr lapukhov
Petr lapukhov Petr dejmek
Petr dejmek Vnve
Vnve Petr dokáže udělat celou práci sám za 6 hodin
Petr dokáže udělat celou práci sám za 6 hodin Hlsie
Hlsie Petr dejmek
Petr dejmek Koleso text
Koleso text Petr paukner
Petr paukner Petr knapp
Petr knapp Petr kolenko
Petr kolenko Petr kolenko
Petr kolenko Petr rikov
Petr rikov Petr vech
Petr vech Petr gapko
Petr gapko Petr vech
Petr vech Petr jahn
Petr jahn