Enzyme Kinetics Protein Folding 972004 Protein folding is














- Slides: 54

Enzyme Kinetics & Protein Folding 9/7/2004

Protein folding is “one of the great unsolved problems of science” Alan Fersht

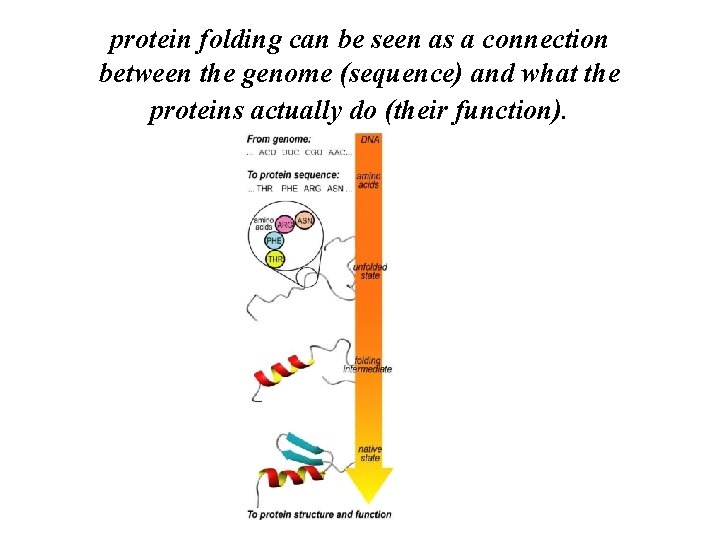
protein folding can be seen as a connection between the genome (sequence) and what the proteins actually do (their function).

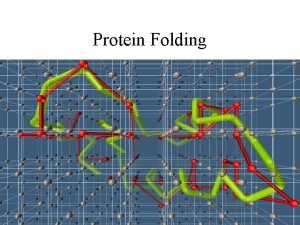
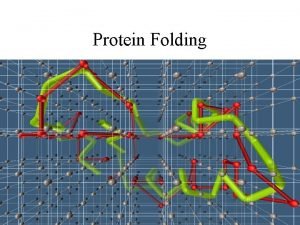
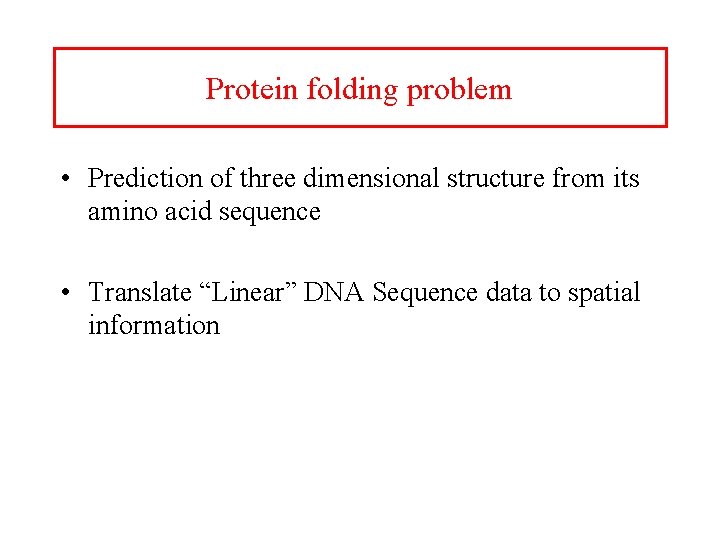
Protein folding problem • Prediction of three dimensional structure from its amino acid sequence • Translate “Linear” DNA Sequence data to spatial information

Why solve the folding problem? • Acquisition of sequence data relatively quick • Acquisition of experimental structural information slow • Limited to proteins that crystallize or stable in solution for NMR

Protein folding dynamics Electrostatics, hydrogen bonds and van der Waals forces hold a protein together. Hydrophobic effects force global protein conformation. Peptide chains can be cross-linked by disulfides, Zinc, heme or other liganding compounds. Zinc has a complete d orbital , one stable oxidation state and forms ligands with sulfur, nitrogen and oxygen. Proteins refold very rapidly and generally in only one stable conformation.

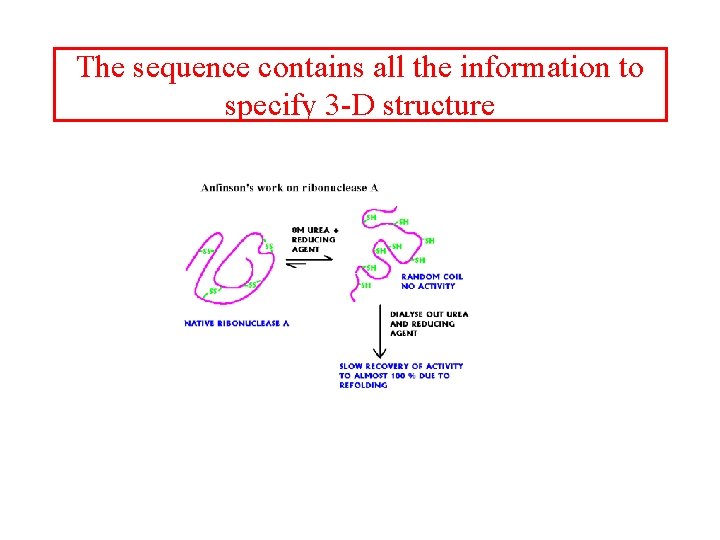
The sequence contains all the information to specify 3 -D structure

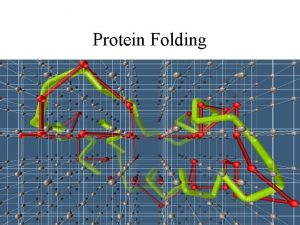
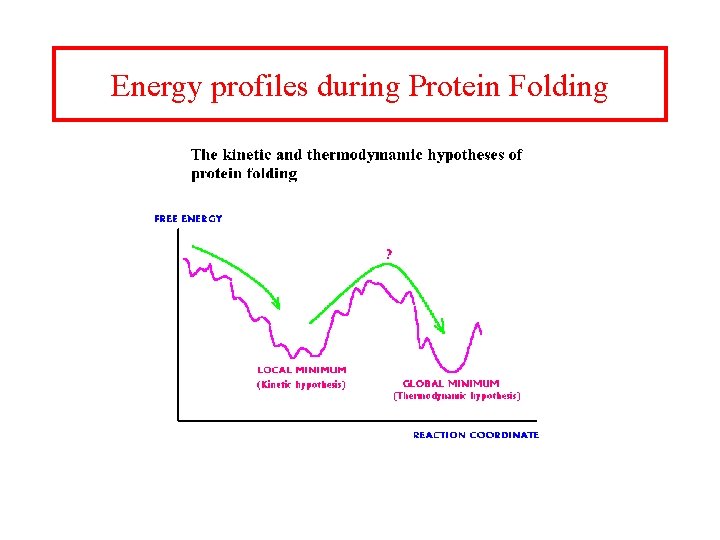
Random search and the Levinthal paradox • The initial stages of folding must be nearly random, but if the entire process was a random search it would require too much time. Consider a 100 residue protein. If each residue is considered to have just 3 possible conformations the total number of conformations of the protein is 3100. Conformational changes occur on a time scale of 10 -13 seconds i. e. the time required to sample all possible conformations would be 3100 x 10 -13 seconds which is about 1027 years. Even if a significant proportion of these conformations are sterically disallowed the folding time would still be astronomical. Proteins are known to fold on a time scale of seconds to minutes and hence energy barriers probably cause the protein to fold along a definite pathway.

Energy profiles during Protein Folding


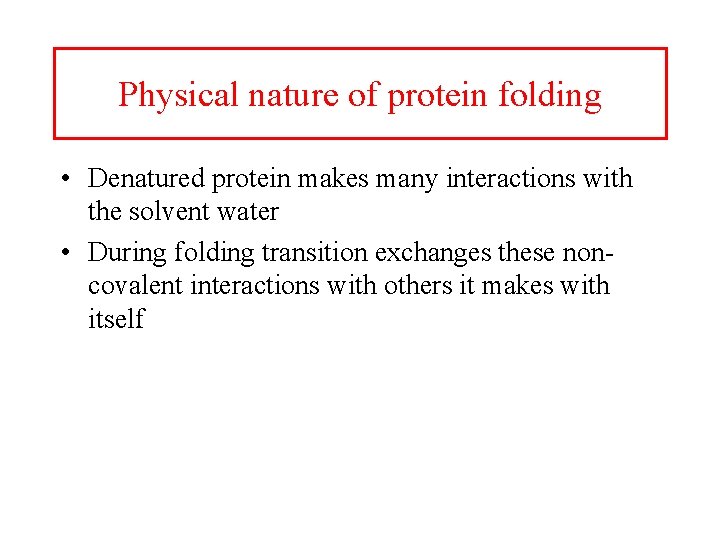
Physical nature of protein folding • Denatured protein makes many interactions with the solvent water • During folding transition exchanges these noncovalent interactions with others it makes with itself

What happens if proteins don't fold correctly? • Diseases such as Alzheimer's disease, cystic fibrosis, Mad Cow disease, an inherited form of emphysema, and even many cancers are believed to result from protein misfolding

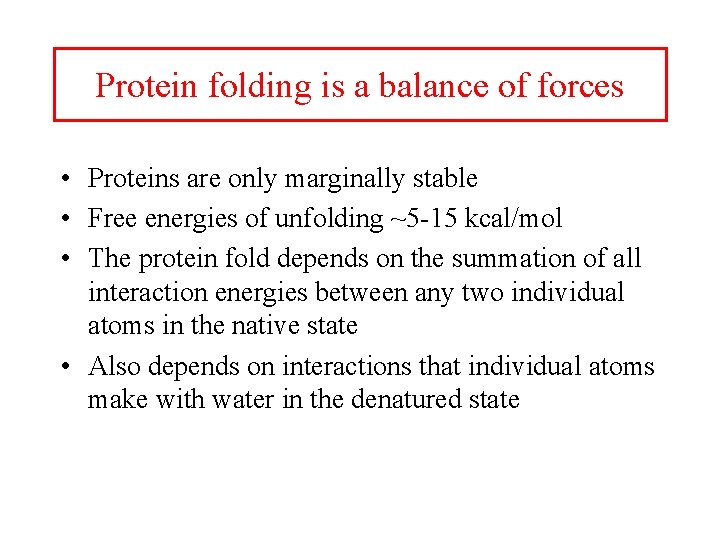
Protein folding is a balance of forces • Proteins are only marginally stable • Free energies of unfolding ~5 -15 kcal/mol • The protein fold depends on the summation of all interaction energies between any two individual atoms in the native state • Also depends on interactions that individual atoms make with water in the denatured state

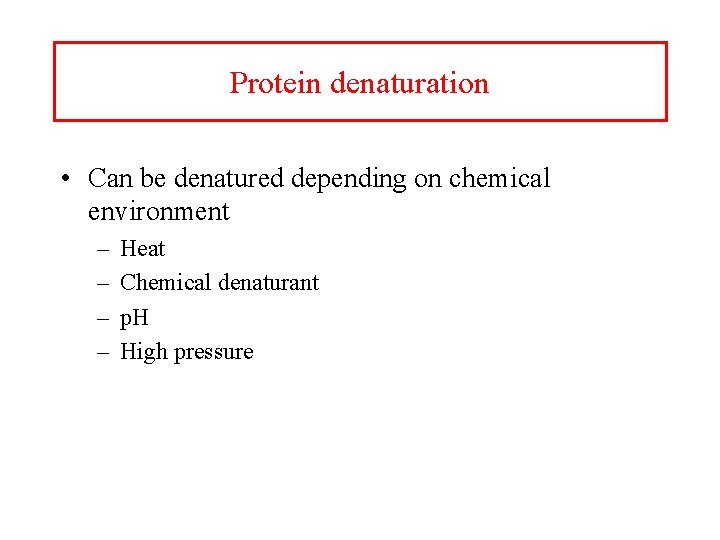
Protein denaturation • Can be denatured depending on chemical environment – – Heat Chemical denaturant p. H High pressure

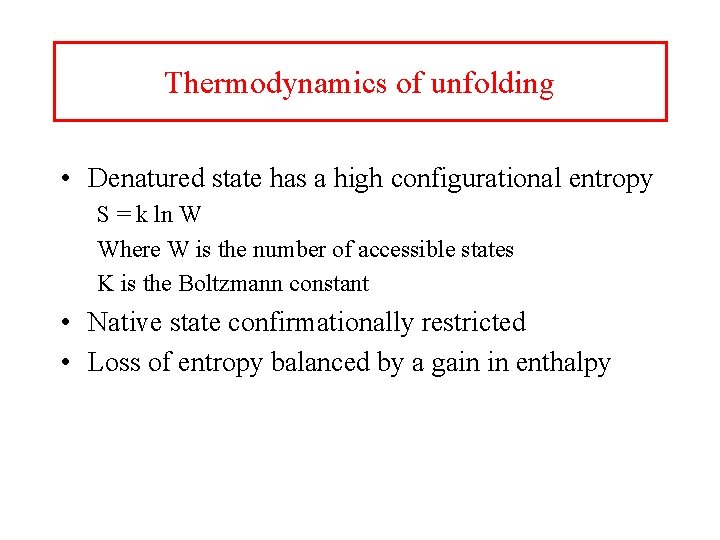
Thermodynamics of unfolding • Denatured state has a high configurational entropy S = k ln W Where W is the number of accessible states K is the Boltzmann constant • Native state confirmationally restricted • Loss of entropy balanced by a gain in enthalpy

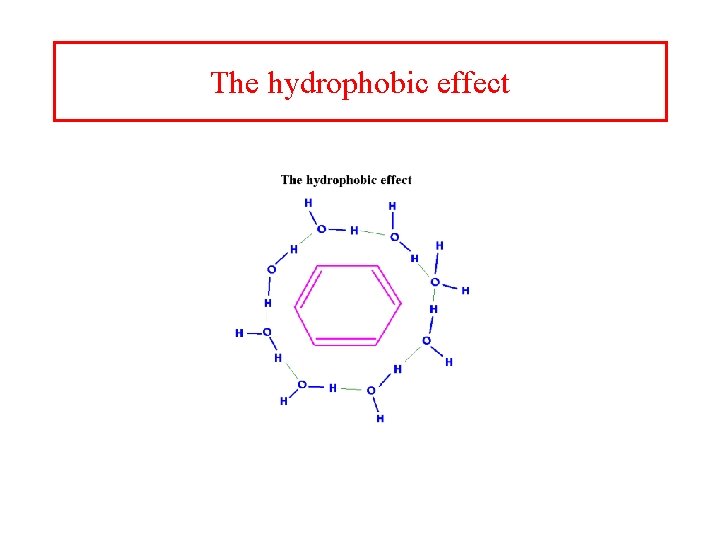
Entropy and enthaply of water must be added • The contribution of water has two important consequences – Entropy of release of water upon folding – The specific heat of unfolding (ΔCp) • “icebergs” of solvent around exposed hydrophobics • Weakly structured regions in the denatured state

The hydrophobic effect

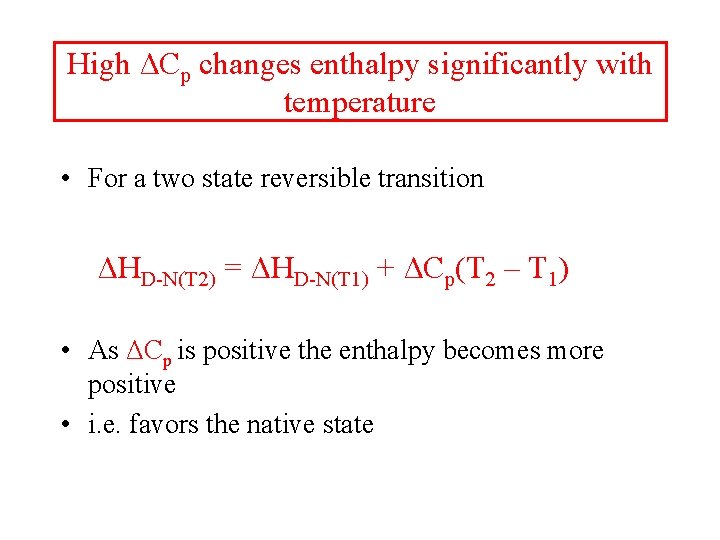
High ΔCp changes enthalpy significantly with temperature • For a two state reversible transition ΔHD-N(T 2) = ΔHD-N(T 1) + ΔCp(T 2 – T 1) • As ΔCp is positive the enthalpy becomes more positive • i. e. favors the native state

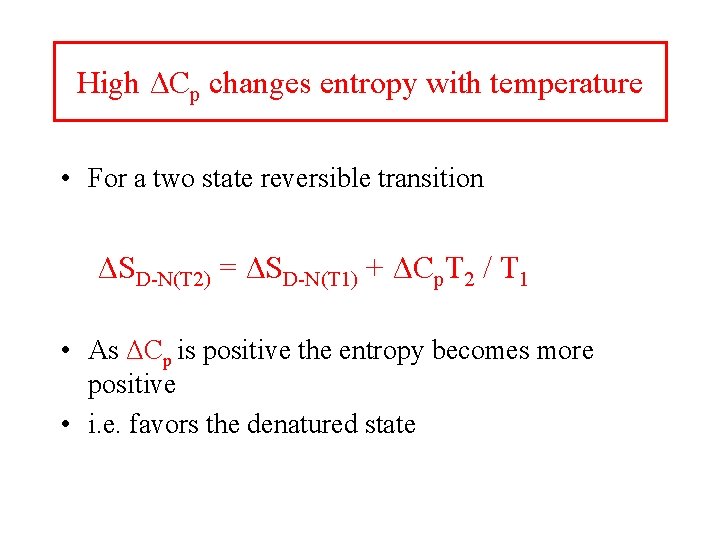
High ΔCp changes entropy with temperature • For a two state reversible transition ΔSD-N(T 2) = ΔSD-N(T 1) + ΔCp. T 2 / T 1 • As ΔCp is positive the entropy becomes more positive • i. e. favors the denatured state

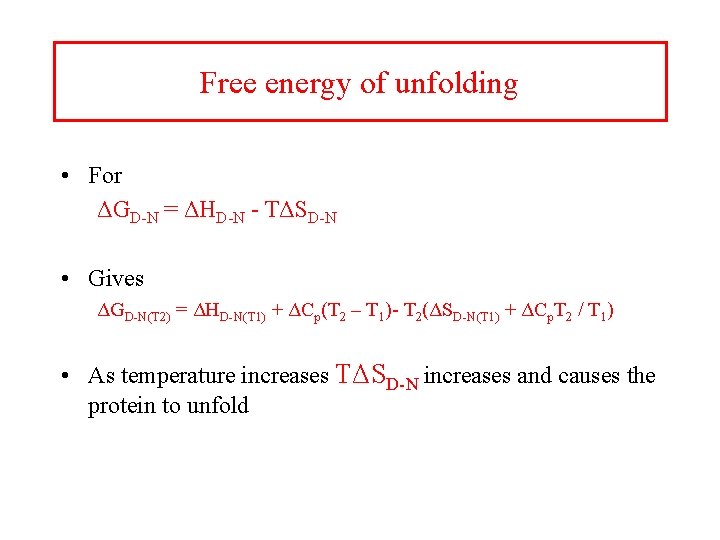
Free energy of unfolding • For ΔGD-N = ΔHD-N - TΔSD-N • Gives ΔGD-N(T 2) = ΔHD-N(T 1) + ΔCp(T 2 – T 1)- T 2(ΔSD-N(T 1) + ΔCp. T 2 / T 1) • As temperature increases TΔSD-N increases and causes the protein to unfold

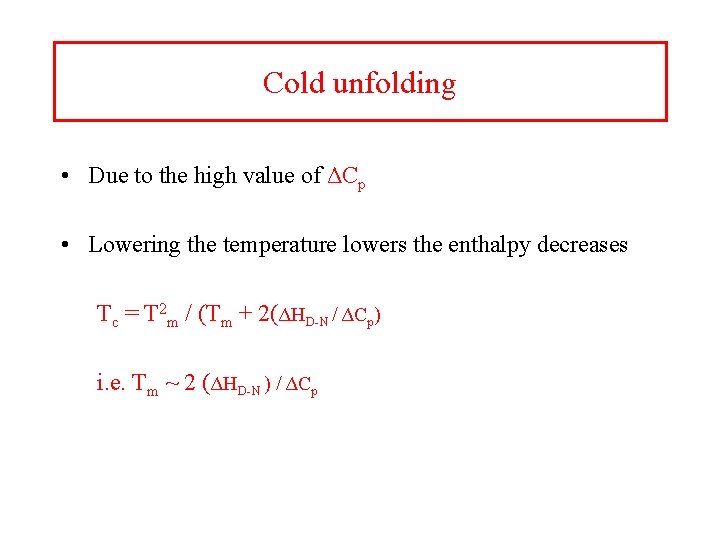
Cold unfolding • Due to the high value of ΔCp • Lowering the temperature lowers the enthalpy decreases Tc = T 2 m / (Tm + 2(ΔHD-N / ΔCp) i. e. Tm ~ 2 (ΔHD-N ) / ΔCp

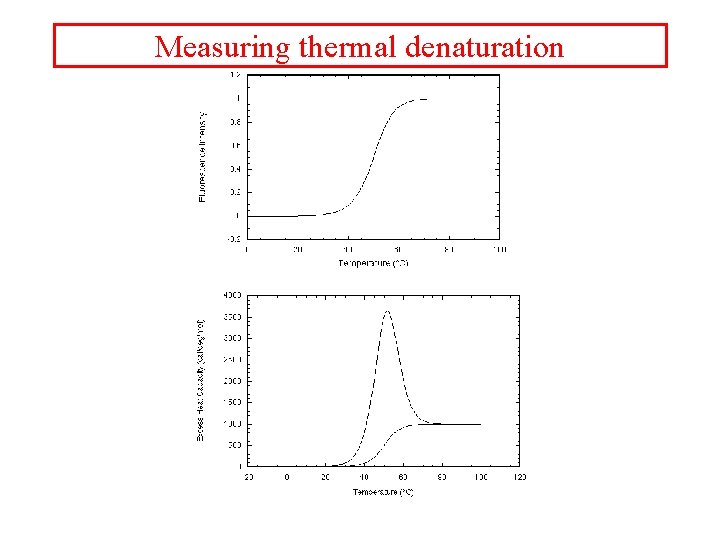
Measuring thermal denaturation

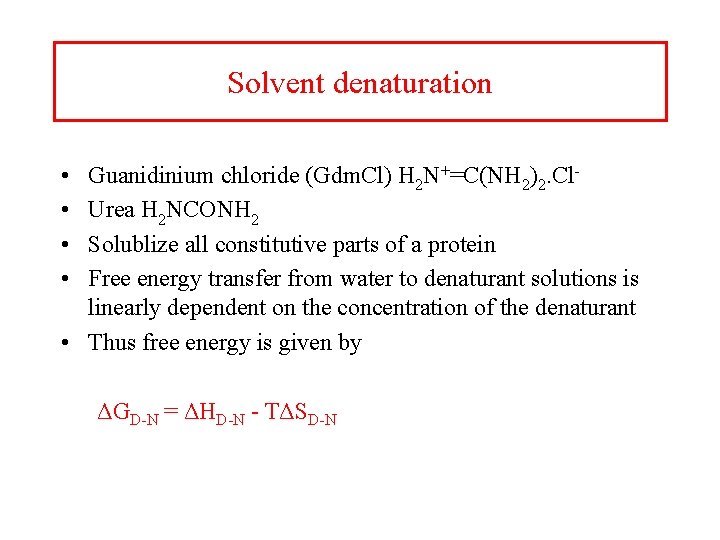
Solvent denaturation • • Guanidinium chloride (Gdm. Cl) H 2 N+=C(NH 2)2. Cl- Urea H 2 NCONH 2 Solublize all constitutive parts of a protein Free energy transfer from water to denaturant solutions is linearly dependent on the concentration of the denaturant • Thus free energy is given by ΔGD-N = ΔHD-N - TΔSD-N

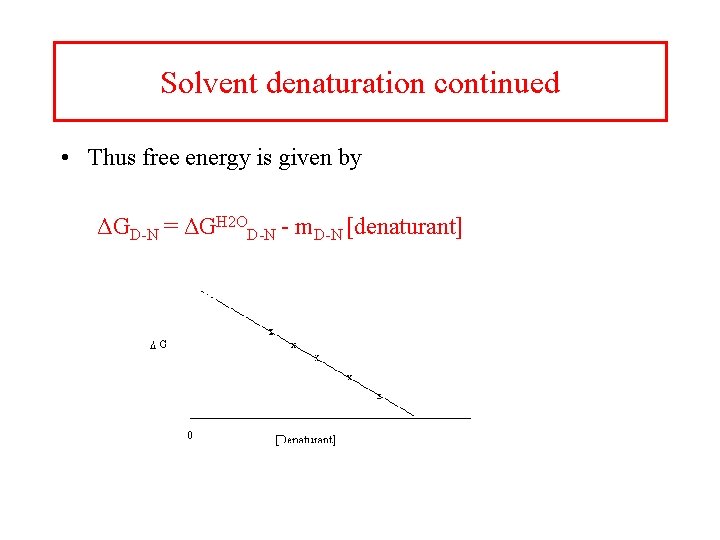
Solvent denaturation continued • Thus free energy is given by ΔGD-N = ΔGH 2 OD-N - m. D-N [denaturant]

Acid - Base denaturation • Most protein’s denature at extremes of p. H • Primarily due to perturbed p. Ka’s of buried groups • e. g. buried salt bridges

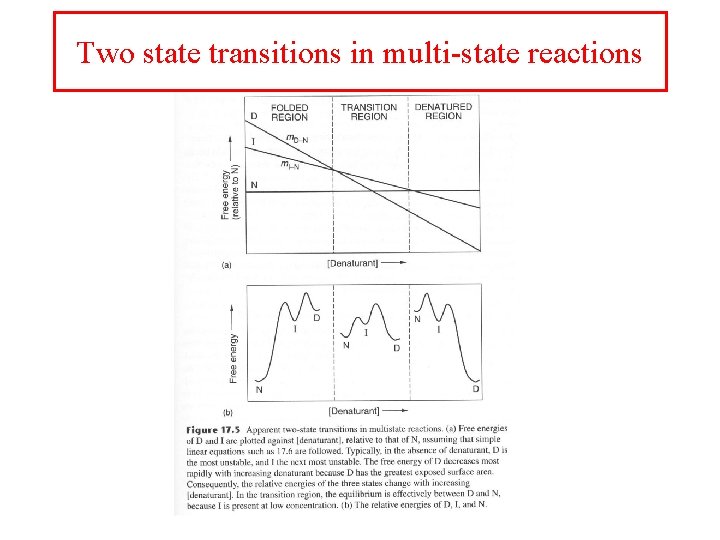
Two state transitions • Proteins have a folded (N) and unfolded (D) state • May have an intermediate state (I) • Many proteins undergo a simple two state transition D <—> N

Folding of a 20 -mer poly Ala

Unfolding of the DNA Binding Domain of HIV Integrase

Two state transitions in multi-state reactions

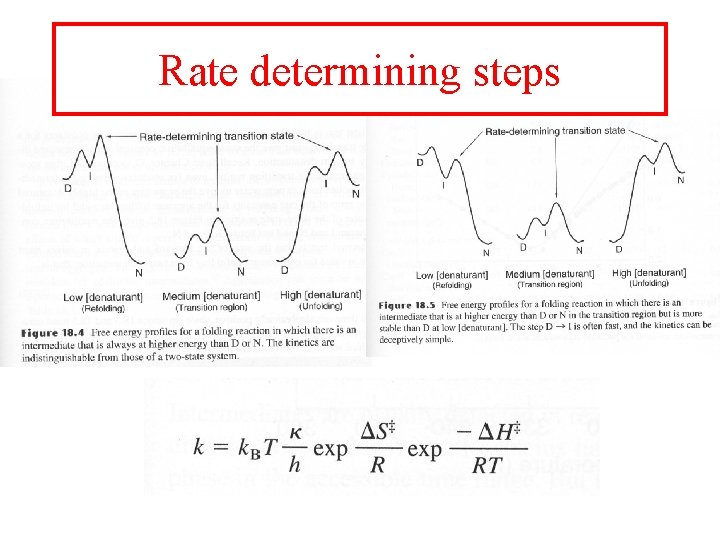
Rate determining steps



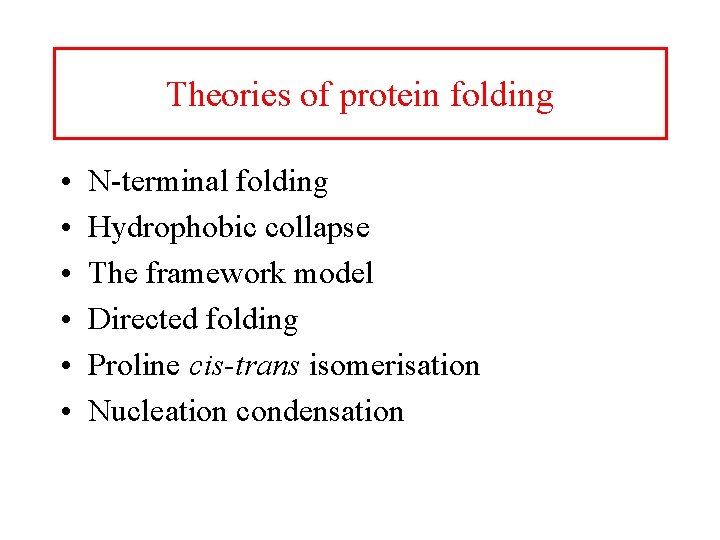
Theories of protein folding • • • N-terminal folding Hydrophobic collapse The framework model Directed folding Proline cis-trans isomerisation Nucleation condensation

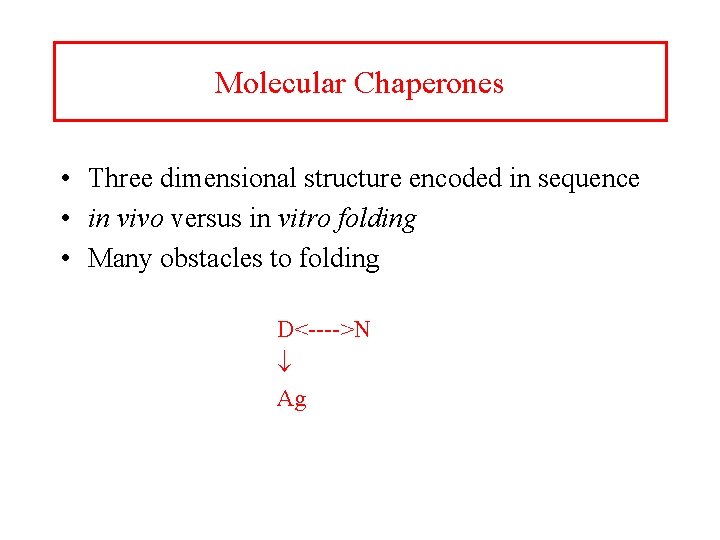
Molecular Chaperones • Three dimensional structure encoded in sequence • in vivo versus in vitro folding • Many obstacles to folding D<---->N Ag

Molecular Chaperone Function • • • Disulfide isomerases Peptidyl-prolyl isomerases (cyclophilin, FK 506) Bind the denatured state formed on ribozome Heat shock proteins Hsp (Dna. K) Protein export & delivery Sec. B

What happens if proteins don't fold correctly? • Diseases such as Alzheimer's disease, cystic fibrosis, Mad Cow disease, an inherited form of emphysema, and even many cancers are believed to result from protein misfolding

Gro. EL

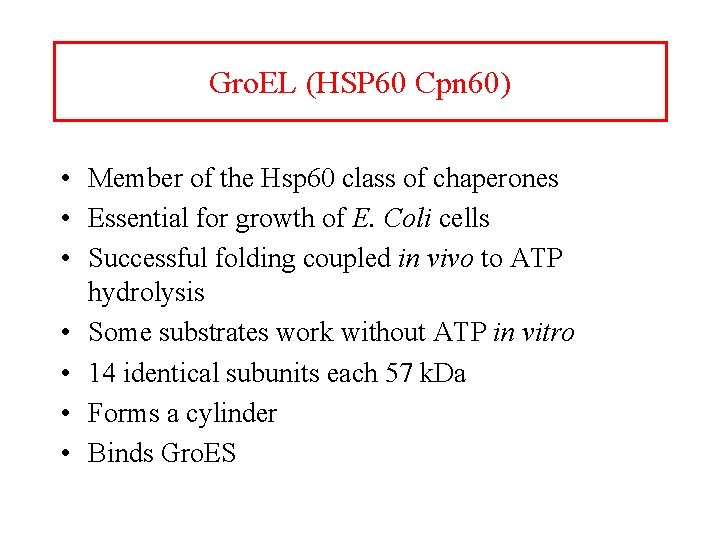
Gro. EL (HSP 60 Cpn 60) • Member of the Hsp 60 class of chaperones • Essential for growth of E. Coli cells • Successful folding coupled in vivo to ATP hydrolysis • Some substrates work without ATP in vitro • 14 identical subunits each 57 k. Da • Forms a cylinder • Binds Gro. ES

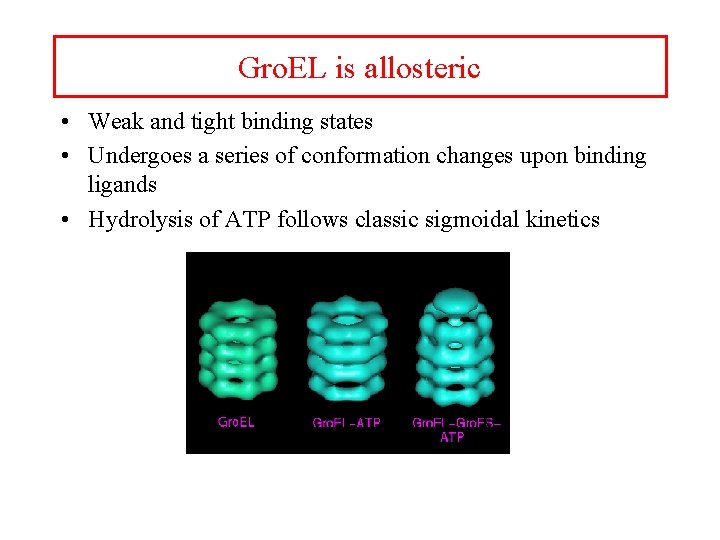
Gro. EL is allosteric • Weak and tight binding states • Undergoes a series of conformation changes upon binding ligands • Hydrolysis of ATP follows classic sigmoidal kinetics

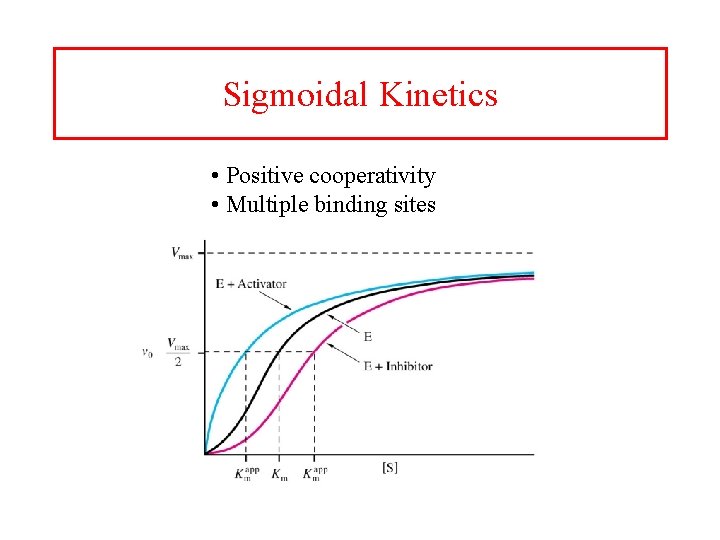
Sigmoidal Kinetics • Positive cooperativity • Multiple binding sites

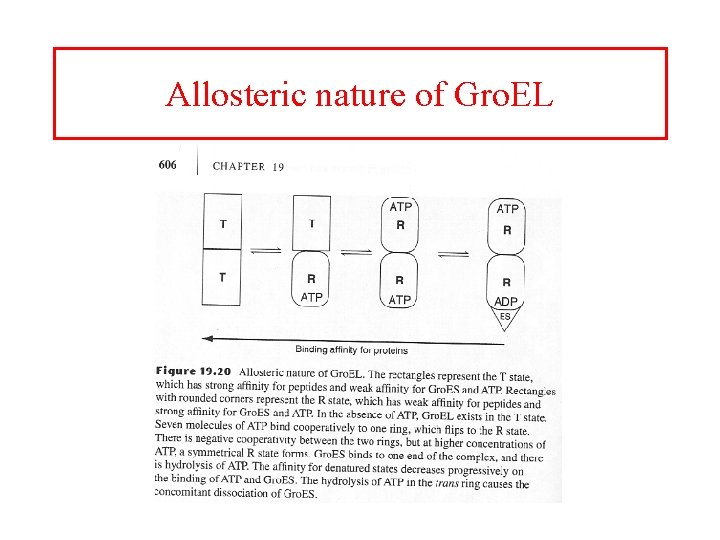
Allosteric nature of Gro. EL

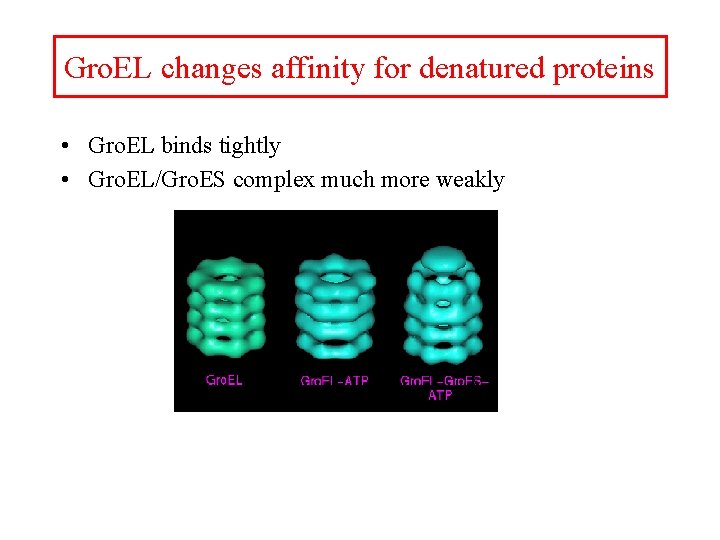
Gro. EL changes affinity for denatured proteins • Gro. EL binds tightly • Gro. EL/Gro. ES complex much more weakly

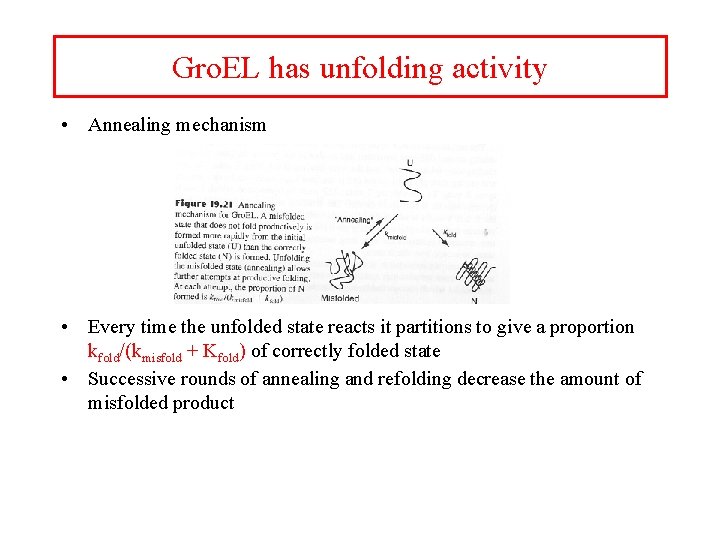
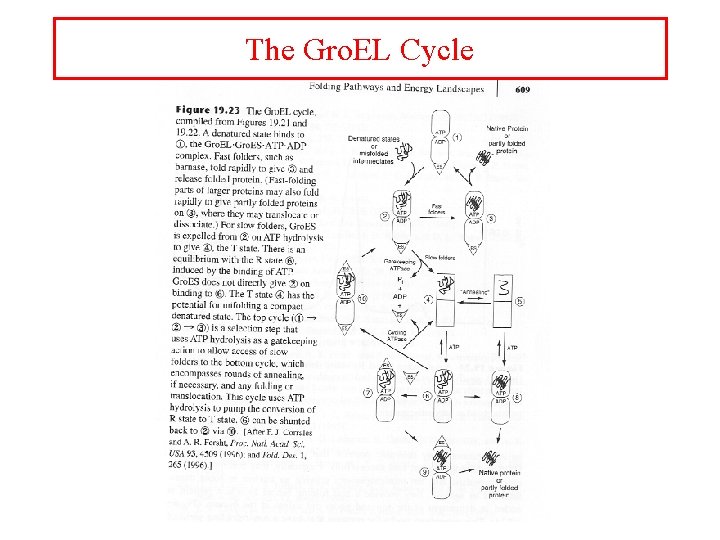
Gro. EL has unfolding activity • Annealing mechanism • Every time the unfolded state reacts it partitions to give a proportion kfold/(kmisfold + Kfold) of correctly folded state • Successive rounds of annealing and refolding decrease the amount of misfolded product

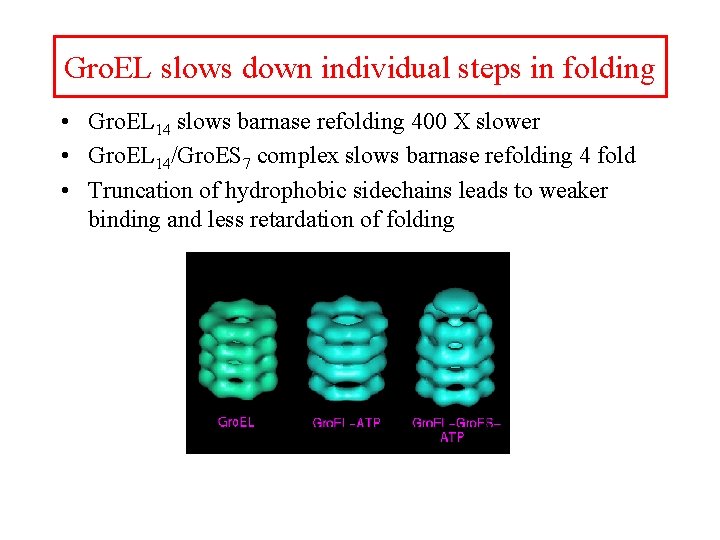
Gro. EL slows down individual steps in folding • Gro. EL 14 slows barnase refolding 400 X slower • Gro. EL 14/Gro. ES 7 complex slows barnase refolding 4 fold • Truncation of hydrophobic sidechains leads to weaker binding and less retardation of folding

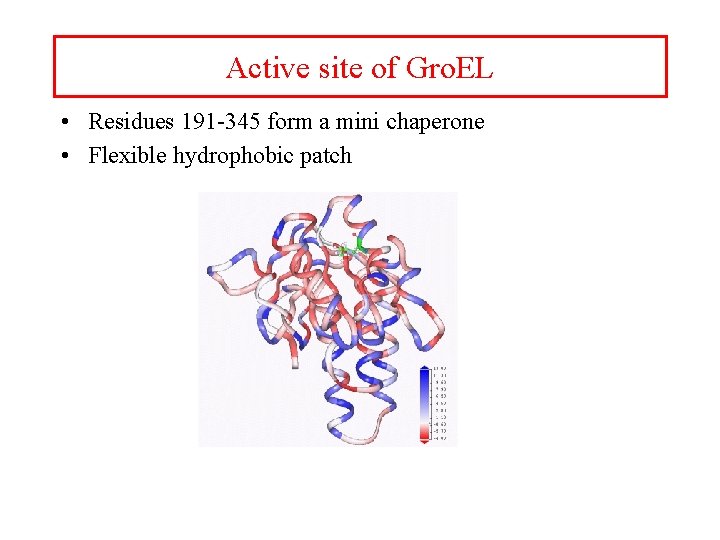
Active site of Gro. EL • Residues 191 -345 form a mini chaperone • Flexible hydrophobic patch

Role of ATP hydrolysis

The Gro. EL Cycle

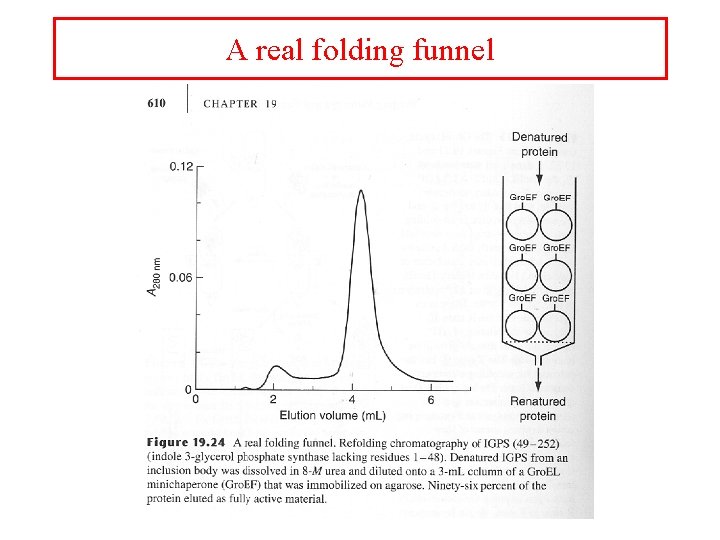
A real folding funnel

Amyloids • A last type of effect of misfolded protein • protein deposits in the cells as fibrils • A number of common diseases of old age, such as Alzheimer's disease fit into this category, and in some cases an inherited version occurs, which has enabled study of the defective protein

Known amyloidogenic peptides CJD spongiform encepalopathies prion protein fragments APP Alzheimer beta protein fragment 1 -40/43 HRA hemodialysis-related amyloidosis beta-2 microglobin* PSA primary systmatic amyloidosis immunoglobulin light chain and fragments SAA 1 secondary systmatic amyloidosis serum amyloid A 78 residue fragment FAP I** familial amyloid polyneuropathy I transthyretin fragments, 50+ allels FAP III familial amyloid polyneuropathy III apolipoprotein A-1 fragments CAA cerebral amyloid angiopathy cystatin C minus 10 residues FHSA Finnish hereditary systemic amyloidosis gelsolin 71 aa fragment IAPP type II diabetes islet amyloid polypeptide fragment (amylin) ILA injection-localized amyloidosis insulin CAL medullary thyroid carcinoma calcitonin fragments ANF atrial amyloidosis atrial natriuretic factor NNSA non-neuropathic systemic amylodosis lysozyme and fragments HRA hereditary renal amyloidosis fibrinogen fragments

Transthyretin • transports thyroxin and retinol binding protein in the bloodstream and cerebrospinal fluid • senile systemic amyloidosis, which affects people over 80, transtherytin forms fibrillar deposits in the heart. which leads to congestive heart failure • Familial amyloid polyneuropathy (FAP) affects much younger people; causing protein deposits in the heart, and in many other tissues; deposits around nerves can lead to paralysis

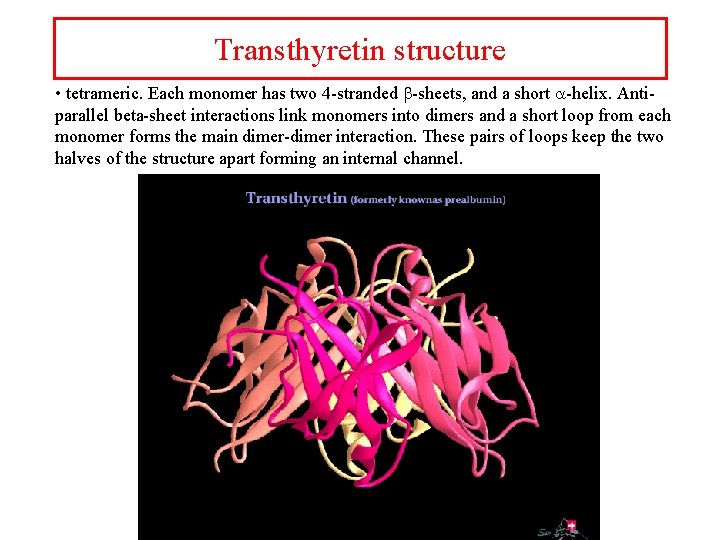
Transthyretin structure • tetrameric. Each monomer has two 4 -stranded b-sheets, and a short a-helix. Antiparallel beta-sheet interactions link monomers into dimers and a short loop from each monomer forms the main dimer-dimer interaction. These pairs of loops keep the two halves of the structure apart forming an internal channel.

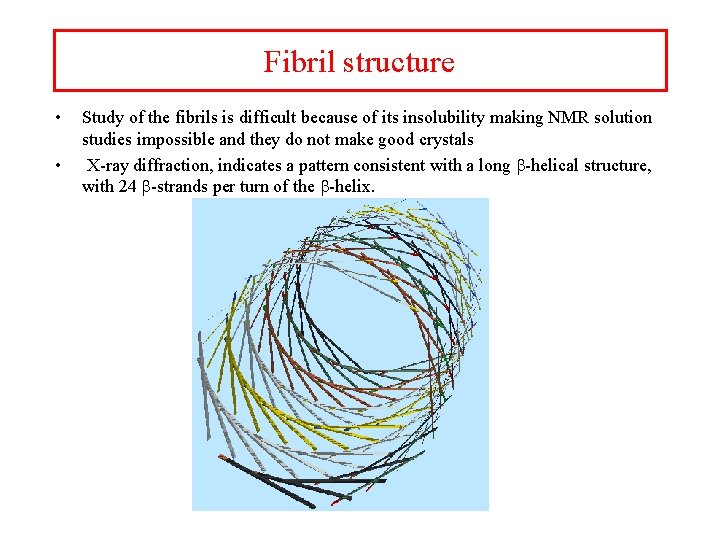
Fibril structure • • Study of the fibrils is difficult because of its insolubility making NMR solution studies impossible and they do not make good crystals X-ray diffraction, indicates a pattern consistent with a long b-helical structure, with 24 b-strands per turn of the b-helix.

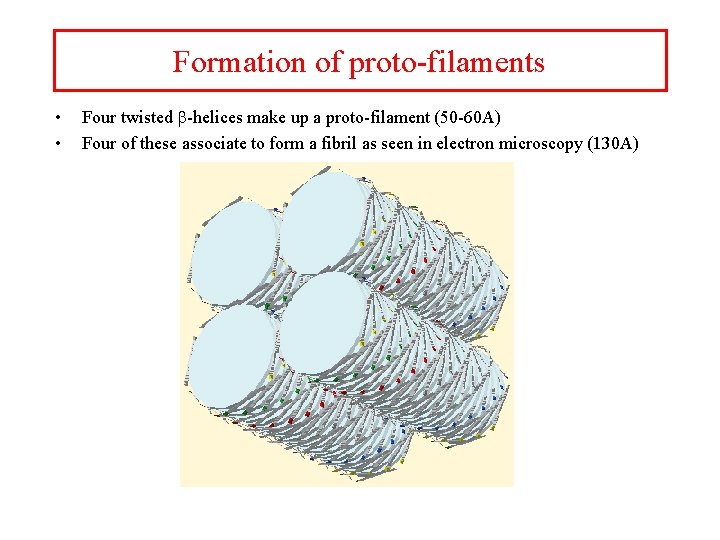
Formation of proto-filaments • • Four twisted b-helices make up a proto-filament (50 -60 A) Four of these associate to form a fibril as seen in electron microscopy (130 A)
 Pseudo 1st order reaction
Pseudo 1st order reaction Enzyme kinetics
Enzyme kinetics Km in enzyme kinetics
Km in enzyme kinetics Enzyme vs protein
Enzyme vs protein Enzymes affect the reactions in living cells by
Enzymes affect the reactions in living cells by Define enzyme
Define enzyme Hydrophobic collapse in protein folding
Hydrophobic collapse in protein folding Protein folding
Protein folding Role of chaperones in protein folding ppt
Role of chaperones in protein folding ppt Hydrophobic collapse in protein folding
Hydrophobic collapse in protein folding Enzyme-linked receptor
Enzyme-linked receptor Denaturation and protein folding
Denaturation and protein folding How do proteins fold
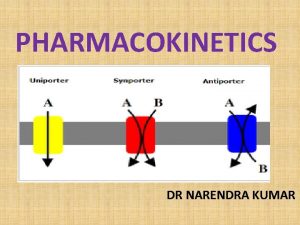
How do proteins fold Channel vs carrier proteins
Channel vs carrier proteins Protein-protein docking
Protein-protein docking Pre equilibrium approximation
Pre equilibrium approximation Vampnets for deep learning of molecular kinetics
Vampnets for deep learning of molecular kinetics Kinematics and kinetics of rigid bodies
Kinematics and kinetics of rigid bodies Kinetics of rigid bodies engineering mechanics
Kinetics of rigid bodies engineering mechanics Kinetics of crystal violet fading
Kinetics of crystal violet fading First order drug elimination
First order drug elimination Kinetics reaction
Kinetics reaction Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Order of kinetics
Order of kinetics Factors affecting distribution of drugs
Factors affecting distribution of drugs Data kinetics ltd
Data kinetics ltd Kinetics of a particle: force and acceleration
Kinetics of a particle: force and acceleration Octet biosensors
Octet biosensors Collision theory of kinetics
Collision theory of kinetics Applications of chemical kinetics
Applications of chemical kinetics Kinetics is the branch of:
Kinetics is the branch of: Dynafit kinetics
Dynafit kinetics Chemical kinetics definition
Chemical kinetics definition Fbd and kd
Fbd and kd Kcat
Kcat Kinetics ap chemistry
Kinetics ap chemistry Kinetics of particles newton's second law
Kinetics of particles newton's second law Types of reactions grade 11
Types of reactions grade 11 Molecularity
Molecularity Growth kinetics
Growth kinetics Fermenter
Fermenter Leukocyte development kinetics and functions
Leukocyte development kinetics and functions Planar kinetics of a rigid body: force and acceleration
Planar kinetics of a rigid body: force and acceleration Difference between zero and first order kinetics
Difference between zero and first order kinetics Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy Chemical kinetics experiment
Chemical kinetics experiment Kinetics flotation chemicals
Kinetics flotation chemicals Kinetics and equilibrium
Kinetics and equilibrium Secondary bronchi
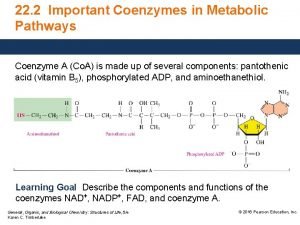
Secondary bronchi Function of co enzyme
Function of co enzyme Ec number in enzyme
Ec number in enzyme Enzyme
Enzyme Zinc is cofactor for which enzyme
Zinc is cofactor for which enzyme Carbohydrates digestion
Carbohydrates digestion Grandfather derek mahon poem
Grandfather derek mahon poem