Enzyme Kinetic Zhi Hui Enzyme Kinetics is the

![Important Conclusions of Michaels Menten Kinetics • when [S]= KM, the equation reduces to Important Conclusions of Michaels Menten Kinetics • when [S]= KM, the equation reduces to](https://slidetodoc.com/presentation_image/339789e1400f759c189a8a5948759f38/image-4.jpg)



![Influence of enzyme concentration v = k 3 [E], as [S]>>[E] Influence of enzyme concentration v = k 3 [E], as [S]>>[E]](https://slidetodoc.com/presentation_image/339789e1400f759c189a8a5948759f38/image-15.jpg)








- Slides: 34

Enzyme Kinetic Zhi Hui

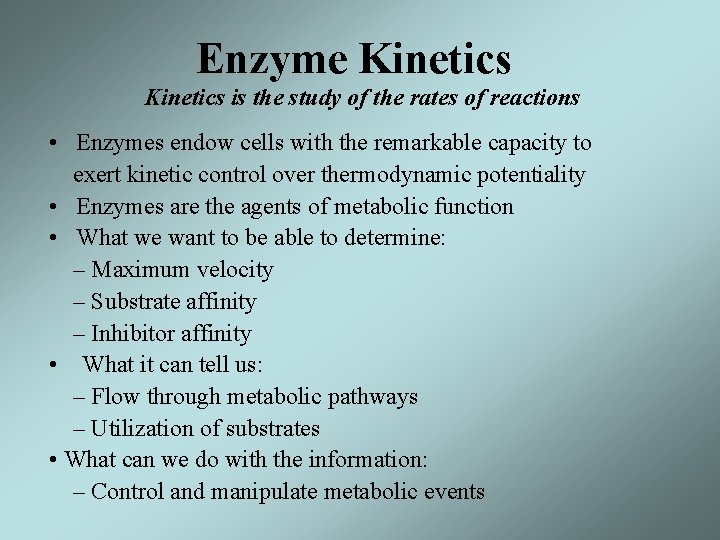
Enzyme Kinetics is the study of the rates of reactions • Enzymes endow cells with the remarkable capacity to exert kinetic control over thermodynamic potentiality • Enzymes are the agents of metabolic function • What we want to be able to determine: – Maximum velocity – Substrate affinity – Inhibitor affinity • What it can tell us: – Flow through metabolic pathways – Utilization of substrates • What can we do with the information: – Control and manipulate metabolic events

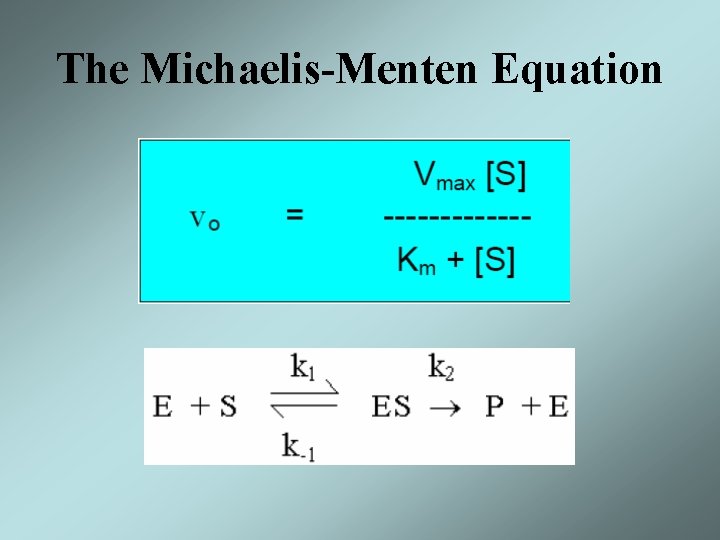
The Michaelis-Menten Equation
![Important Conclusions of Michaels Menten Kinetics when S KM the equation reduces to Important Conclusions of Michaels Menten Kinetics • when [S]= KM, the equation reduces to](https://slidetodoc.com/presentation_image/339789e1400f759c189a8a5948759f38/image-4.jpg)
Important Conclusions of Michaels Menten Kinetics • when [S]= KM, the equation reduces to • when [S] >> KM, the equation reduces to • when [S] << KM, the equation reduces to

Important Conclusions of Michaels Menten Kinetics

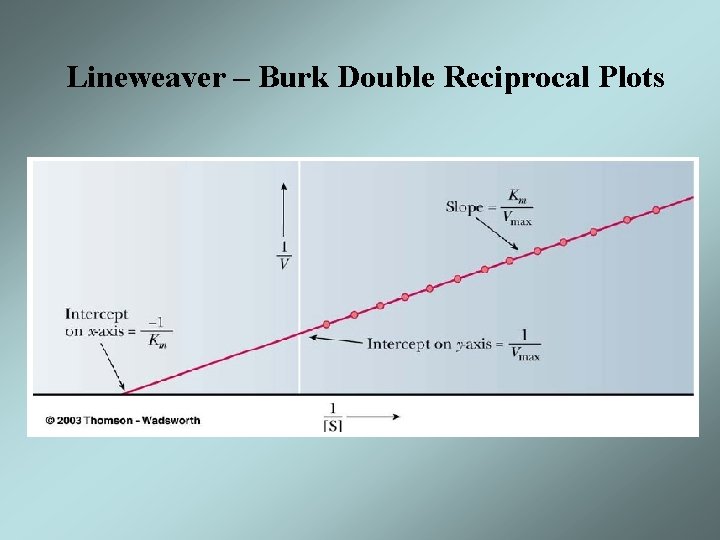
Lineweaver – Burk Double Reciprocal Plots • It is difficult to determine Vmax experimentally • The equation for a hyperbola can be transformed into the equation for a straight line by taking the reciprocal of each side • The formula for a straight line is y = mx + b • A plot of 1/V versus 1/[S] will give a straight line with slope of KM/Vmax and y intercept of 1/Vmax • Such a plot is known as a Lineweaver-Burk double reciprocal plot

Lineweaver – Burk Double Reciprocal Plots

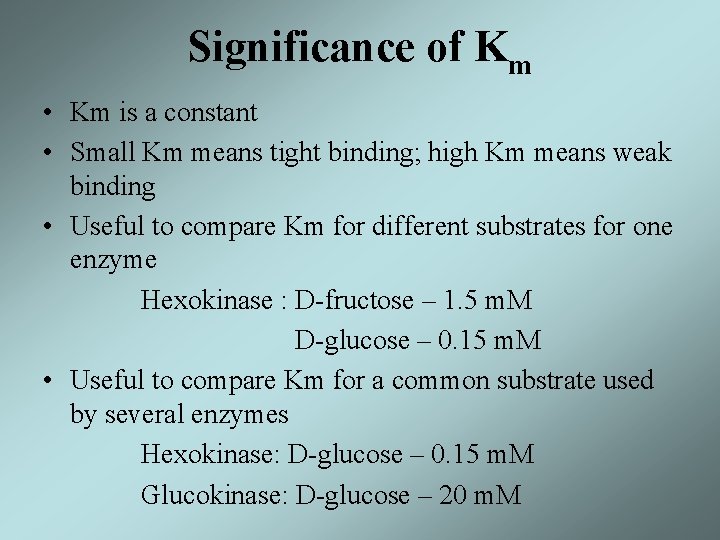
Significance of Km • Km is a constant • Small Km means tight binding; high Km means weak binding • Useful to compare Km for different substrates for one enzyme Hexokinase : D-fructose – 1. 5 m. M D-glucose – 0. 15 m. M • Useful to compare Km for a common substrate used by several enzymes Hexokinase: D-glucose – 0. 15 m. M Glucokinase: D-glucose – 20 m. M

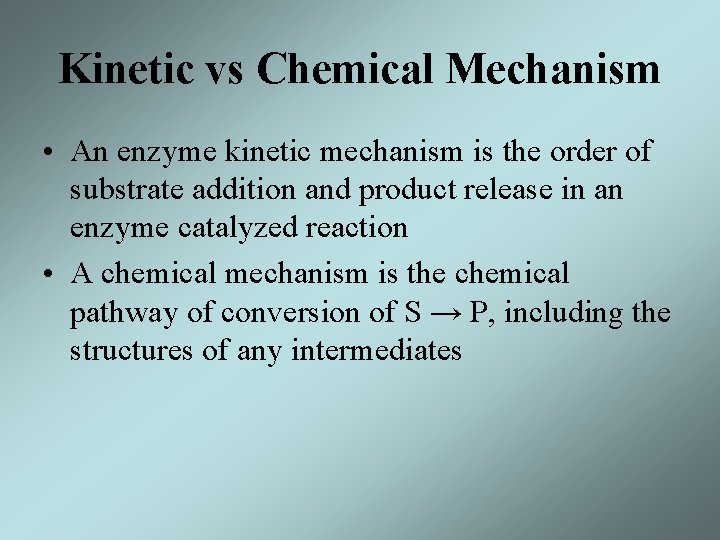
Kinetic vs Chemical Mechanism • An enzyme kinetic mechanism is the order of substrate addition and product release in an enzyme catalyzed reaction • A chemical mechanism is the chemical pathway of conversion of S → P, including the structures of any intermediates

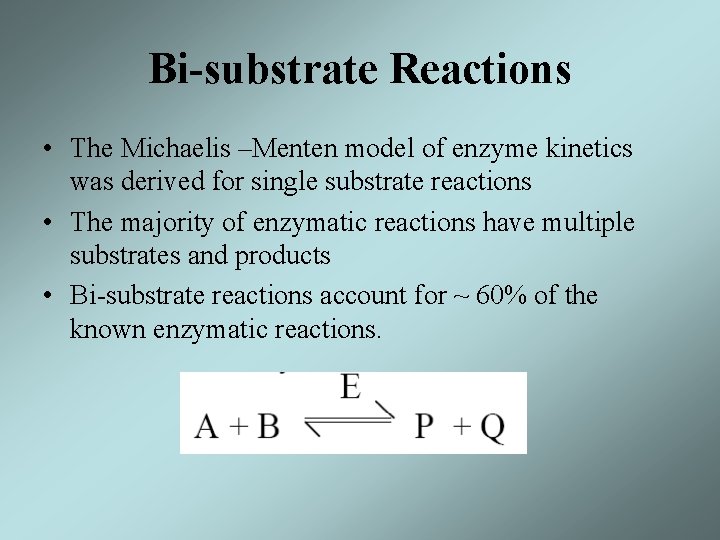
Bi-substrate Reactions • The Michaelis –Menten model of enzyme kinetics was derived for single substrate reactions • The majority of enzymatic reactions have multiple substrates and products • Bi-substrate reactions account for ~ 60% of the known enzymatic reactions.

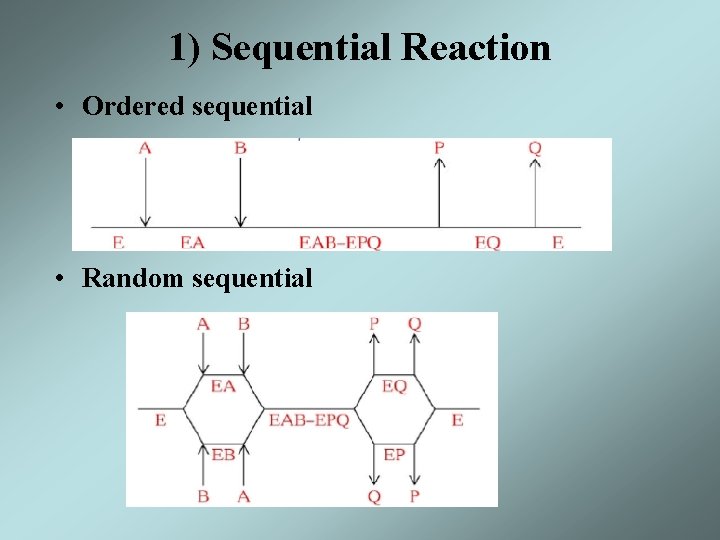
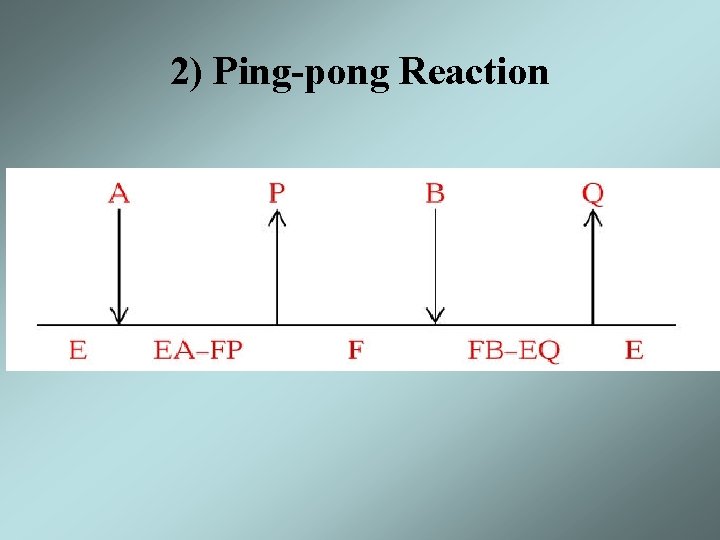
Substrate Addition / Product Release • The order of substrate addition and product release in most enzymatic reactions follow two reaction mechanism – Sequential reaction - all substrates must bind to the enzyme before the reaction occurs and products are released • Ordered sequential • Random sequential – Ping-pong reaction - one or more products are released before all substrates have been added an alternate stable enzyme form, F, is produced in the half reaction

1) Sequential Reaction • Ordered sequential • Random sequential

2) Ping-pong Reaction

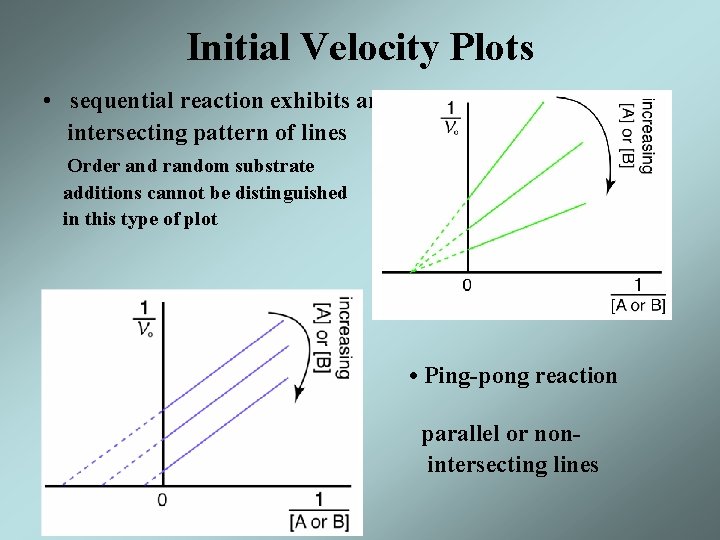
Initial Velocity Plots • sequential reaction exhibits an intersecting pattern of lines Order and random substrate additions cannot be distinguished in this type of plot shows • Ping-pong reaction parallel or nonintersecting lines
![Influence of enzyme concentration v k 3 E as SE Influence of enzyme concentration v = k 3 [E], as [S]>>[E]](https://slidetodoc.com/presentation_image/339789e1400f759c189a8a5948759f38/image-15.jpg)
Influence of enzyme concentration v = k 3 [E], as [S]>>[E]

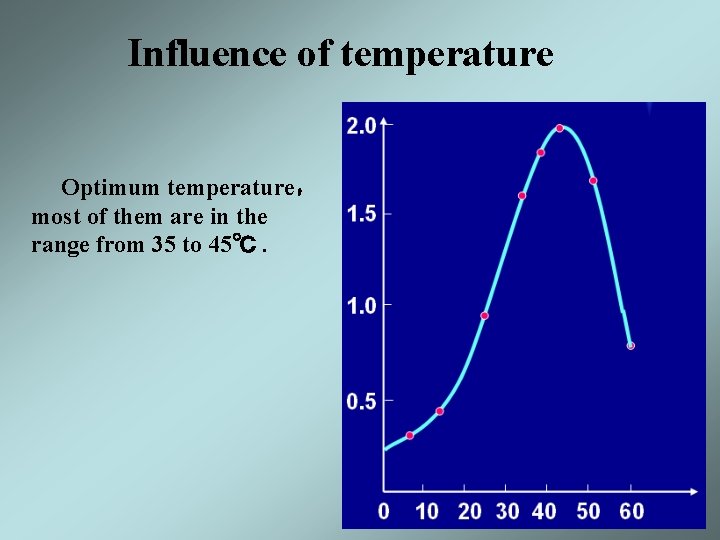
Influence of temperature Optimum temperature, most of them are in the range from 35 to 45℃.

Influence of p. H Optimum p. H

Enzyme Inhibition Enzyme inhibitors are important for a variety of reasons 1) they can be used to gain information about the shape on the enzyme active site and the amino acid residues in the active site. 2) they can be used to gain information about the chemical mechanism. 3) they can be used to gain information about the regulation or control of a metabolic pathway. 4) they can be very important in drug design.

Enzyme Inhibition • Reversible inhibitor: a substance that binds to an enzyme to inhibit it, but can be released – usually involves formation of non-covalent bonds – Generally two types • Dead end • Product • Irreversible inhibitor: a substance that causes inhibition that cannot be reversed – usually involves formation or breaking of covalent bonds to or on the enzyme

Inhibitors Irreversible inhibition Reversible inhibition competitive inhibition non-competitive inhibition uncompetitive inhibition

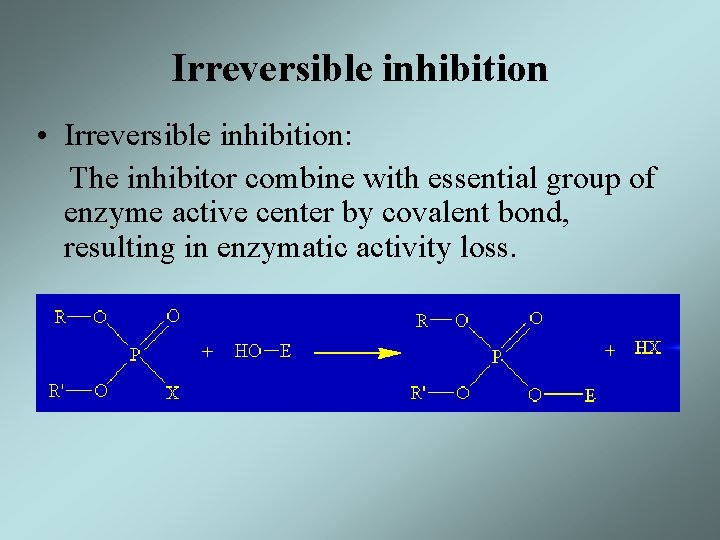
Irreversible inhibition • Irreversible inhibition: The inhibitor combine with essential group of enzyme active center by covalent bond, resulting in enzymatic activity loss.

Inhibition Patterns Inhibitors act in a variety of mechanisms • An inhibitor may bind at the same site as one of the substrates – these inhibitors structurally resemble the substrate • An inhibitor may bind at an alternate site affecting catalytic activity without affecting substrate binding • Many inhibitors do both • Most common types – Competitive – Mixed or Non-competitive – Uncompetitive

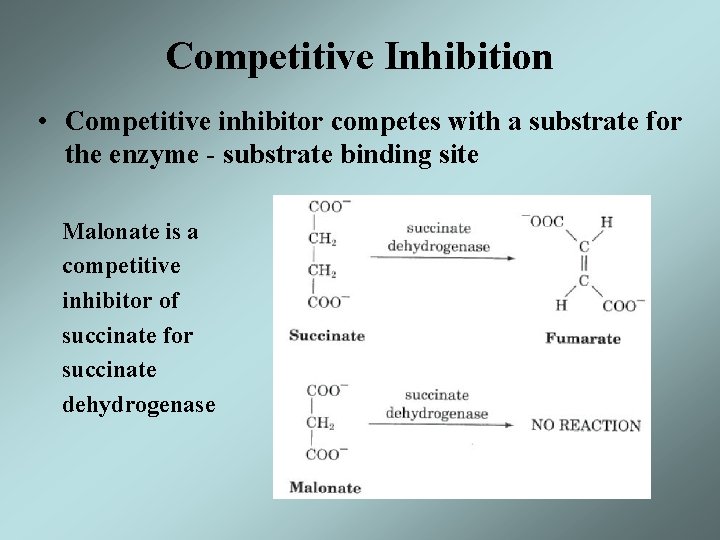
Competitive Inhibition • Competitive inhibitor competes with a substrate for the enzyme - substrate binding site Malonate is a competitive inhibitor of succinate for succinate dehydrogenase

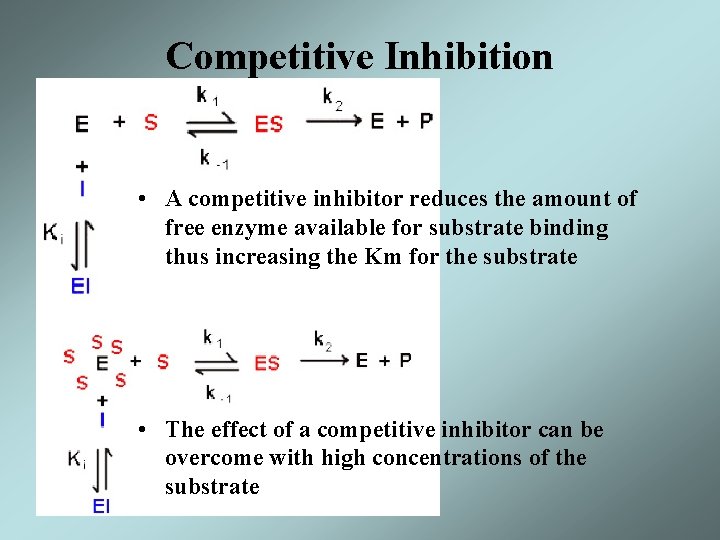
Competitive Inhibition • A competitive inhibitor reduces the amount of free enzyme available for substrate binding thus increasing the Km for the substrate • The effect of a competitive inhibitor can be overcome with high concentrations of the substrate

Competitive Inhibition

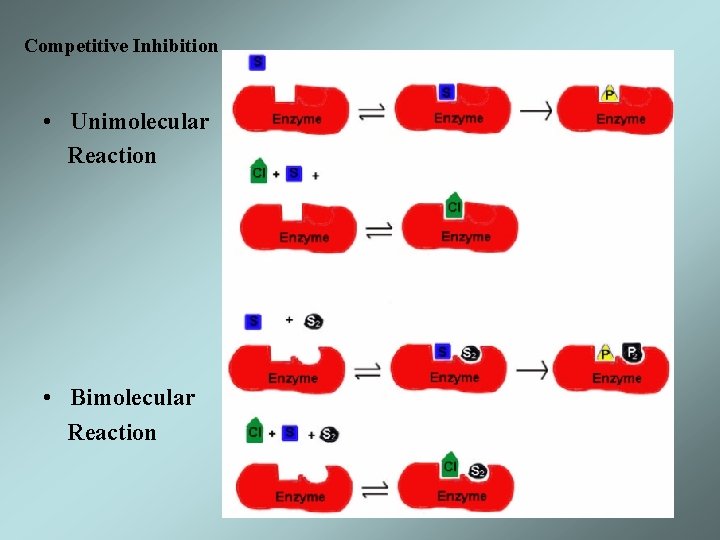
Competitive Inhibition • Unimolecular Reaction • Bimolecular Reaction

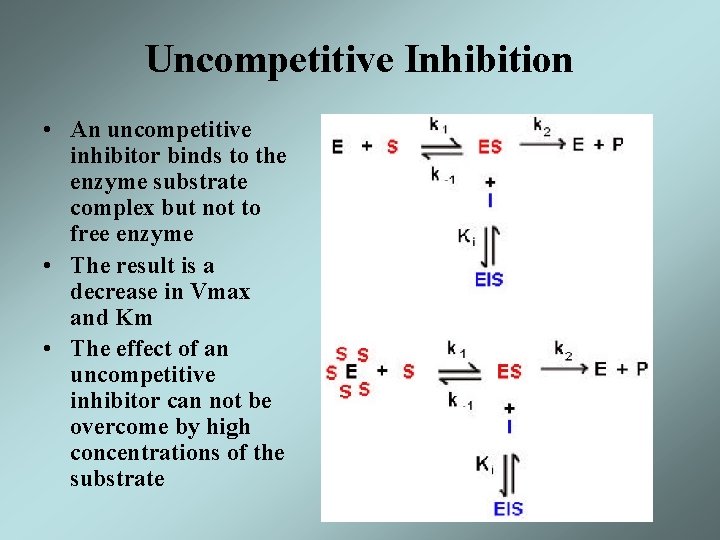
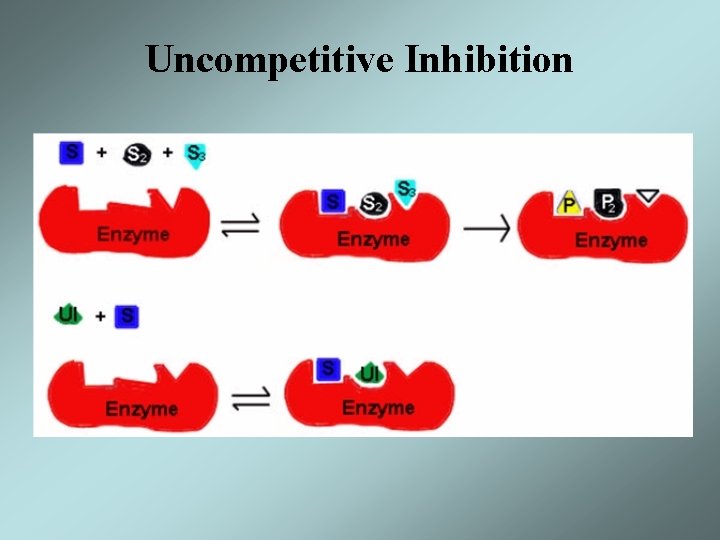
Uncompetitive Inhibition • An uncompetitive inhibitor binds to the enzyme substrate complex but not to free enzyme • The result is a decrease in Vmax and Km • The effect of an uncompetitive inhibitor can not be overcome by high concentrations of the substrate

Uncompetitive Inhibition

Uncompetitive

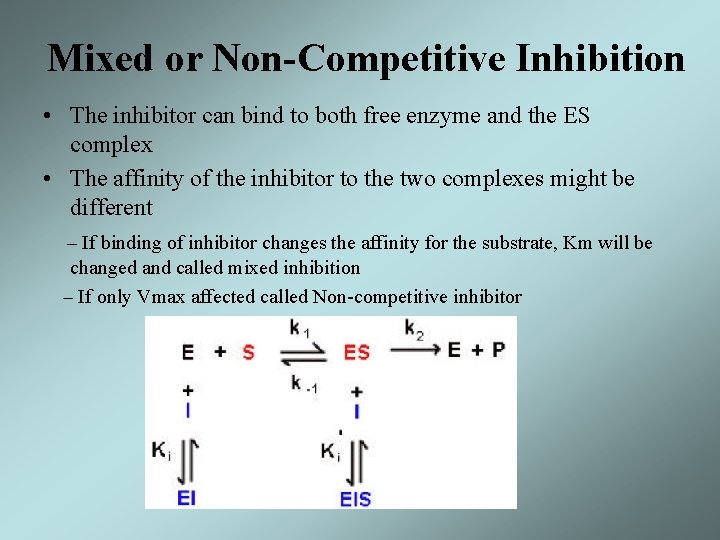
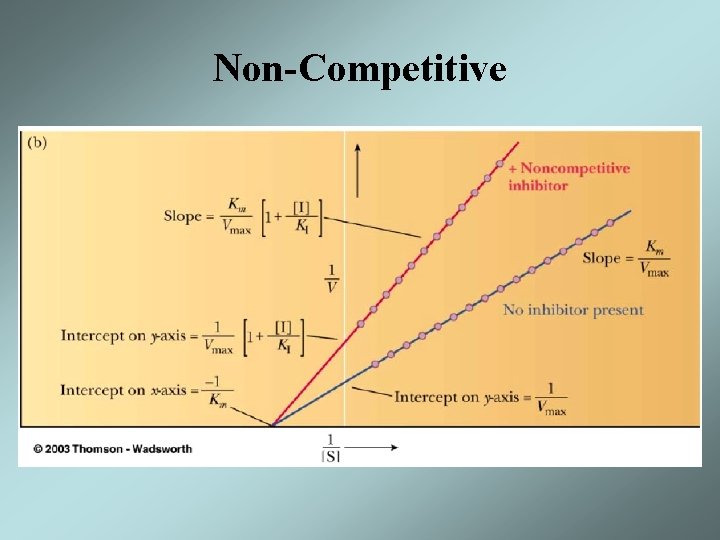
Mixed or Non-Competitive Inhibition • The inhibitor can bind to both free enzyme and the ES complex • The affinity of the inhibitor to the two complexes might be different – If binding of inhibitor changes the affinity for the substrate, Km will be changed and called mixed inhibition – If only Vmax affected called Non-competitive inhibitor

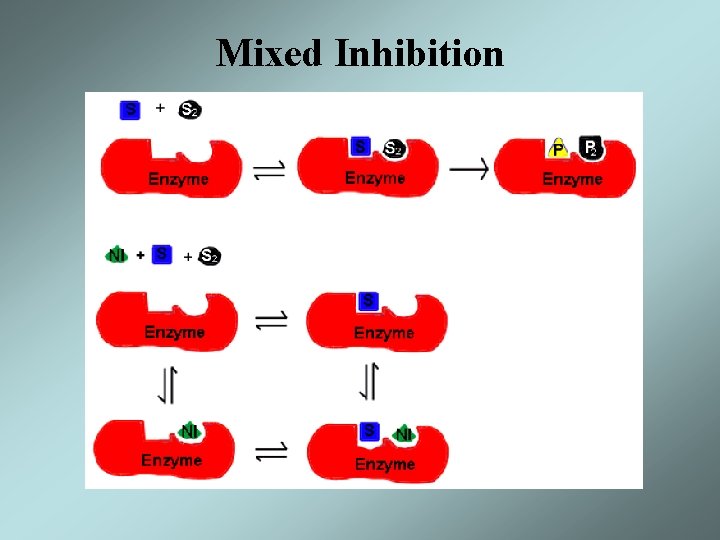
Mixed Inhibition

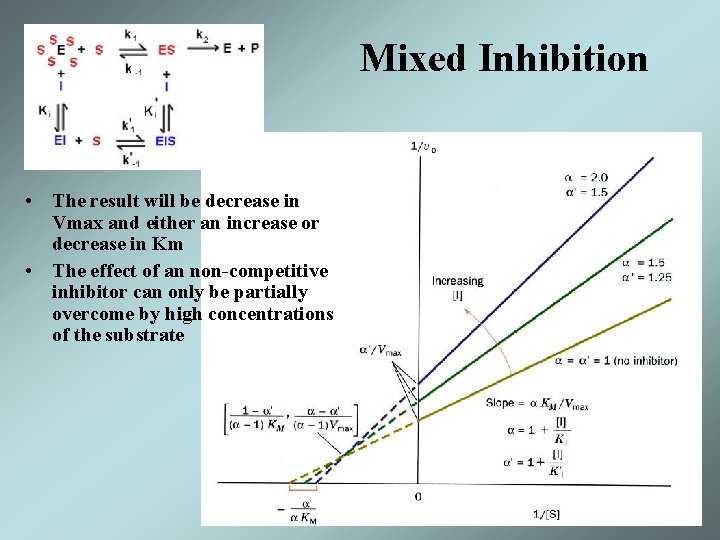
Mixed Inhibition • The result will be decrease in Vmax and either an increase or decrease in Km • The effect of an non-competitive inhibitor can only be partially overcome by high concentrations of the substrate

Non-Competitive

Thank you !
 Yi zhi zou
Yi zhi zou En dian zhi lu
En dian zhi lu General principles of catalysis
General principles of catalysis Km in enzyme kinetics
Km in enzyme kinetics Enzyme kinetics
Enzyme kinetics Unity hui
Unity hui Hui lin financial services professional
Hui lin financial services professional Hui zhang indiana
Hui zhang indiana Qu'est-ce qu'on va faire aujourd'hui
Qu'est-ce qu'on va faire aujourd'hui Chia hui chang
Chia hui chang Liv3lo
Liv3lo Sonu hyang-hui
Sonu hyang-hui Hui zhang cmu
Hui zhang cmu Seung-hui cho sister
Seung-hui cho sister Lam hui columbia
Lam hui columbia Grace yang georgetown
Grace yang georgetown Virginia tech shooter
Virginia tech shooter Cest mardi
Cest mardi Hui zhang cmu
Hui zhang cmu Woon xin hui
Woon xin hui Shihhui lim
Shihhui lim Liu hui mathematician
Liu hui mathematician Sandy hui
Sandy hui Christ aujourd'hui nous appelle
Christ aujourd'hui nous appelle Harmony hui
Harmony hui Lek hsiang hui
Lek hsiang hui Xu hui
Xu hui Qu'est-ce qu'on va faire aujourd'hui
Qu'est-ce qu'on va faire aujourd'hui Jav huai yan
Jav huai yan Aujourd'hui dieu te fait prophète paroles
Aujourd'hui dieu te fait prophète paroles Musique tunisienne moderne
Musique tunisienne moderne Hui transmit data
Hui transmit data Hui whakaoranga
Hui whakaoranga Heidi hui
Heidi hui Aujourd'hui levons nous
Aujourd'hui levons nous