Protein Folding PROTEIN FOLDING Process in which a







- Slides: 22

Protein Folding

PROTEIN FOLDING • Process in which a polypeptide chain goes from a linear chain of amino acids with vast number of more or less random conformations in solution to the native, folded tertiary (and for multichain proteins, quaternary) structure

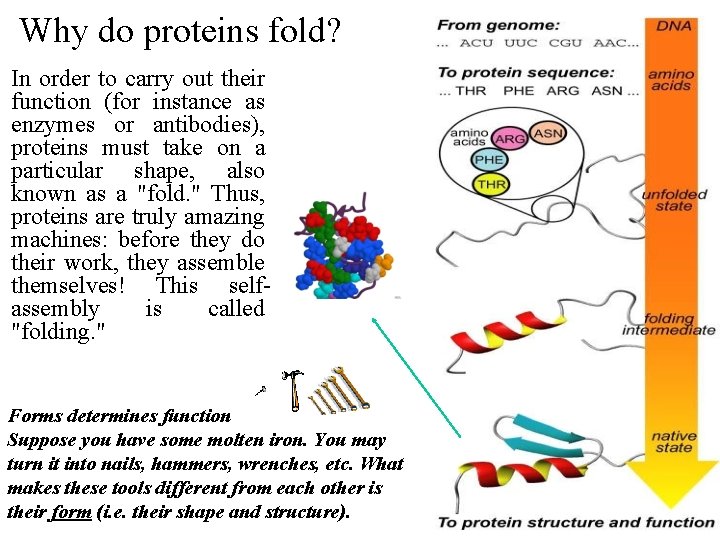
Protein Folding • Protein folding considers the question of how the process of protein folding occurs, i. e. unfolded native state. • This very challenging problem has been described as the second half of the genetic code, and as the three-dimensional code, as opposed to the one-dimensional code involved in nucleotide/amino acid sequence. • Importance: – Predict 3 D structure from primary sequence – Avoid misfolding related to human diseases – Design proteins with novel functions

Why do proteins fold? In order to carry out their function (for instance as enzymes or antibodies), proteins must take on a particular shape, also known as a "fold. " Thus, proteins are truly amazing machines: before they do their work, they assemble themselves! This self assembly is called "folding. " Forms determines function Suppose you have some molten iron. You may turn it into nails, hammers, wrenches, etc. What makes these tools different from each other is their form (i. e. their shape and structure).

PRIMARY STRUCTURE DETERMINES TERTIARY (AND QUATERNARY) STRUCTURES. – demonstrated by the fact that many proteins can refold from a more or less "random coil" set of conformations without "instructions" from any other cellular components – All the information for 3 dimensional structure is provided by the amino acid sequence. Proteins fold on a defined pathway (or a small number of alternative pathways); they don't randomly search all possible conformations until they arrive at the most stable (lowest free energy) structure.

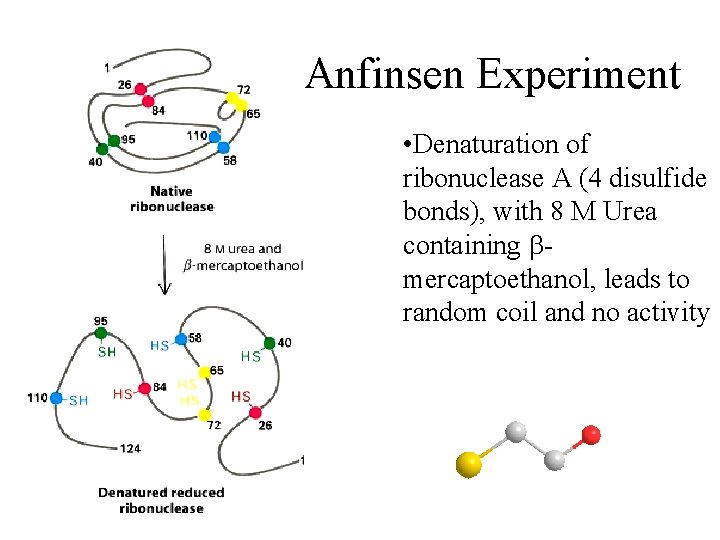
Anfinsen Experiment • Denaturation of ribonuclease A (4 disulfide bonds), with 8 M Urea containing b mercaptoethanol, leads to random coil and no activity

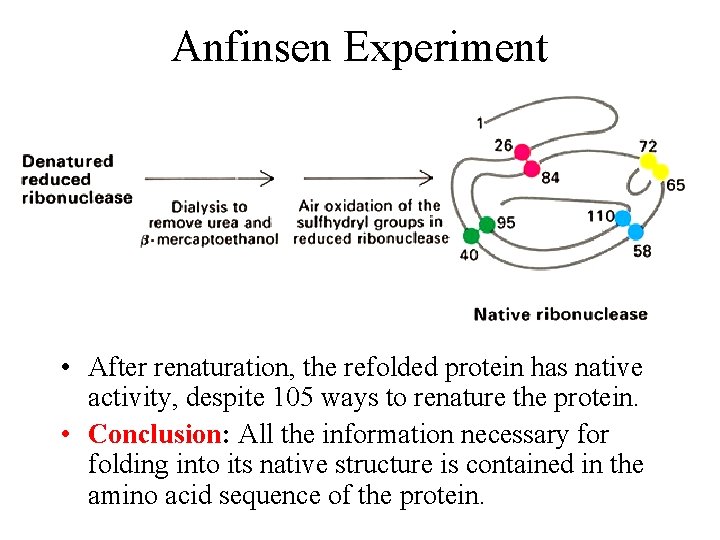
Anfinsen Experiment • After renaturation, the refolded protein has native activity, despite 105 ways to renature the protein. • Conclusion: All the information necessary for folding into its native structure is contained in the amino acid sequence of the protein.

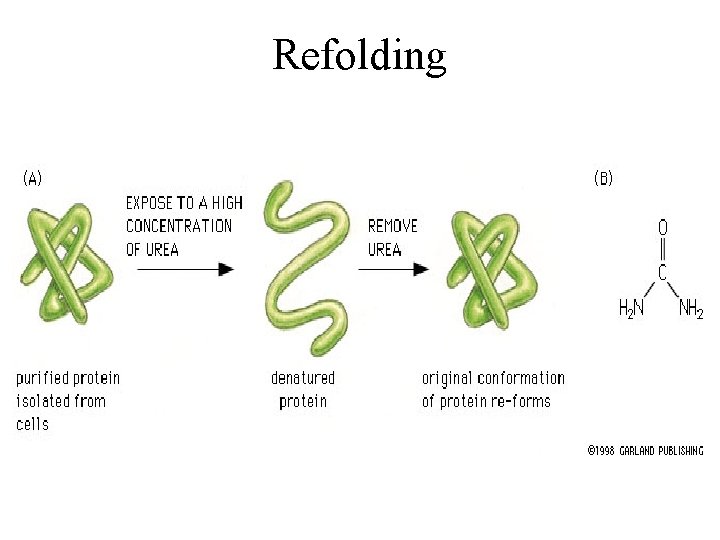
Refolding

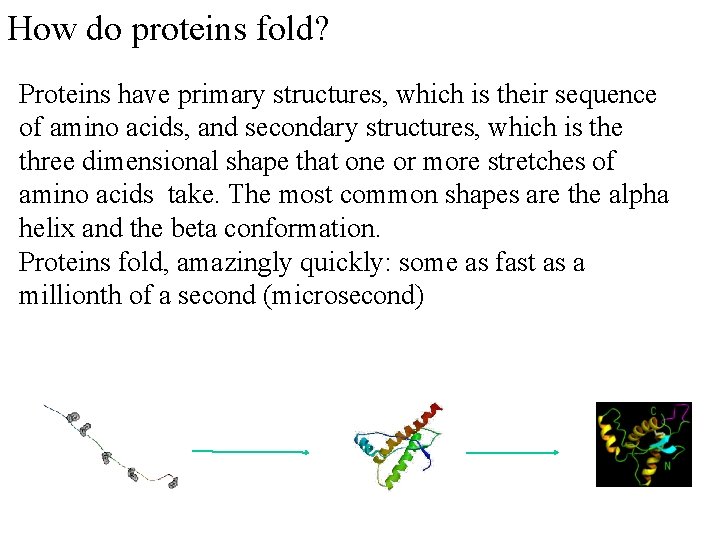
How do proteins fold? Proteins have primary structures, which is their sequence of amino acids, and secondary structures, which is the three dimensional shape that one or more stretches of amino acids take. The most common shapes are the alpha helix and the beta conformation. Proteins fold, amazingly quickly: some as fast as a millionth of a second (microsecond)

• Folding means arriving at the right combinations of angles for every amino acid residue in the sequence. • Proteins fold on a defined pathway (or a small number of alternative pathways); they don't randomly search all possible conformations until they arrive at the most stable (lowest free energy) structure. • Proteins that don't (re)fold on their own, need "molecular chaperones" (which are also proteins) to keep them from slipping off the folding pathway or to help them to get back on it. –Some chaperones require ATP to carry out their function.

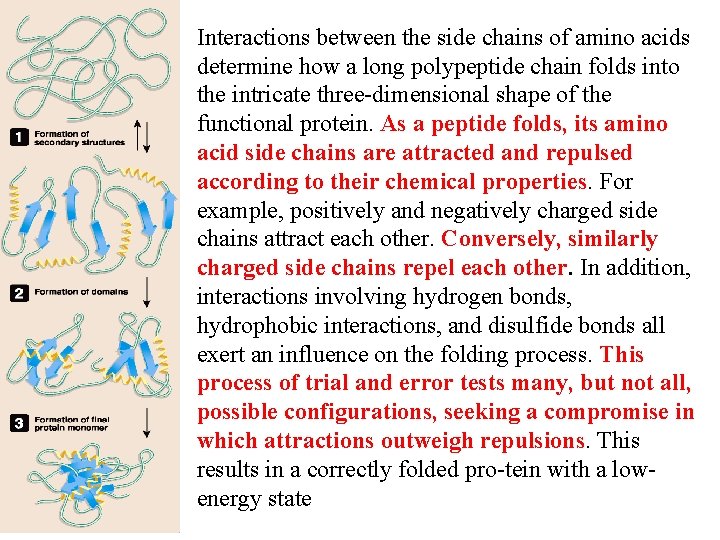
Interactions between the side chains of amino acids determine how a long polypeptide chain folds into the intricate three dimensional shape of the functional protein. As a peptide folds, its amino acid side chains are attracted and repulsed according to their chemical properties. For example, positively and negatively charged side chains attract each other. Conversely, similarly charged side chains repel each other. In addition, interactions involving hydrogen bonds, hydrophobic interactions, and disulfide bonds all exert an influence on the folding process. This process of trial and error tests many, but not all, possible configurations, seeking a compromise in which attractions outweigh repulsions. This results in a correctly folded pro tein with a low energy state

CHAPERONES It is generally accepted that the information needed for correct protein folding is contained in the primary structure of the polypeptide. Given that premise, it is difficult to explain why most proteins when denatured do not resume their native conformations under favorable environmental conditions. One answer to this problem is that a protein begins to fold in stages during its synthesis, rather than waiting for synthesis of the entire chain to be totally completed. This limits competing folding configurations made available by longer stretches of nascent peptide.

In addition, a specialized group of proteins, named “chaperones, ” are required for the proper folding of many species of proteins. The chaperones—also known as “heat shock” proteins—interact with the polypeptide at various stages during the folding process. They help guide the folding and can help keep the new protein from associating with the wrong partner

Some chaperones are important in keeping the protein unfolded until its synthesis is finished, or act as catalysts by increasing the rates of the final stages in the folding process. Others protect proteins as they fold so that their vulnerable, exposed regions do not become tangled in unproductive interactions

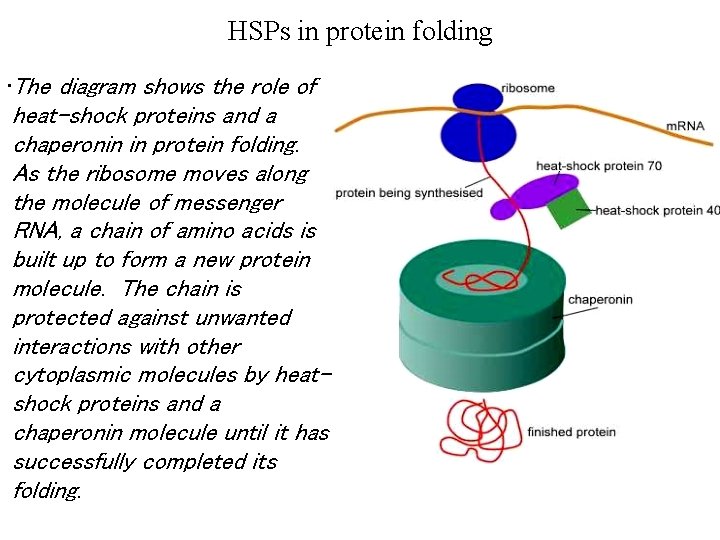
HSPs in protein folding • The diagram shows the role of heat-shock proteins and a chaperonin in protein folding. As the ribosome moves along the molecule of messenger RNA, a chain of amino acids is built up to form a new protein molecule. The chain is protected against unwanted interactions with other cytoplasmic molecules by heatshock proteins and a chaperonin molecule until it has successfully completed its folding.

One major function of chaperones is to prevent both newly synthesised polypeptide chains and assembled subunits from aggregating into nonfunctional structures. It is for this reason that many chaperones, but by no means all, are heat-shock proteins because the tendency to aggregate increases as proteins are denatured by stress.

Many chaperones are heat shock proteins, that is, proteins expressed in response to elevated temperatures or other cellular stresses. The reason for this behaviour is that protein folding is severely affected by heat and, therefore, some chaperones act to prevent or correct damage caused by misfolding. Other chaperones are involved in folding newly made proteins as they are extruded from the ribosome. Although most newly synthesized proteins can fold in absence of chaperones, a minority strictly requires them for the same.

Protein denaturation

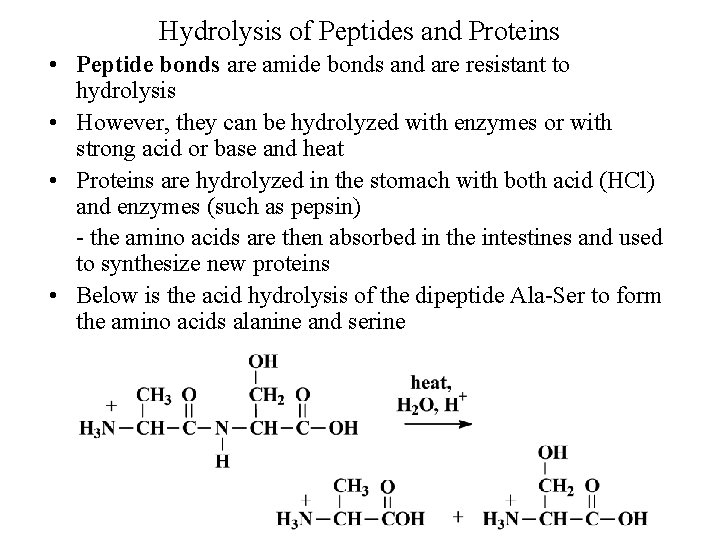
Hydrolysis of Peptides and Proteins • Peptide bonds are amide bonds and are resistant to hydrolysis • However, they can be hydrolyzed with enzymes or with strong acid or base and heat • Proteins are hydrolyzed in the stomach with both acid (HCl) and enzymes (such as pepsin) the amino acids are then absorbed in the intestines and used to synthesize new proteins • Below is the acid hydrolysis of the dipeptide Ala Ser to form the amino acids alanine and serine

Denaturation of Proteins • Denaturation causes proteins to lose their 3 D structure and so they lose their function • Denaturation involves the disruption of cross linking in the secondary, tertiary and quaternary protein structures • Heat and organic compounds disrupt H bonding and hydrophobic interactions • Acids and bases disrupt H bonding between polar R groups and break ionic bonds • Heavy metal ions break S S bonds by reacting with the sulfur • Agitation such as whipping stretches chains, disrupting all types of cross linking

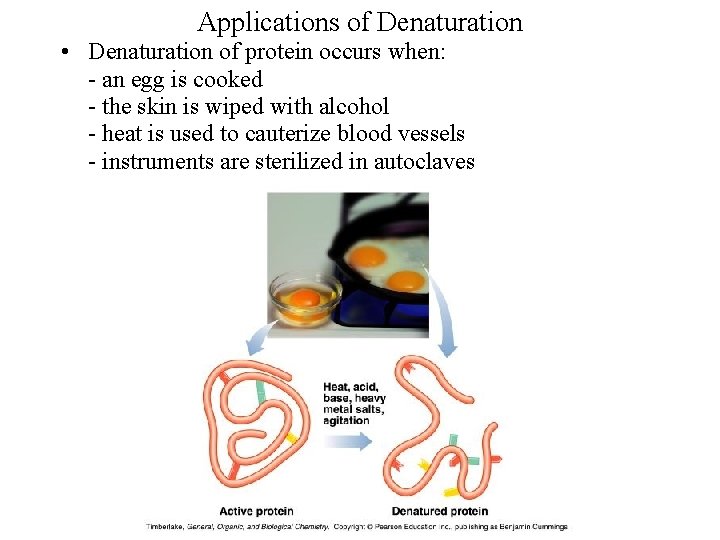
Applications of Denaturation • Denaturation of protein occurs when: an egg is cooked the skin is wiped with alcohol heat is used to cauterize blood vessels instruments are sterilized in autoclaves
