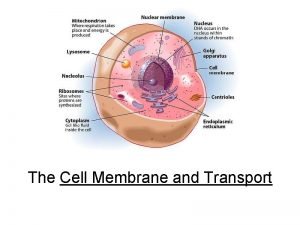
Protein Folding The production of a mature protein



- Slides: 68

Protein Folding * The production of a mature protein is a multistep process in eukaryotic cells. * The final folded configuration, or shape, of a protein is determined by its amino acid sequence. * Most proteins require the assistance of molecular chaperones, like Hsp 70 & Hsp 60, to reach their final folded form. * These molecular chaperones bind to exposed hydrophobic patches on the surface of incompletely folded proteins. * Hsp 70 and Hsp 60 act sequentially on proteins to help them achieve their correct folded state. * Proteins that fail to be properly folded are ultimately targeted for destruction.

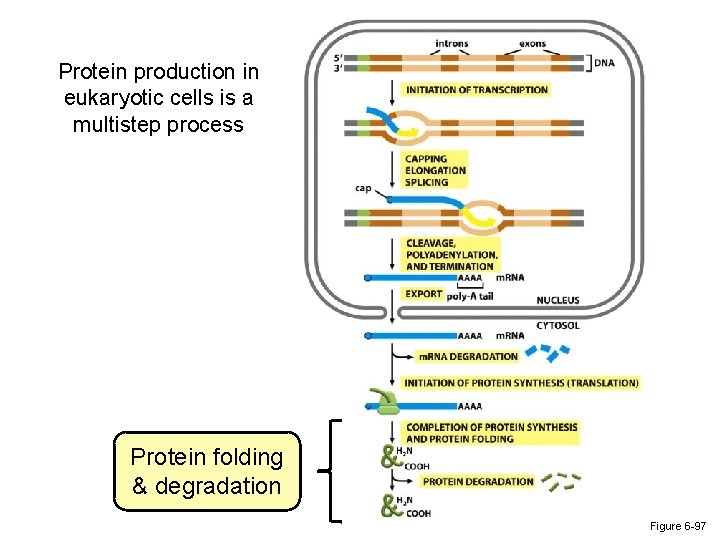
Protein production in eukaryotic cells is a multistep process Protein folding & degradation Figure 6 -97

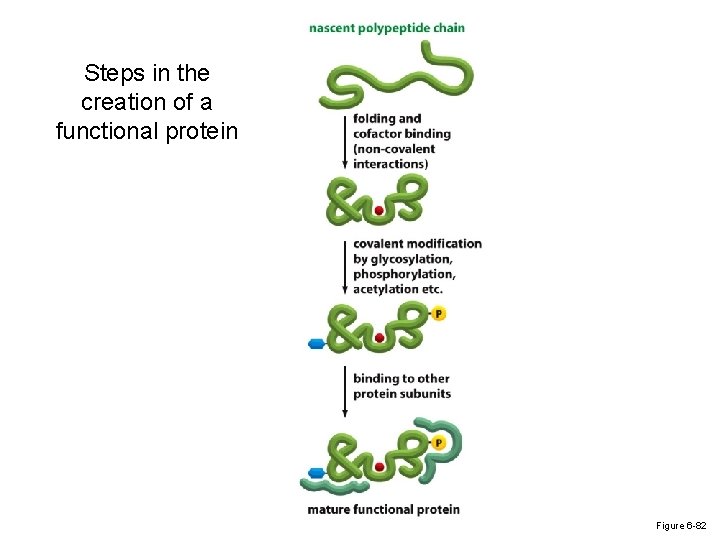
Steps in the creation of a functional protein Figure 6 -82

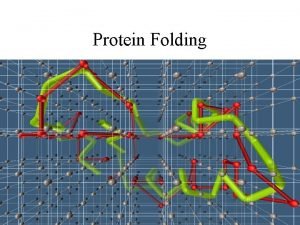
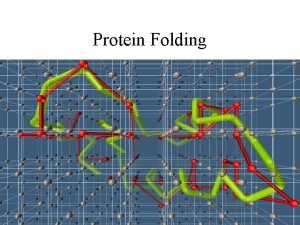
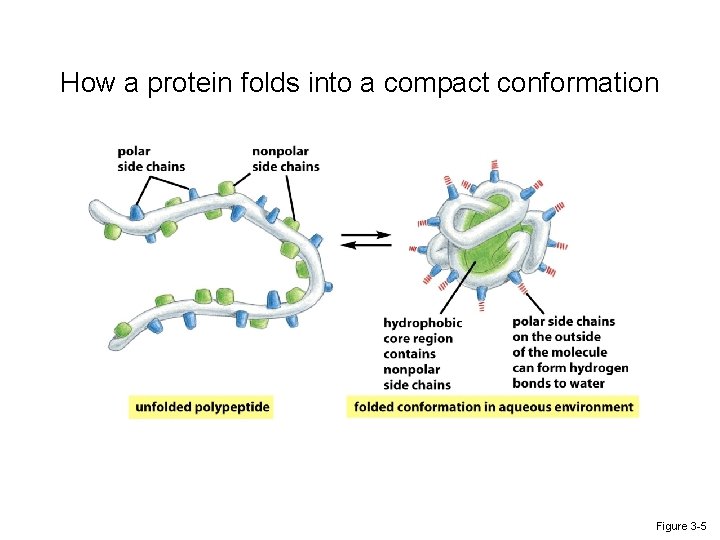
How a protein folds into a compact conformation Figure 3 -5

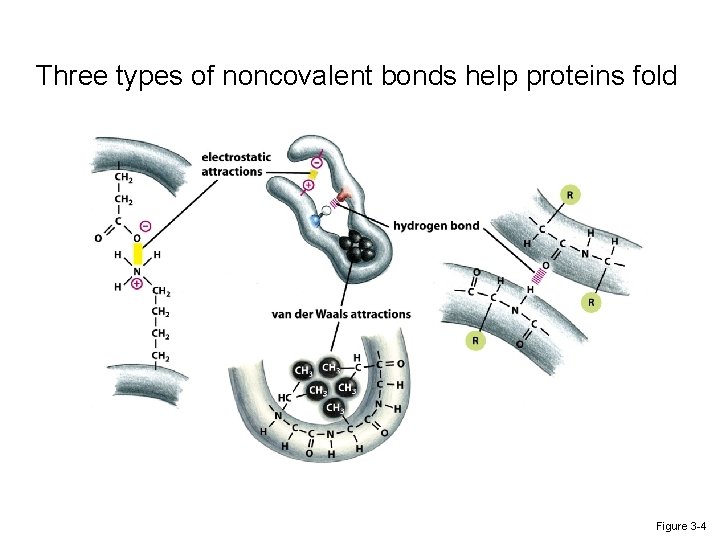
Three types of noncovalent bonds help proteins fold Figure 3 -4

A current view of the protein folding process Structure of a molten globule Figure 6 -85

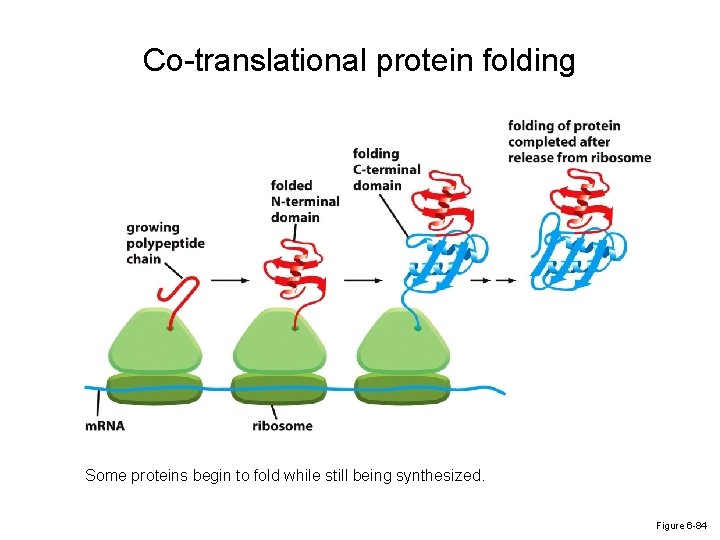
Co-translational protein folding Some proteins begin to fold while still being synthesized. Figure 6 -84

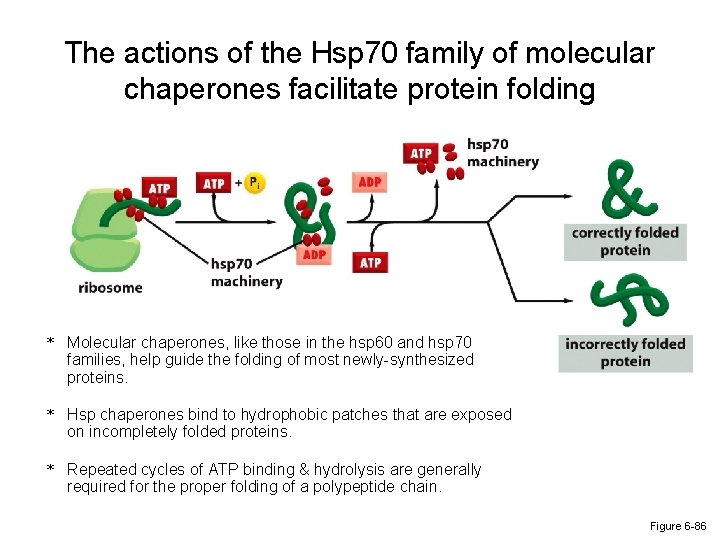
The actions of the Hsp 70 family of molecular chaperones facilitate protein folding * Molecular chaperones, like those in the hsp 60 and hsp 70 families, help guide the folding of most newly-synthesized proteins. * Hsp chaperones bind to hydrophobic patches that are exposed on incompletely folded proteins. * Repeated cycles of ATP binding & hydrolysis are generally required for the proper folding of a polypeptide chain. Figure 6 -86

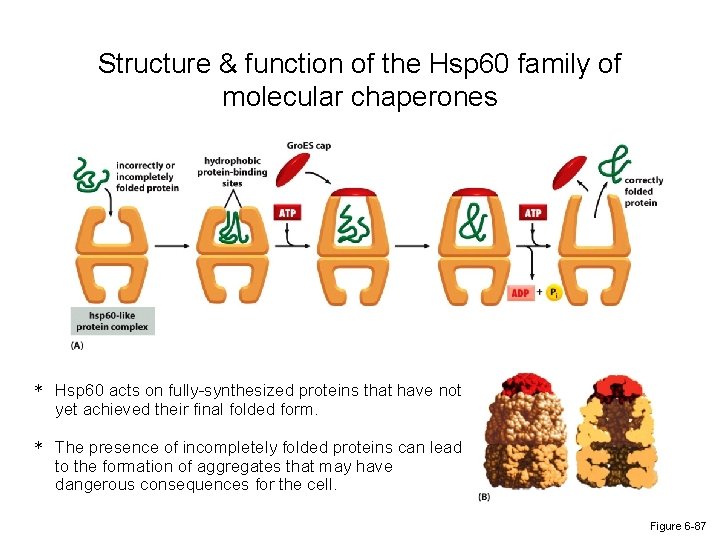
Structure & function of the Hsp 60 family of molecular chaperones * Hsp 60 acts on fully-synthesized proteins that have not yet achieved their final folded form. * The presence of incompletely folded proteins can lead to the formation of aggregates that may have dangerous consequences for the cell. Figure 6 -87

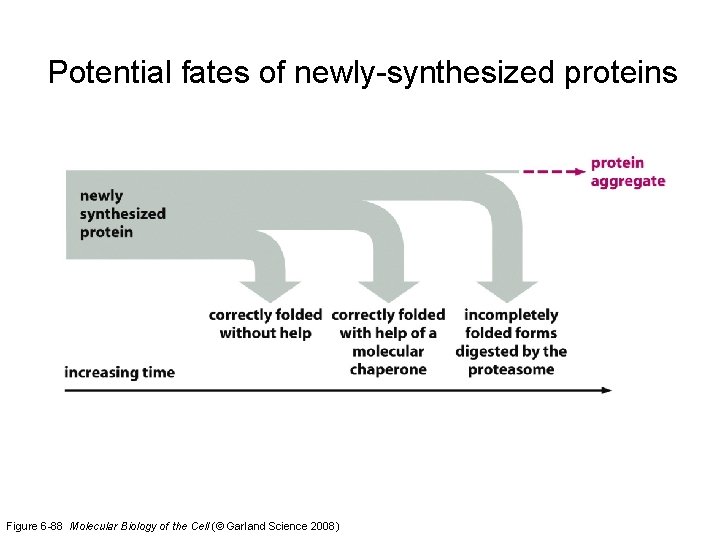
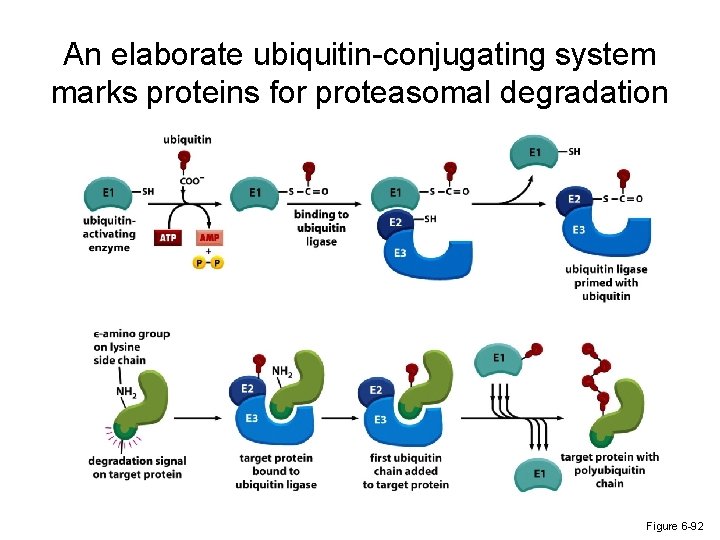
Proteasome-mediated protein turnover * The ultimate turnover of incompletely folded proteins is mediated by an abundant ATP-dependent protease, known as the proteasome. * The proteasome is a highly compartmentalized protease with sequestered active sites. * The proteasome acts processively to convert the entire protein substrate into short peptides. * Proteins destined for degradation are marked by polyubiquitin chains that are added via a multistep conjugation process. * The process of degradation can be regulated by altering the activity of the E 3 ubiquitin ligase or the availability of the degradation signal on the target protein.

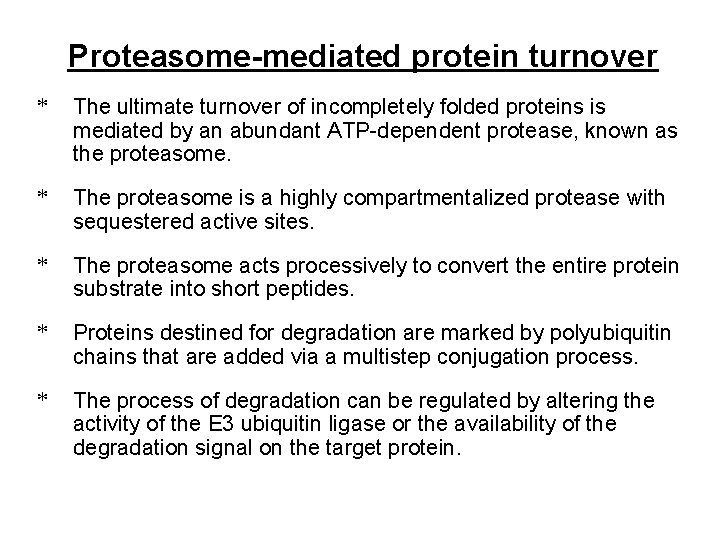
Potential fates of newly-synthesized proteins Figure 6 -88 Molecular Biology of the Cell (© Garland Science 2008)

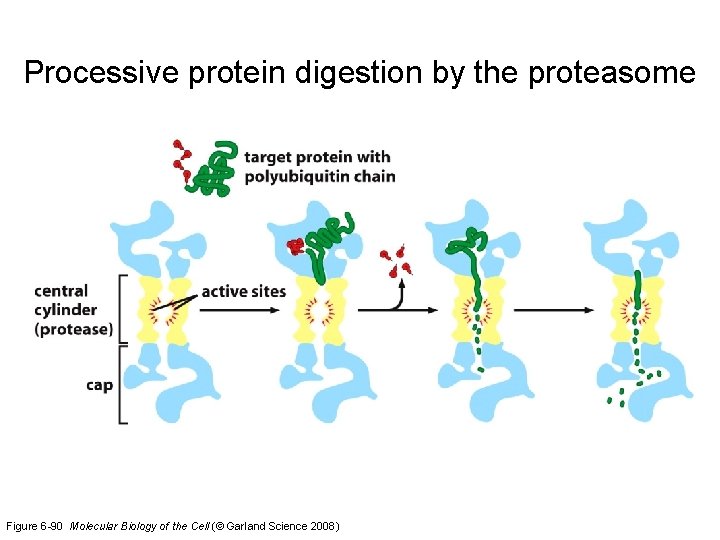
Processive protein digestion by the proteasome Figure 6 -90 Molecular Biology of the Cell (© Garland Science 2008)

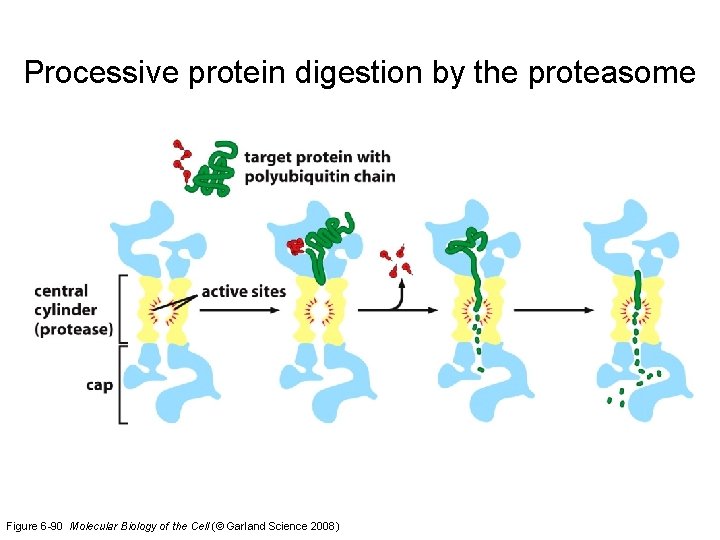
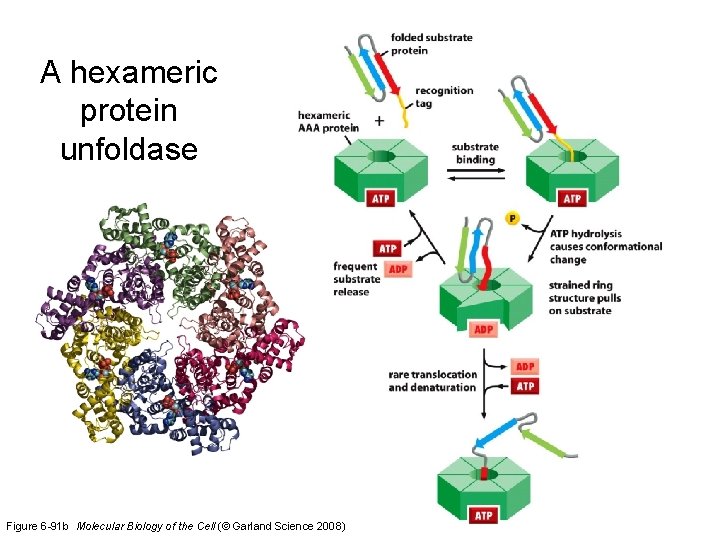
A hexameric protein unfoldase Figure 6 -91 b Molecular Biology of the Cell (© Garland Science 2008)

Processive protein digestion by the proteasome Figure 6 -90 Molecular Biology of the Cell (© Garland Science 2008)

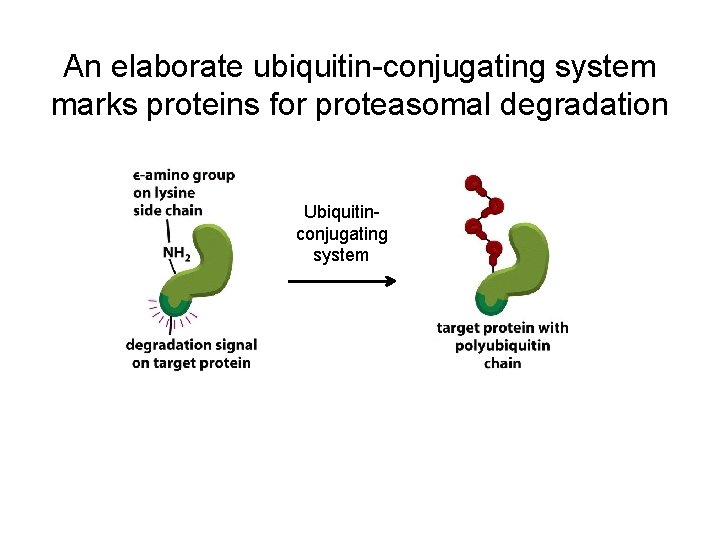
An elaborate ubiquitin-conjugating system marks proteins for proteasomal degradation Figure 6 -92

An elaborate ubiquitin-conjugating system marks proteins for proteasomal degradation Ubiquitinconjugating system

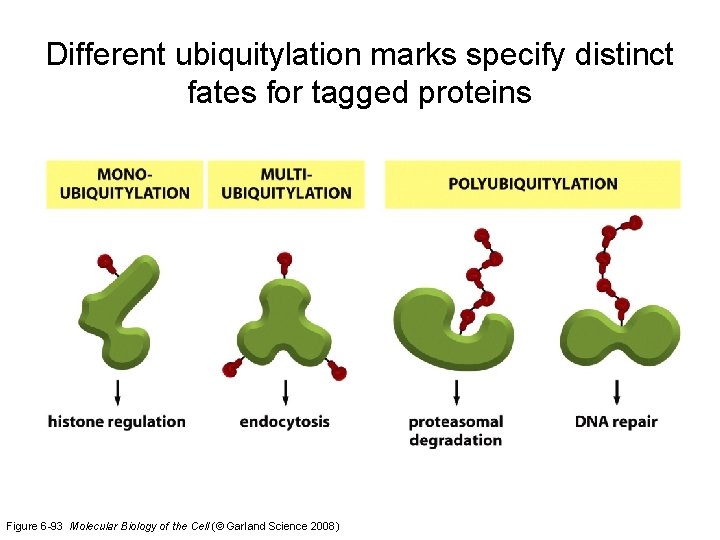
Different ubiquitylation marks specify distinct fates for tagged proteins Figure 6 -93 Molecular Biology of the Cell (© Garland Science 2008)

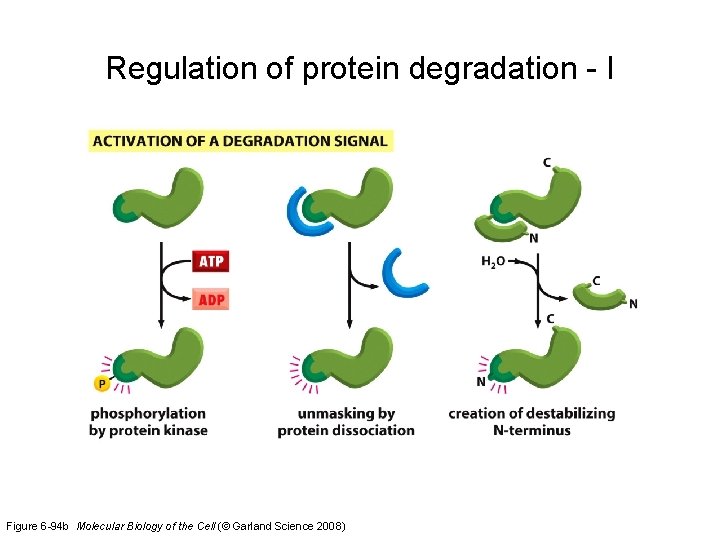
Regulation of protein degradation - I Figure 6 -94 b Molecular Biology of the Cell (© Garland Science 2008)

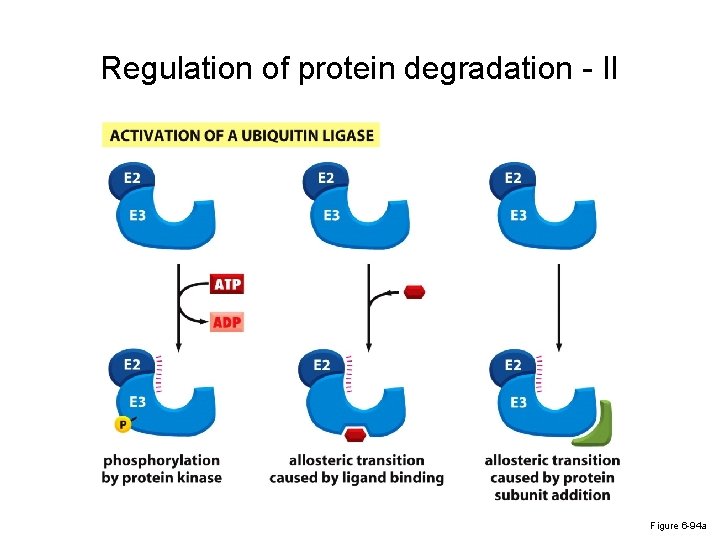
Regulation of protein degradation - II Figure 6 -94 a

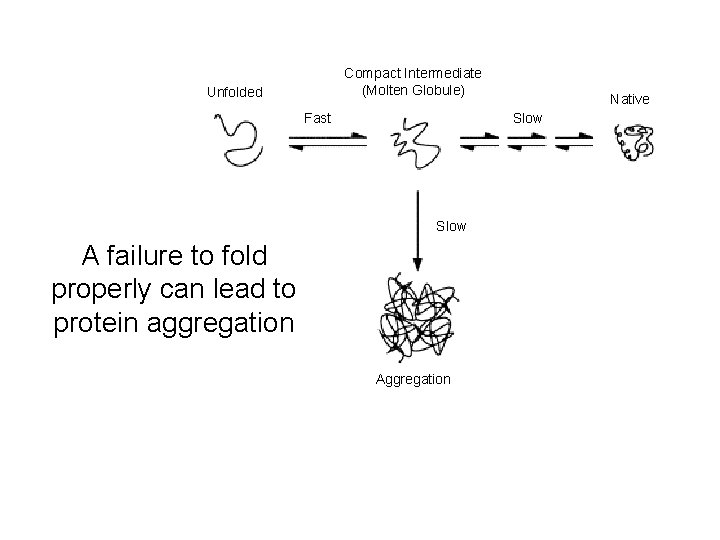
Compact Intermediate (Molten Globule) Unfolded Fast Native Slow A failure to fold properly can lead to protein aggregation Aggregation

Protein Misfolding Diseases (PMDs) * Many inherited diseases result from mutant proteins that evade quality control processes, fold abnormally and ultimately form aggregates. * The gradual decline of protein quality controls with age can also lead to disease by permitting normal proteins to form aggregates that can impair cellular functions. * Many neurodegenerative diseases, including Huntington’s and Alzheimer’s, are associated with the presence of misfolded protein aggregates in neuronal tissue. * The aggregates in patients can be intracellular (nuclear and/or cytoplasmic) or extracellular.

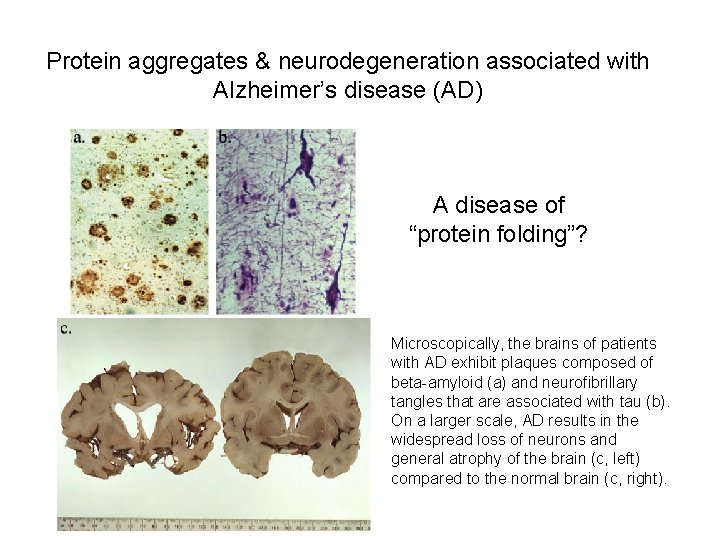
Protein aggregates & neurodegeneration associated with Alzheimer’s disease (AD) A disease of “protein folding”? Microscopically, the brains of patients with AD exhibit plaques composed of beta-amyloid (a) and neurofibrillary tangles that are associated with tau (b). On a larger scale, AD results in the widespread loss of neurons and general atrophy of the brain (c, left) compared to the normal brain (c, right).

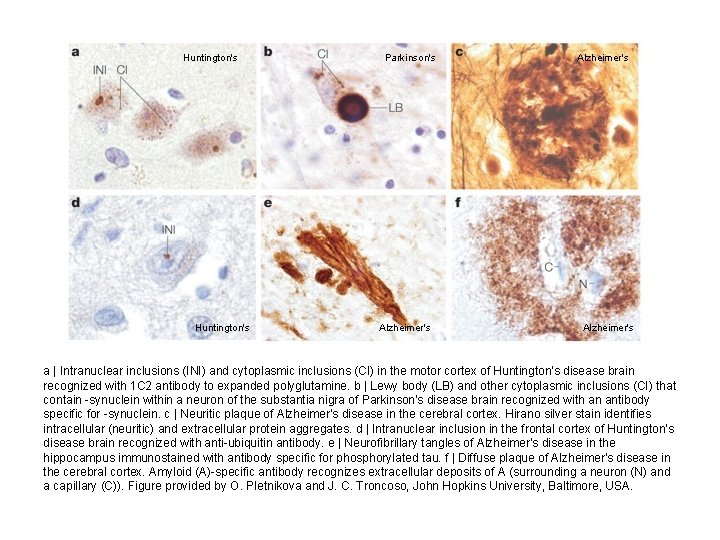
Huntington's Parkinson's Alzheimer's a | Intranuclear inclusions (INI) and cytoplasmic inclusions (CI) in the motor cortex of Huntington's disease brain recognized with 1 C 2 antibody to expanded polyglutamine. b | Lewy body (LB) and other cytoplasmic inclusions (CI) that contain -synuclein within a neuron of the substantia nigra of Parkinson's disease brain recognized with an antibody specific for -synuclein. c | Neuritic plaque of Alzheimer's disease in the cerebral cortex. Hirano silver stain identifies intracellular (neuritic) and extracellular protein aggregates. d | Intranuclear inclusion in the frontal cortex of Huntington's disease brain recognized with anti-ubiquitin antibody. e | Neurofibrillary tangles of Alzheimer's disease in the hippocampus immunostained with antibody specific for phosphorylated tau. f | Diffuse plaque of Alzheimer's disease in the cerebral cortex. Amyloid (A)-specific antibody recognizes extracellular deposits of A (surrounding a neuron (N) and a capillary (C)). Figure provided by O. Pletnikova and J. C. Troncoso, John Hopkins University, Baltimore, USA.

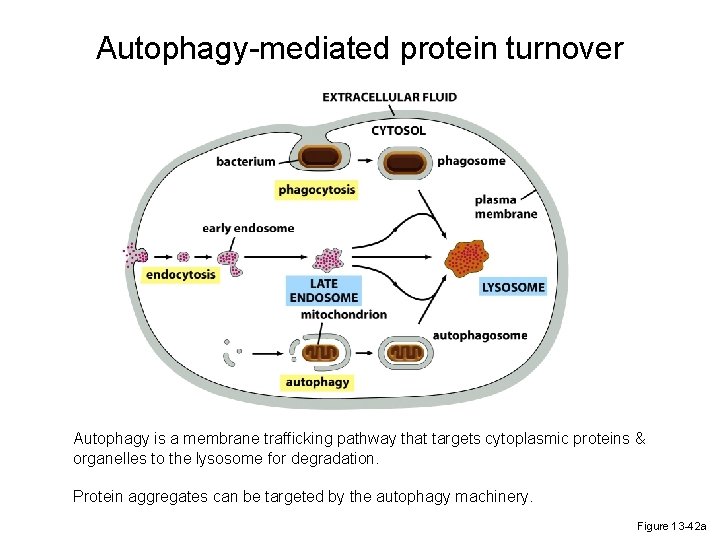
Autophagy-mediated protein turnover Autophagy is a membrane trafficking pathway that targets cytoplasmic proteins & organelles to the lysosome for degradation. Protein aggregates can be targeted by the autophagy machinery. Figure 13 -42 a

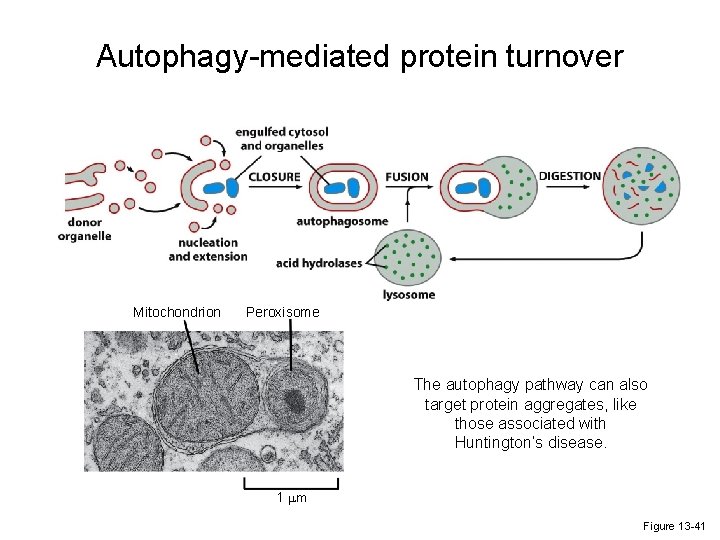
Autophagy-mediated protein turnover Mitochondrion Peroxisome The autophagy pathway can also target protein aggregates, like those associated with Huntington’s disease. 1 mm Figure 13 -41

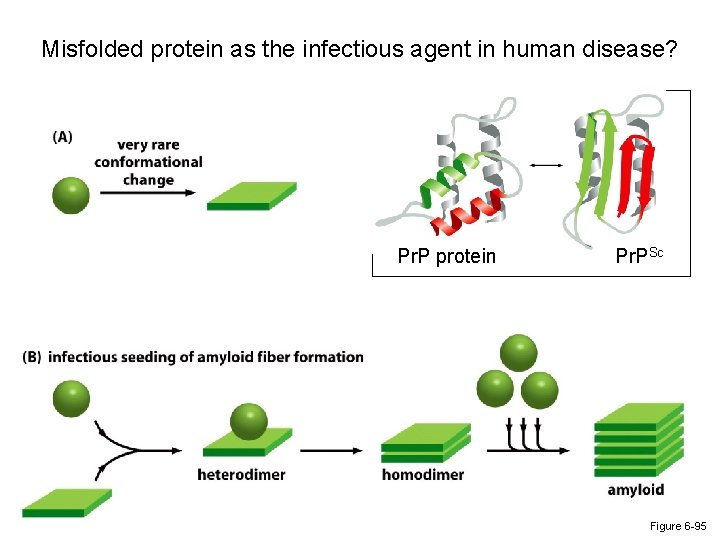
Misfolded protein as the infectious agent in human disease? Stanley Prusiner (UCSF) Nobel Prize in 1997 Protein molecules, known as prions, are the infectious agent responsible for particular neurodegenerative disorders like bovine spongiform encephalopathy ("mad cow disease") and its human equivalent, Creutzfeldt-Jakob disease.

Misfolded protein as the infectious agent in human disease? Pr. P protein Pr. PSc Figure 6 -95

Chapter 15 • Cell Communication

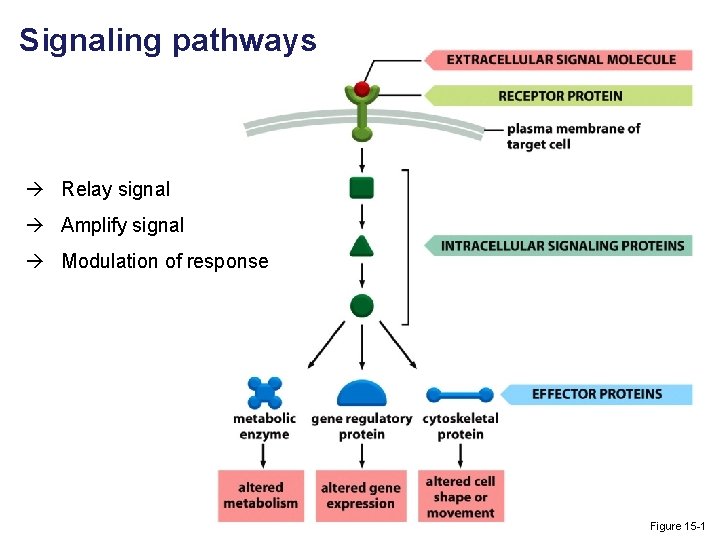
Signaling pathways Relay signal Amplify signal Modulation of response Figure 15 -1

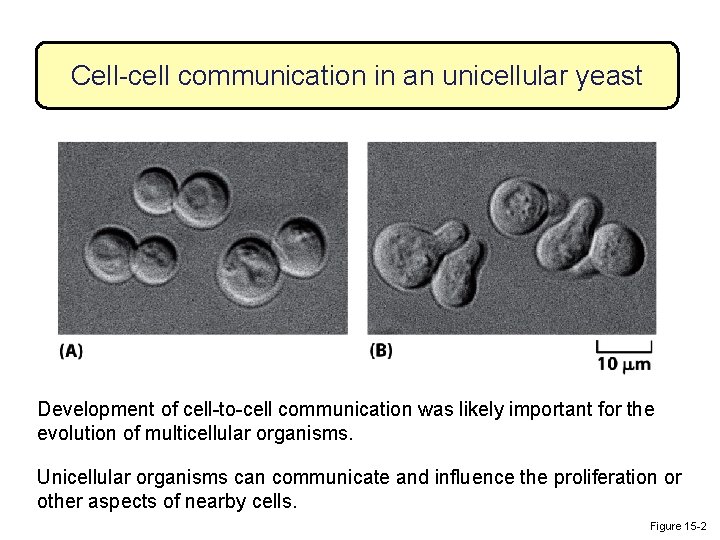
Cell-cell communication in an unicellular yeast Development of cell-to-cell communication was likely important for the evolution of multicellular organisms. Unicellular organisms can communicate and influence the proliferation or other aspects of nearby cells. Figure 15 -2

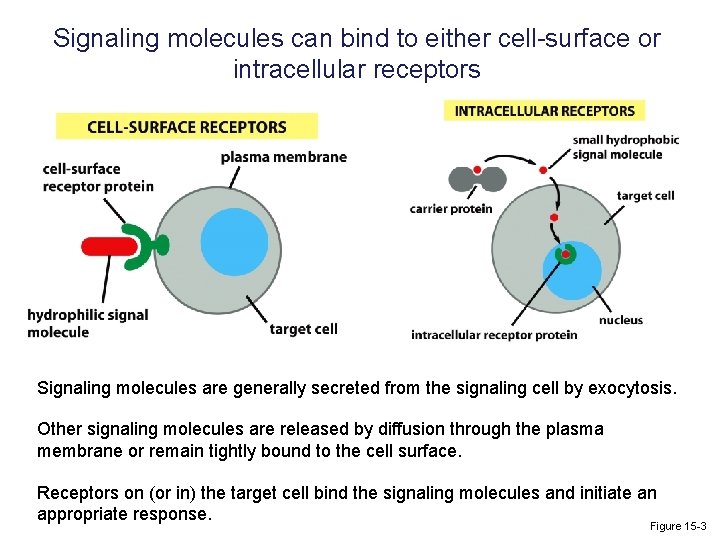
Signaling molecules can bind to either cell-surface or intracellular receptors Signaling molecules are generally secreted from the signaling cell by exocytosis. Other signaling molecules are released by diffusion through the plasma membrane or remain tightly bound to the cell surface. Receptors on (or in) the target cell bind the signaling molecules and initiate an appropriate response. Figure 15 -3

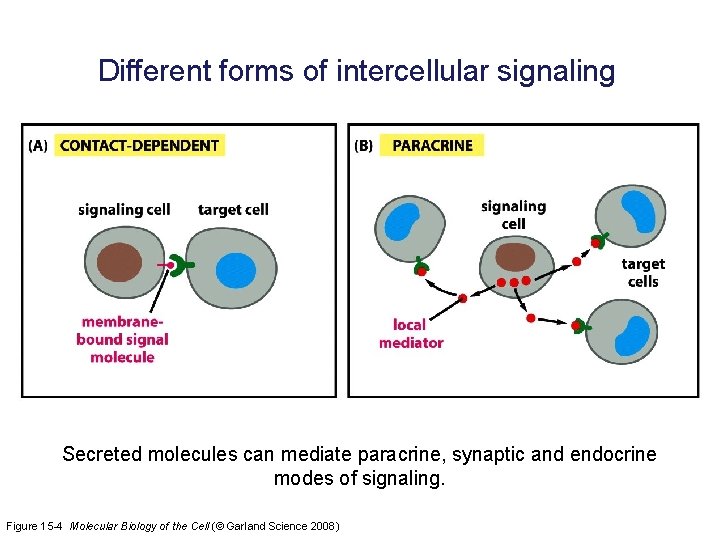
Different forms of intercellular signaling Secreted molecules can mediate paracrine, synaptic and endocrine modes of signaling. Figure 15 -4 Molecular Biology of the Cell (© Garland Science 2008)

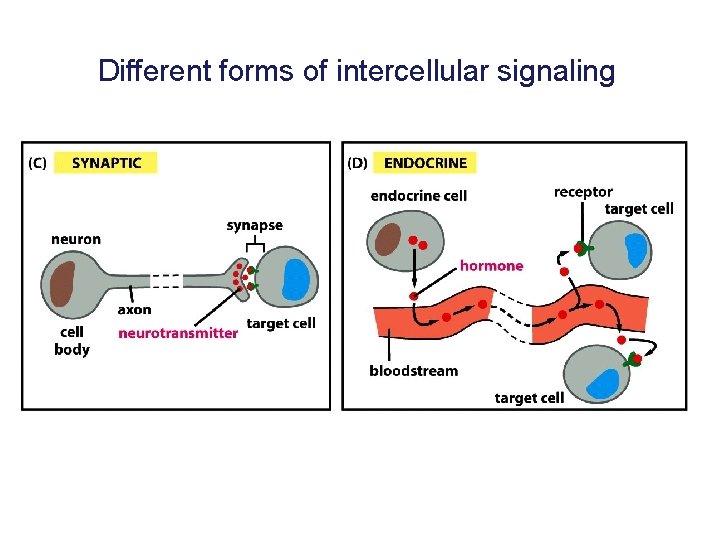
Different forms of intercellular signaling

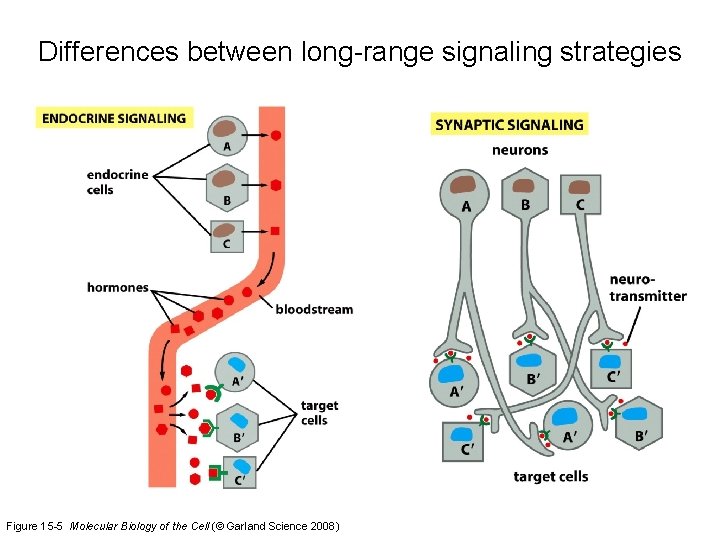
Differences between long-range signaling strategies Figure 15 -5 Molecular Biology of the Cell (© Garland Science 2008)

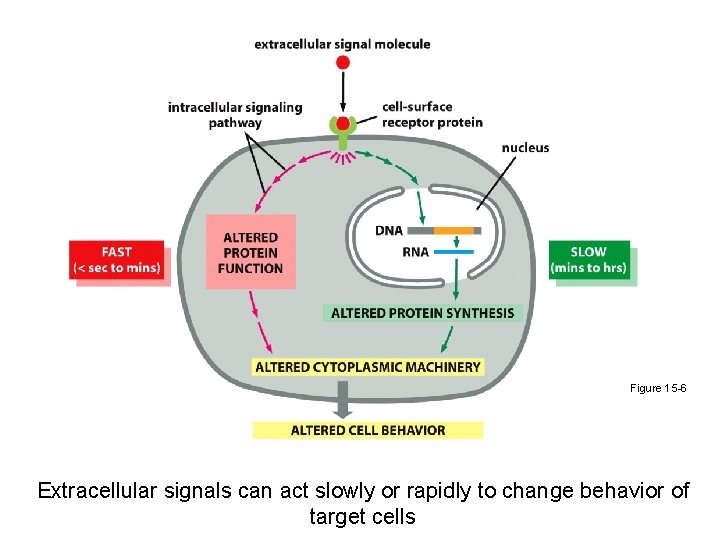
Figure 15 -6 Extracellular signals can act slowly or rapidly to change behavior of target cells

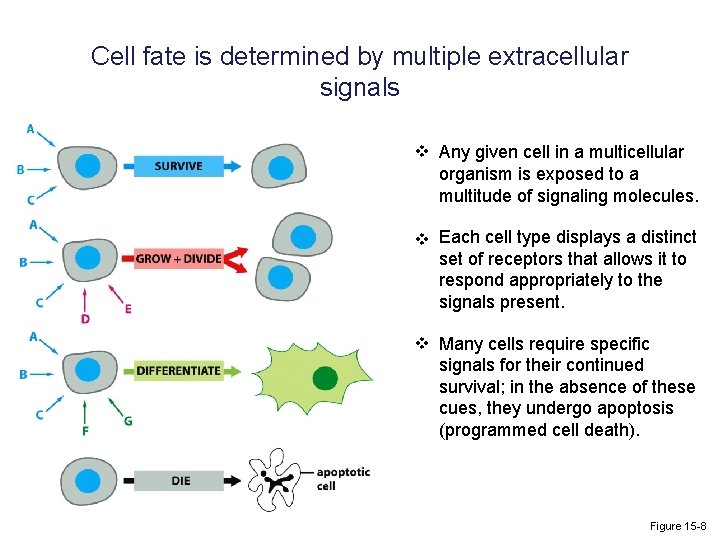
Cell fate is determined by multiple extracellular signals v Any given cell in a multicellular organism is exposed to a multitude of signaling molecules. v Each cell type displays a distinct set of receptors that allows it to respond appropriately to the signals present. v Many cells require specific signals for their continued survival; in the absence of these cues, they undergo apoptosis (programmed cell death). Figure 15 -8

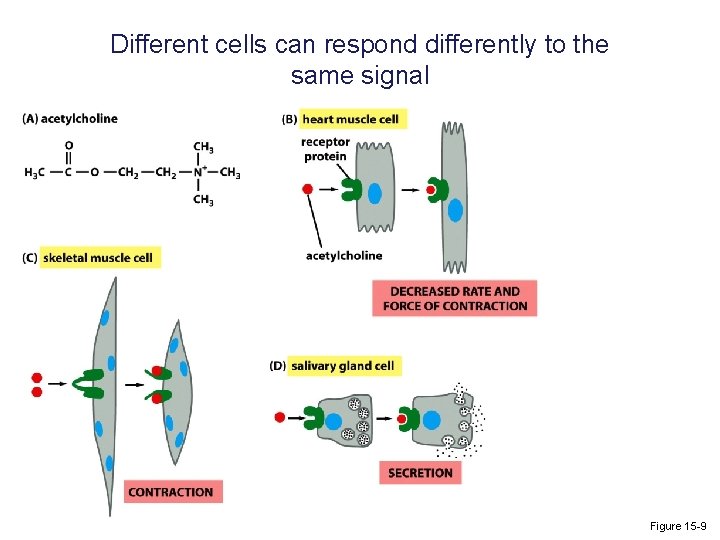
Different cells can respond differently to the same signal Figure 15 -9

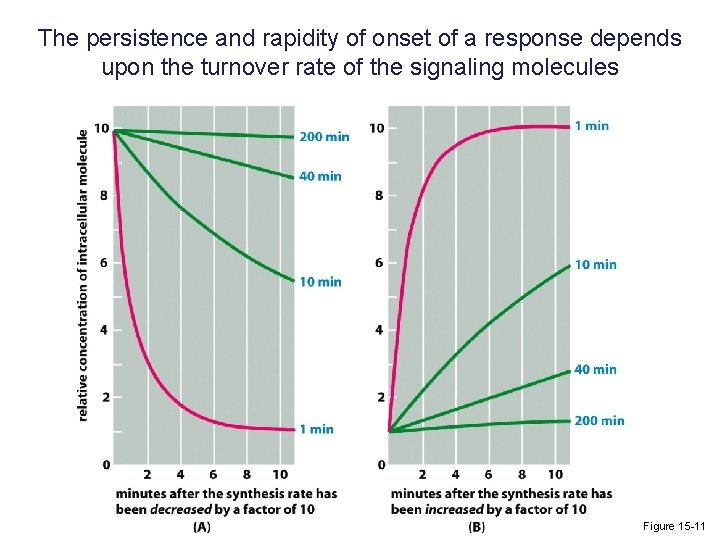
The persistence and rapidity of onset of a response depends upon the turnover rate of the signaling molecules Figure 15 -11

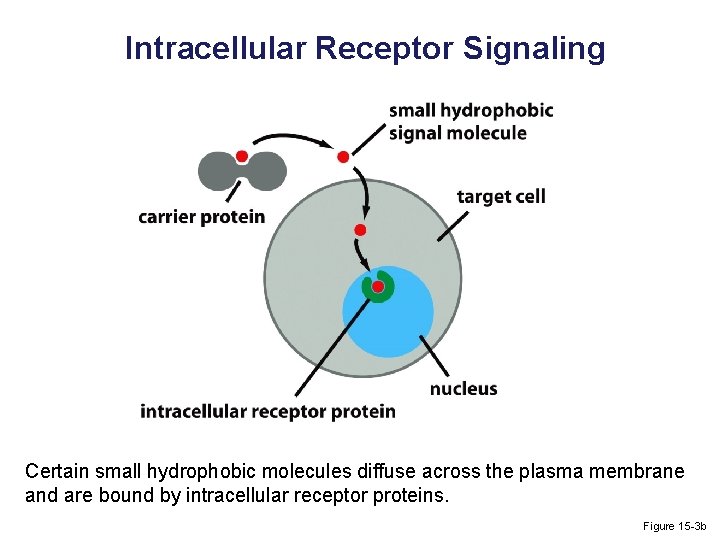
Intracellular Receptor Signaling Certain small hydrophobic molecules diffuse across the plasma membrane and are bound by intracellular receptor proteins. Figure 15 -3 b

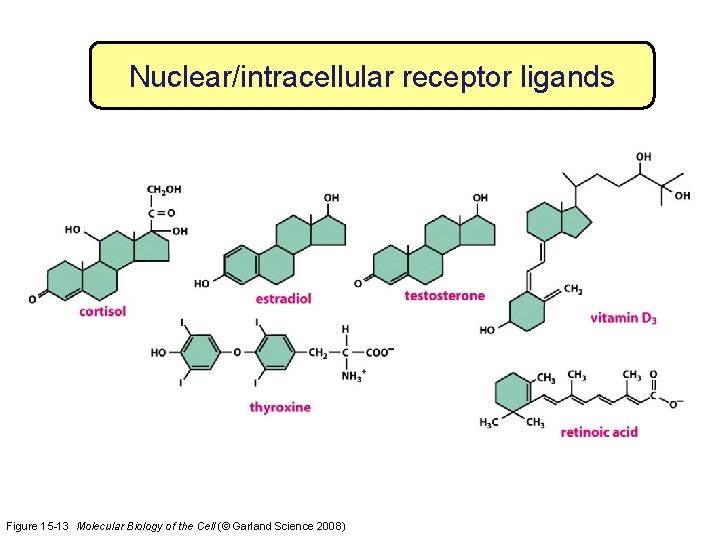
Nuclear/intracellular receptor ligands Figure 15 -13 Molecular Biology of the Cell (© Garland Science 2008)

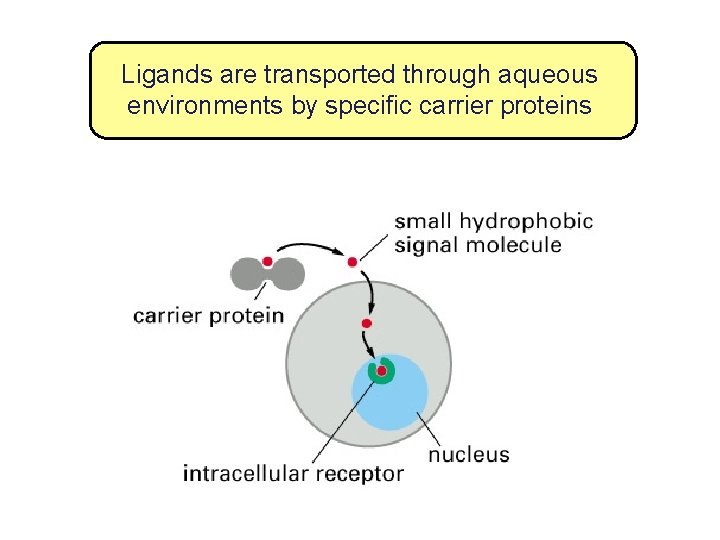
Ligands are transported through aqueous environments by specific carrier proteins

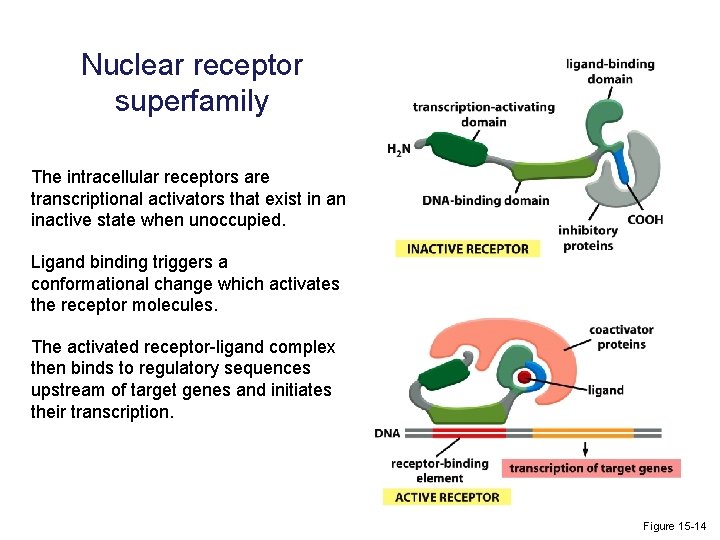
Nuclear receptor superfamily The intracellular receptors are transcriptional activators that exist in an inactive state when unoccupied. Ligand binding triggers a conformational change which activates the receptor molecules. The activated receptor-ligand complex then binds to regulatory sequences upstream of target genes and initiates their transcription. Figure 15 -14

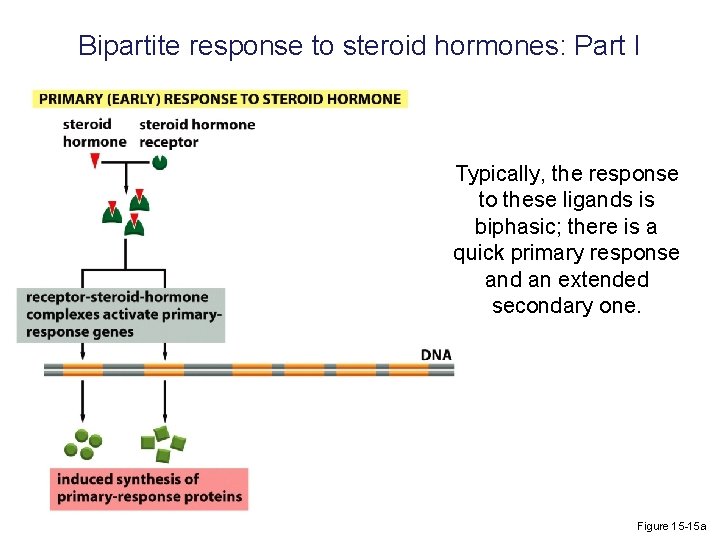
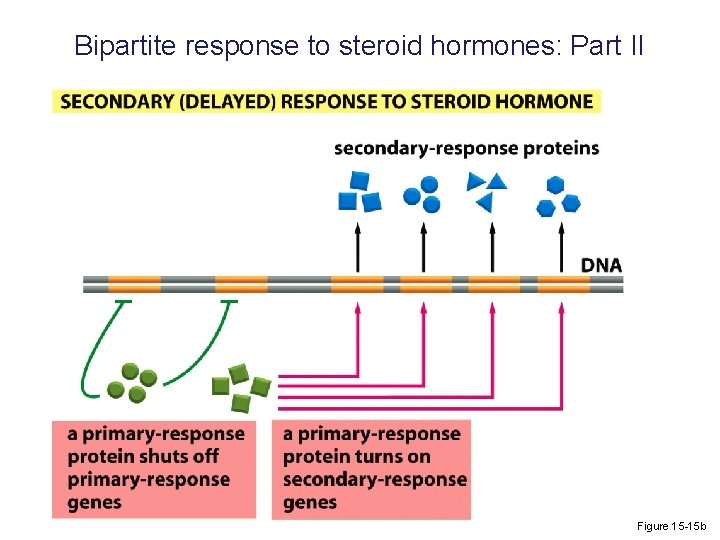
Bipartite response to steroid hormones: Part I Typically, the response to these ligands is biphasic; there is a quick primary response and an extended secondary one. Figure 15 -15 a

Bipartite response to steroid hormones: Part II Figure 15 -15 b

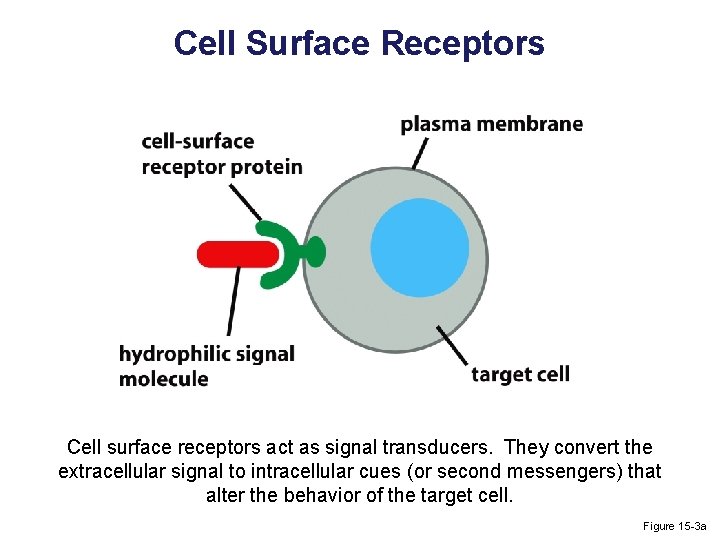
Cell Surface Receptors Cell surface receptors act as signal transducers. They convert the extracellular signal to intracellular cues (or second messengers) that alter the behavior of the target cell. Figure 15 -3 a

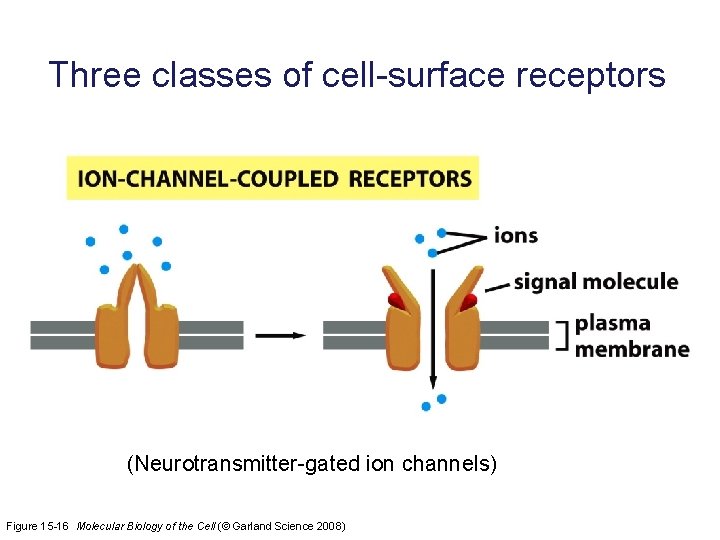
Three classes of cell-surface receptors (Neurotransmitter-gated ion channels) Figure 15 -16 Molecular Biology of the Cell (© Garland Science 2008)

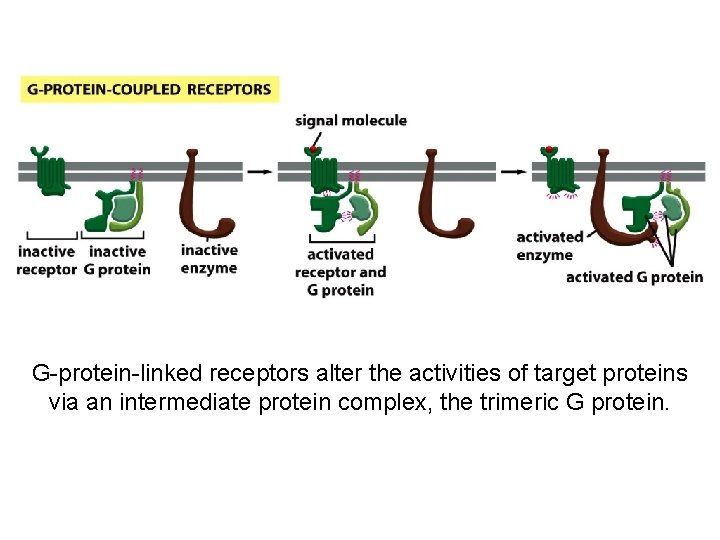
G-protein-linked receptors alter the activities of target proteins via an intermediate protein complex, the trimeric G protein.

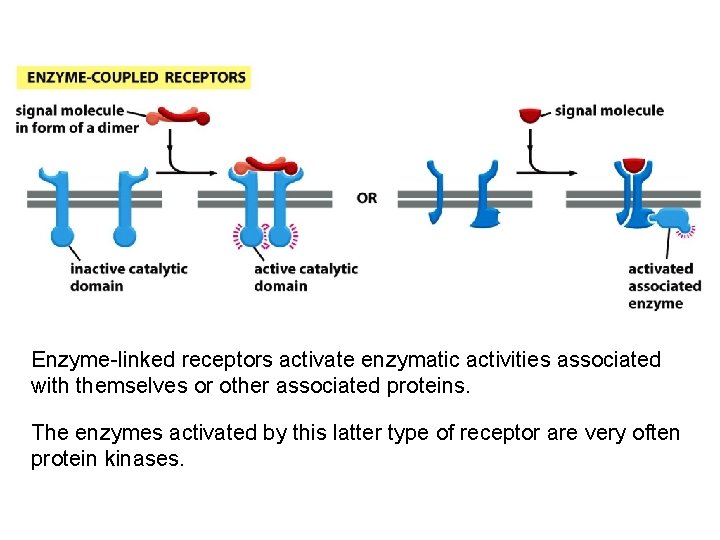
Enzyme-linked receptors activate enzymatic activities associated with themselves or other associated proteins. The enzymes activated by this latter type of receptor are very often protein kinases.

Signal Transduction Pathways * Most signaling events involve a signal transduction pathway where the “message” is passed on from one set of intracellular signaling molecules to another. * The receptor protein performs the primary transduction event by generating a new intracellular signal in response to the binding of an external signal. * The signaling pathway components perform many different functions.

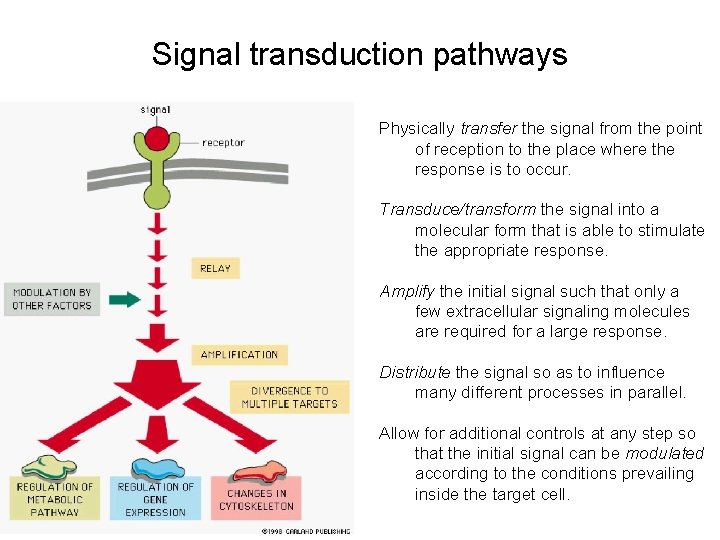
Signal transduction pathways Physically transfer the signal from the point of reception to the place where the response is to occur. Transduce/transform the signal into a molecular form that is able to stimulate the appropriate response. Amplify the initial signal such that only a few extracellular signaling molecules are required for a large response. Distribute the signal so as to influence many different processes in parallel. Allow for additional controls at any step so that the initial signal can be modulated according to the conditions prevailing inside the target cell.

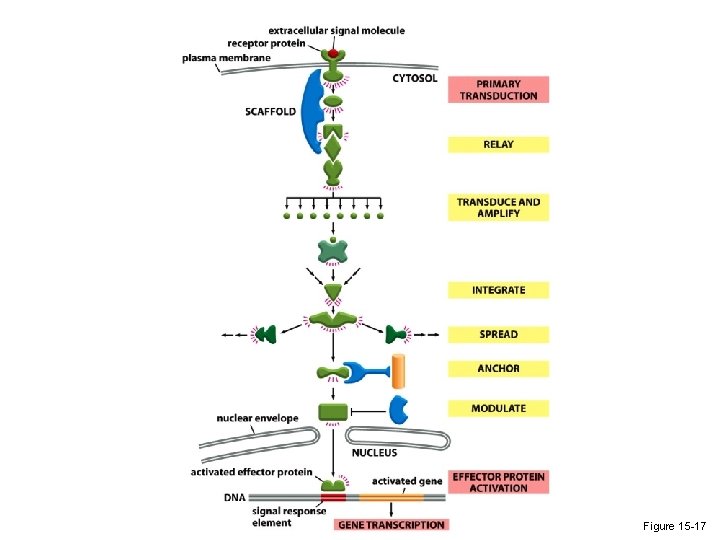
Figure 15 -17

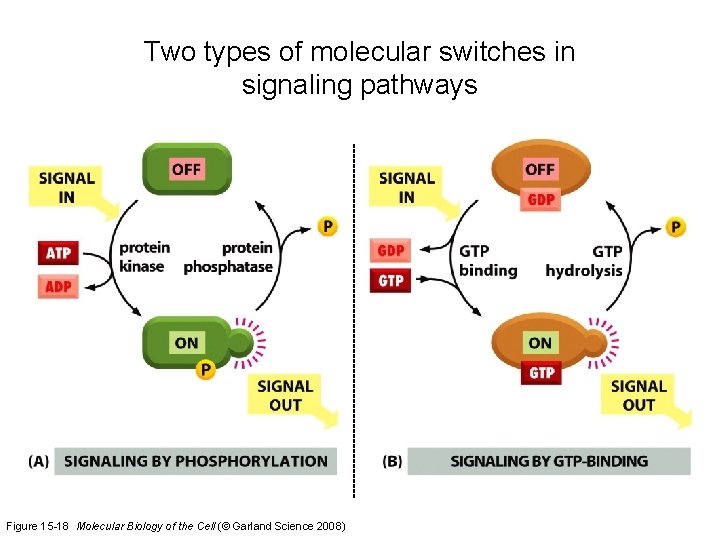
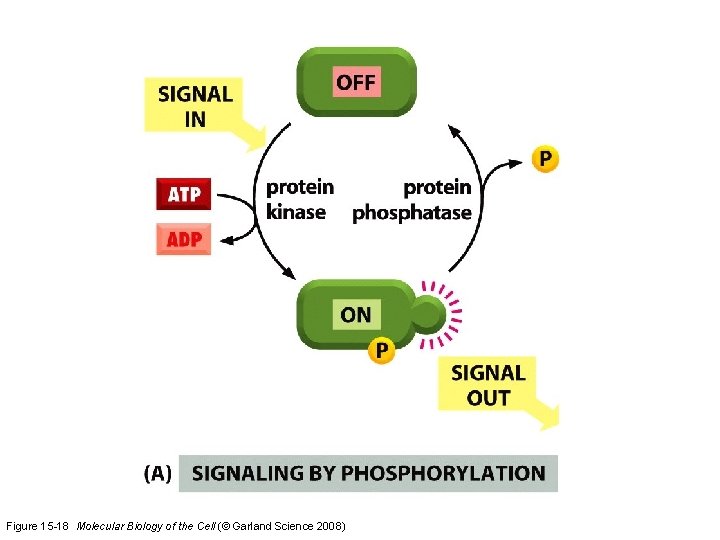
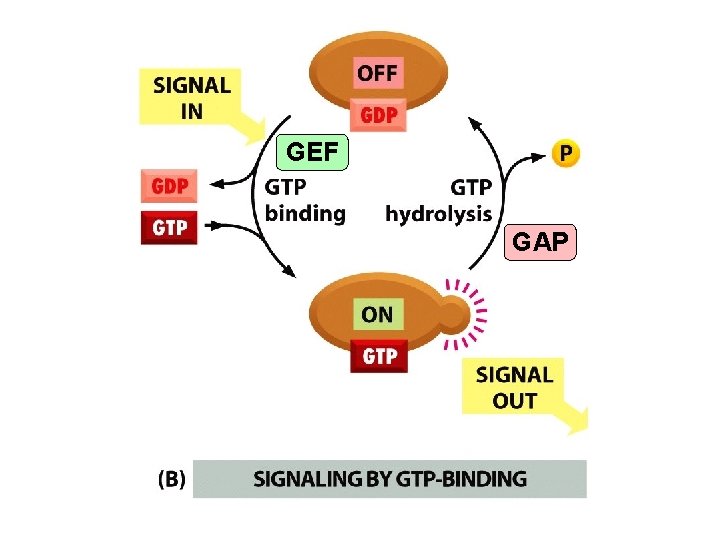
Signaling by Cell Surface Receptors * Signals received by G-protein-linked & enzyme-linked receptors are often relayed to the nucleus where they alter gene transcription. * Most key intracellular signaling proteins act as molecular switches that fall into two main classes. * The first are those proteins regulated by protein phosphorylation. Some of these are protein kinases themselves and are organized into phosphorylation cascades. * Protein kinases fall into two main groups, those that phosphorylate serine and threonine residues and those that phosphorylate tyrosine. * The second group of switch proteins consists of GTP-binding proteins that oscillate between active GTP-bound and inactive GDP -bound states. * Some of the intracellular signaling molecules function as integration devices that allow the cell to respond properly to multiple inputs.

Two types of molecular switches in signaling pathways Figure 15 -18 Molecular Biology of the Cell (© Garland Science 2008)

Figure 15 -18 Molecular Biology of the Cell (© Garland Science 2008)

GEF GAP

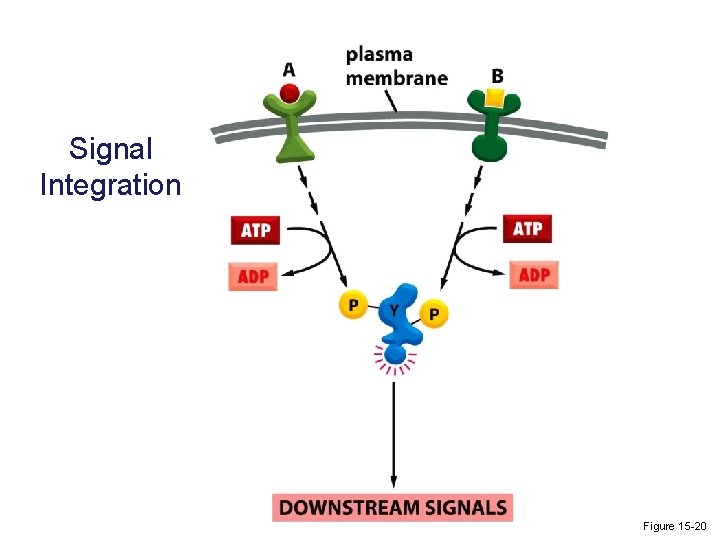
Signal Integration Figure 15 -20

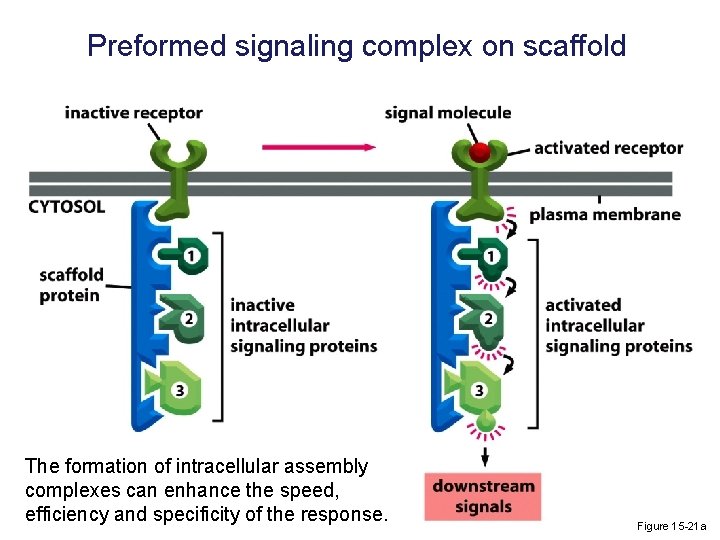
Preformed signaling complex on scaffold The formation of intracellular assembly complexes can enhance the speed, efficiency and specificity of the response. Figure 15 -21 a

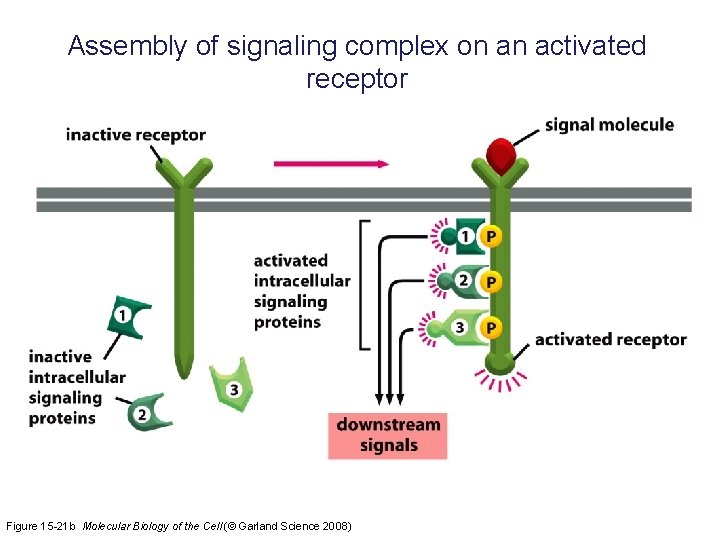
Assembly of signaling complex on an activated receptor Figure 15 -21 b Molecular Biology of the Cell (© Garland Science 2008)

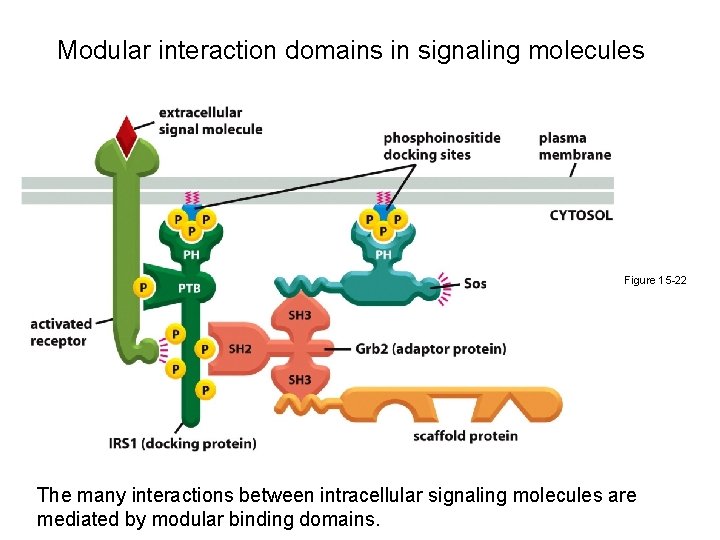
Modular interaction domains in signaling molecules Figure 15 -22 The many interactions between intracellular signaling molecules are mediated by modular binding domains.

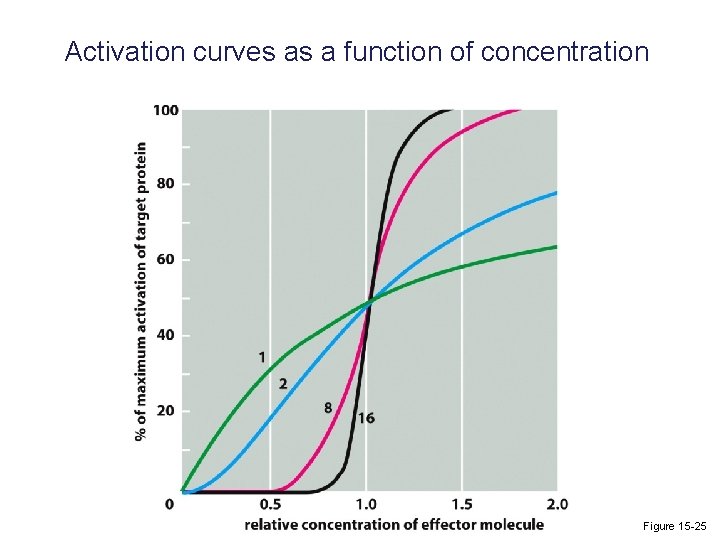
Activation curves as a function of concentration Figure 15 -25

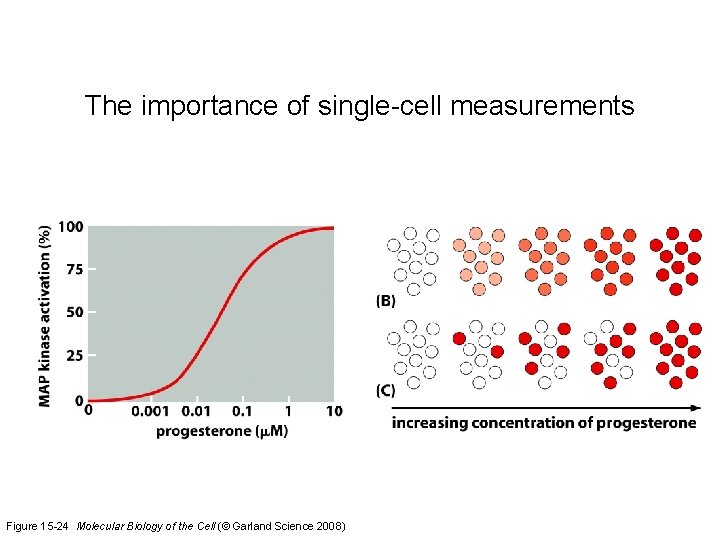
The importance of single-cell measurements Figure 15 -24 Molecular Biology of the Cell (© Garland Science 2008)

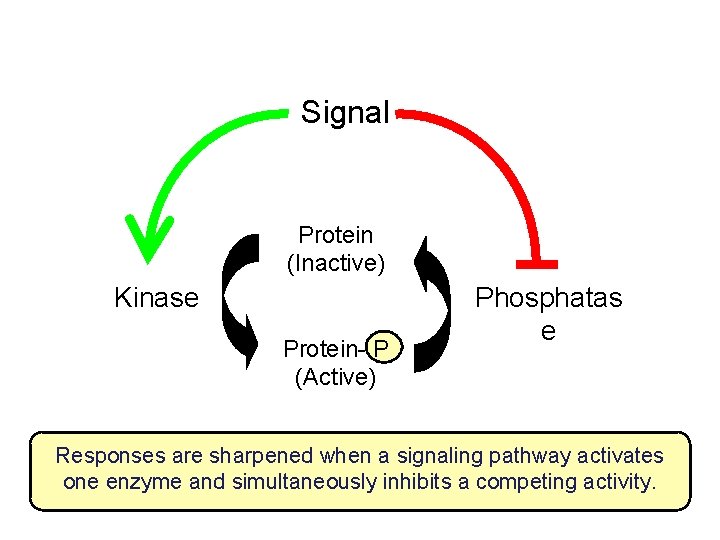
Signal Protein (Inactive) Kinase Protein- P (Active) Phosphatas e Responses are sharpened when a signaling pathway activates one enzyme and simultaneously inhibits a competing activity.

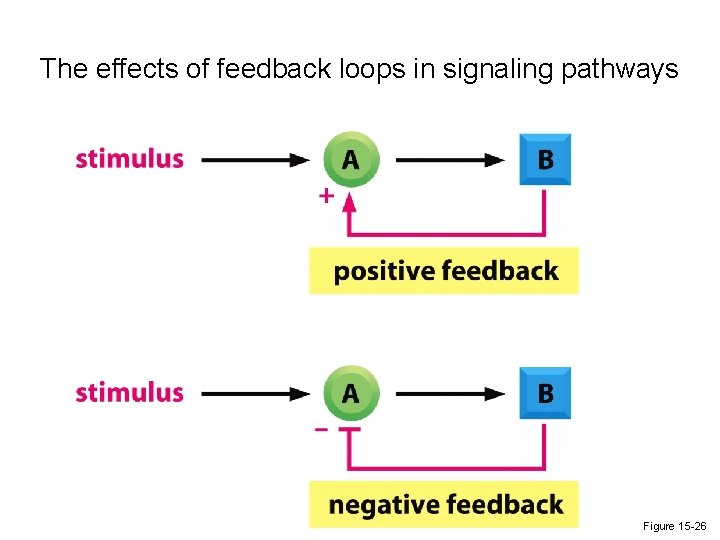
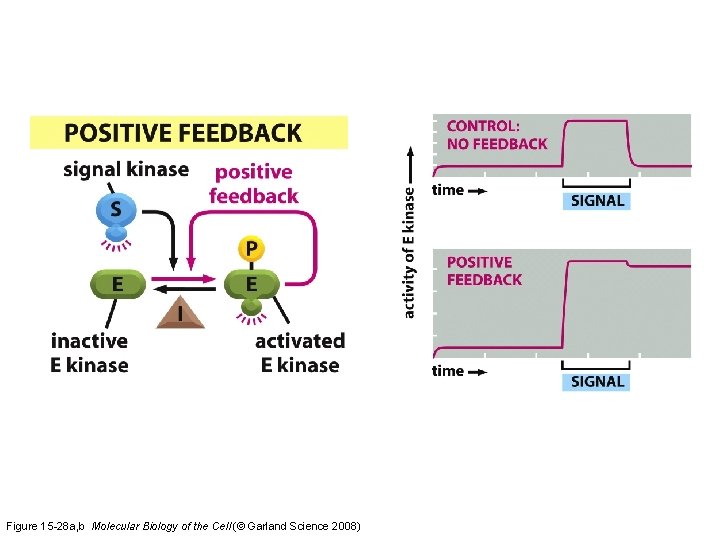
The effects of feedback loops in signaling pathways Figure 15 -26

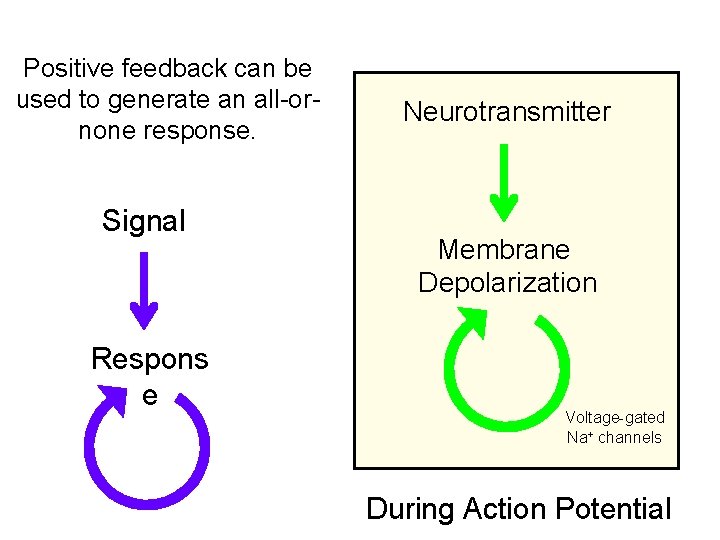
Positive feedback can be used to generate an all-ornone response. Signal Respons e Neurotransmitter Membrane Depolarization Voltage-gated Na+ channels During Action Potential

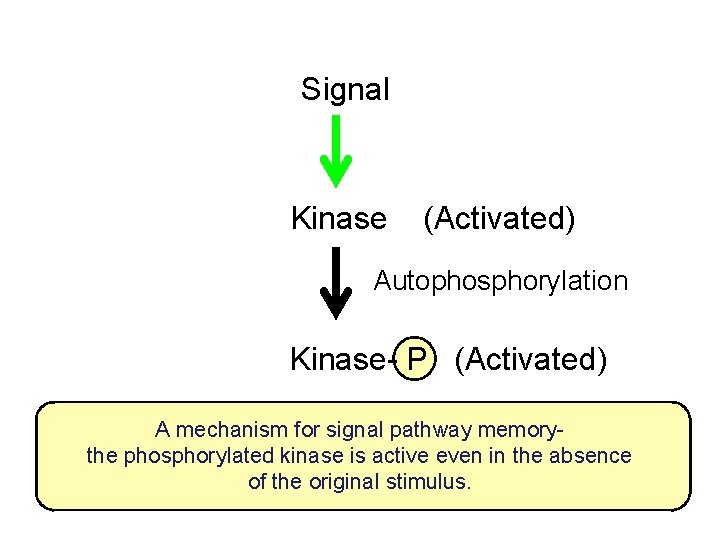
Signal Kinase (Activated) Autophosphorylation Kinase- P (Activated) A mechanism for signal pathway memorythe phosphorylated kinase is active even in the absence of the original stimulus.

Figure 15 -28 a, b Molecular Biology of the Cell (© Garland Science 2008)

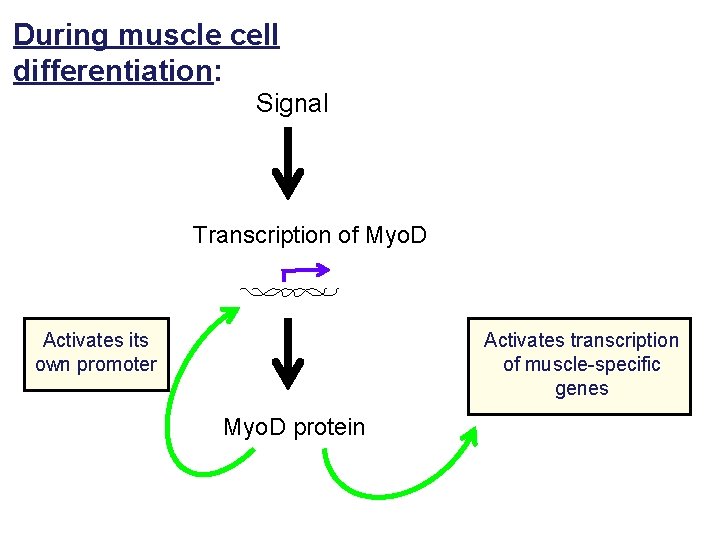
During muscle cell differentiation: Signal Transcription of Myo. D Activates its own promoter Activates transcription of muscle-specific genes Myo. D protein

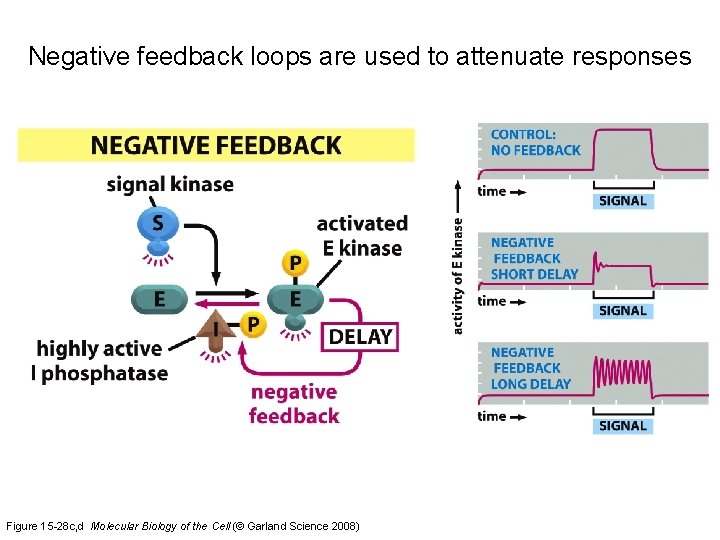
Negative feedback loops are used to attenuate responses Figure 15 -28 c, d Molecular Biology of the Cell (© Garland Science 2008)
 Jelaskan alir proses produksi multimedia
Jelaskan alir proses produksi multimedia Role of chaperones in protein folding ppt
Role of chaperones in protein folding ppt Florys models
Florys models Garland science
Garland science Denaturation of protein
Denaturation of protein How do proteins fold
How do proteins fold Hydrophobic collapse in protein folding
Hydrophobic collapse in protein folding Protein folding
Protein folding Protein pump vs protein channel
Protein pump vs protein channel Protein-protein docking
Protein-protein docking Building vocabulary: protein production
Building vocabulary: protein production Fragmented industry
Fragmented industry What does it mean to be spiritually mature
What does it mean to be spiritually mature Competitive advantage in mature industries
Competitive advantage in mature industries Bill nye hurricanes
Bill nye hurricanes Mature prof
Mature prof Play immature
Play immature Characteristics of mature reasoner
Characteristics of mature reasoner Busty mature women pics
Busty mature women pics Harappa location
Harappa location Mature male names
Mature male names Mature alo
Mature alo Monash seas calculator
Monash seas calculator Mature market strategies
Mature market strategies Mature thinking
Mature thinking Pandora brussels
Pandora brussels Veronika mature
Veronika mature Mature tun
Mature tun Mature gamers
Mature gamers Competitive advantage in mature industries
Competitive advantage in mature industries Structure of mature pollen grain
Structure of mature pollen grain Rodak
Rodak Japanese hot mature
Japanese hot mature Energeticest
Energeticest Cao mature student
Cao mature student Fruit is the mature
Fruit is the mature Strategies for mature and declining markets
Strategies for mature and declining markets Cumulus mature dissipating
Cumulus mature dissipating Messi mature
Messi mature Mature female cow
Mature female cow Mature industry
Mature industry Liverworts
Liverworts Mature follicle
Mature follicle Functions of fruits
Functions of fruits Nucleo multilobato
Nucleo multilobato Mature male cattle
Mature male cattle Cumulus mature dissipating
Cumulus mature dissipating Epithulium
Epithulium Katie bard recruitment
Katie bard recruitment What is commercial maturity?
What is commercial maturity? Male genital
Male genital Mature porcine female
Mature porcine female Types of folding
Types of folding John purdy folding chair
John purdy folding chair Branch folding
Branch folding At a country concert the ratio of the number
At a country concert the ratio of the number Hash folding method
Hash folding method Folding faulting and volcanic activity
Folding faulting and volcanic activity Folding funnel
Folding funnel Bit.ly321
Bit.ly321 British standard for folding drawings
British standard for folding drawings Fold
Fold Apakah proses yang membentuk tanah tinggi di malaysia
Apakah proses yang membentuk tanah tinggi di malaysia Folding
Folding Types of seams in sheet metal
Types of seams in sheet metal Permanent bending and folding of rocks without melting
Permanent bending and folding of rocks without melting Tropopause folding
Tropopause folding Mountain napkin fold
Mountain napkin fold Cloaca
Cloaca