Enzyme Kinetics Chapter 8 Kinetics Study of rxn

![• Assume Vo condition: [S] >>> [E] – Since S used up during • Assume Vo condition: [S] >>> [E] – Since S used up during](https://slidetodoc.com/presentation_image_h2/6e5ad7c6f2de2c489a78f799edfa6cdc/image-4.jpg)
![Exper’l findings: – As incr [S], V incr’s linearly up to some max V Exper’l findings: – As incr [S], V incr’s linearly up to some max V](https://slidetodoc.com/presentation_image_h2/6e5ad7c6f2de2c489a78f799edfa6cdc/image-5.jpg)
![M-M relates [E], [S], [P] exper’ly provable variables • New constant: KM = (k M-M relates [E], [S], [P] exper’ly provable variables • New constant: KM = (k](https://slidetodoc.com/presentation_image_h2/6e5ad7c6f2de2c489a78f799edfa6cdc/image-7.jpg)
![KM (Table 8 -6) • [S] at which ½ enz active sites filled • KM (Table 8 -6) • [S] at which ½ enz active sites filled •](https://slidetodoc.com/presentation_image_h2/6e5ad7c6f2de2c489a78f799edfa6cdc/image-12.jpg)




- Slides: 32

Enzyme Kinetics Chapter 8

Kinetics • Study of rxn rates, changes with changes in experimental conditions • Simplest rxn: S <==> P – Rate meas’d by V = velocity (M/sec) – Depends on k, [S]

Michaelis-Menten • Gen’l theory rxn rate w/ enzymatic catalysis • Add E, ES to rxn: E + S <==> E + P • Assume little reverse rxn E + P ES So E + S <==> ES E + P • Assign rate constants k 1, k-1, k 2
![Assume Vo condition S E Since S used up during • Assume Vo condition: [S] >>> [E] – Since S used up during](https://slidetodoc.com/presentation_image_h2/6e5ad7c6f2de2c489a78f799edfa6cdc/image-4.jpg)
• Assume Vo condition: [S] >>> [E] – Since S used up during rxn, can’t be limiting • Assume: – All E goes to ES • Assume fixed amt enzyme – If all E ES, will see max rate of P formed – At steady state, rate form’n ES = rate breakdown ES
![Experl findings As incr S V incrs linearly up to some max V Exper’l findings: – As incr [S], V incr’s linearly up to some max V](https://slidetodoc.com/presentation_image_h2/6e5ad7c6f2de2c489a78f799edfa6cdc/image-5.jpg)
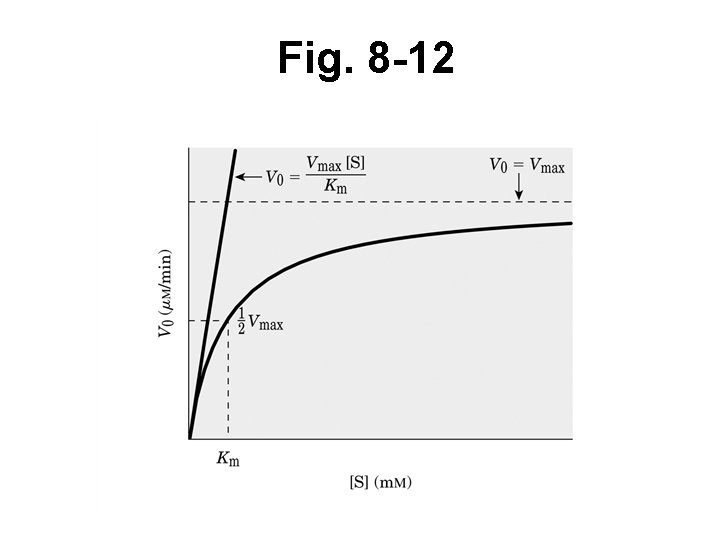
Exper’l findings: – As incr [S], V incr’s linearly up to some max V – At max V, little V incr regardless of [S] added

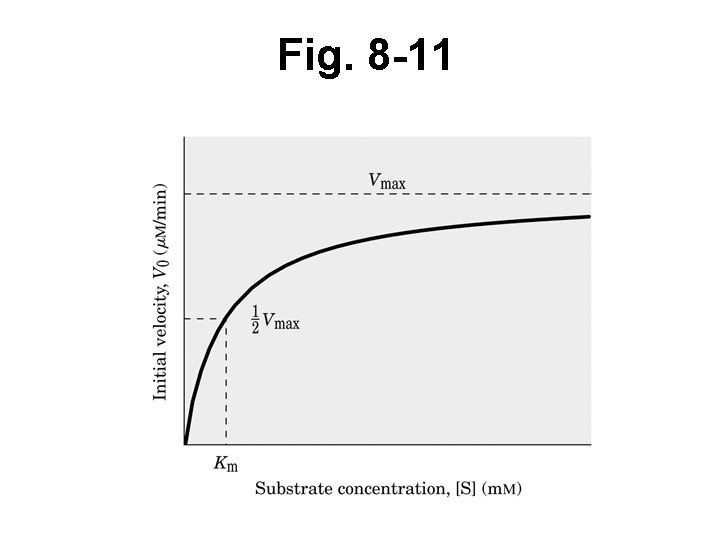
Fig. 8 -11
![MM relates E S P experly provable variables New constant KM k M-M relates [E], [S], [P] exper’ly provable variables • New constant: KM = (k](https://slidetodoc.com/presentation_image_h2/6e5ad7c6f2de2c489a78f799edfa6cdc/image-7.jpg)
M-M relates [E], [S], [P] exper’ly provable variables • New constant: KM = (k 2 + k-1) / k 1 • M-M eq’n: • Vo = (Vmax [S]) / (KM + [S]) • Quantitative relationship between – Initial velocity – Max rate of rxn – Initial [S]

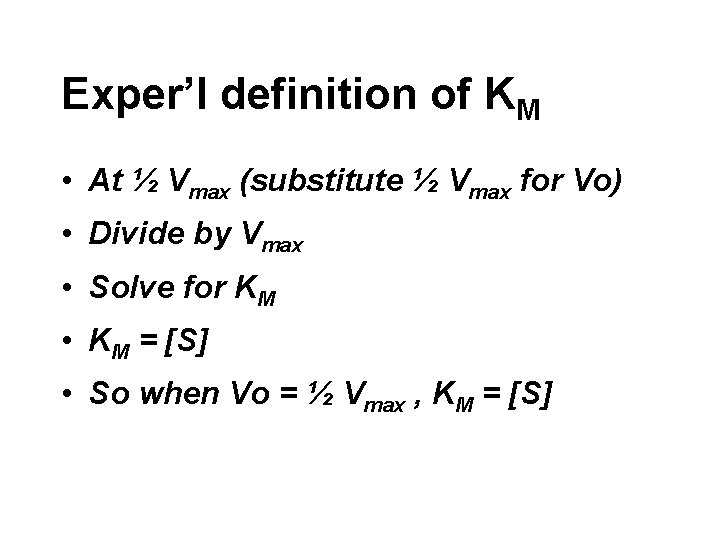
Exper’l definition of KM • At ½ Vmax (substitute ½ Vmax for Vo) • Divide by Vmax • Solve for KM • KM = [S] • So when Vo = ½ Vmax , KM = [S]

Fig. 8 -12

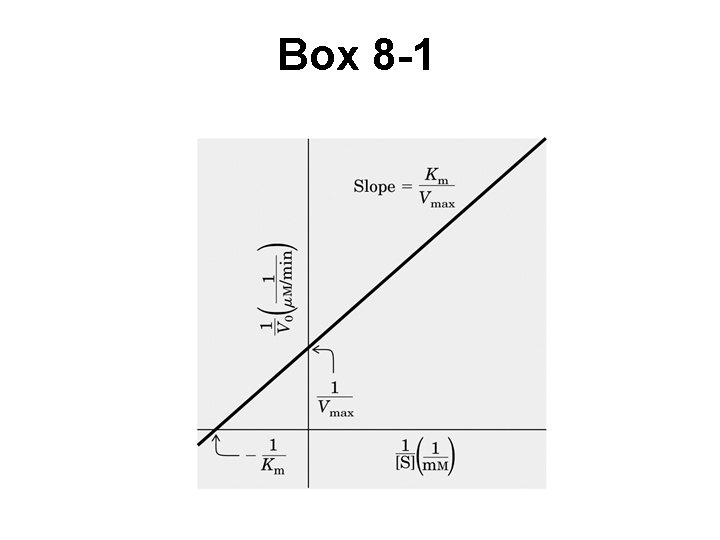
Difficult to determine variables from M-M plot • Hard to measure small changes in V • Use double reciprocal plot straight line • Lineweaver-Burk (Box 8 -1)

Box 8 -1
![KM Table 8 6 S at which ½ enz active sites filled KM (Table 8 -6) • [S] at which ½ enz active sites filled •](https://slidetodoc.com/presentation_image_h2/6e5ad7c6f2de2c489a78f799edfa6cdc/image-12.jpg)
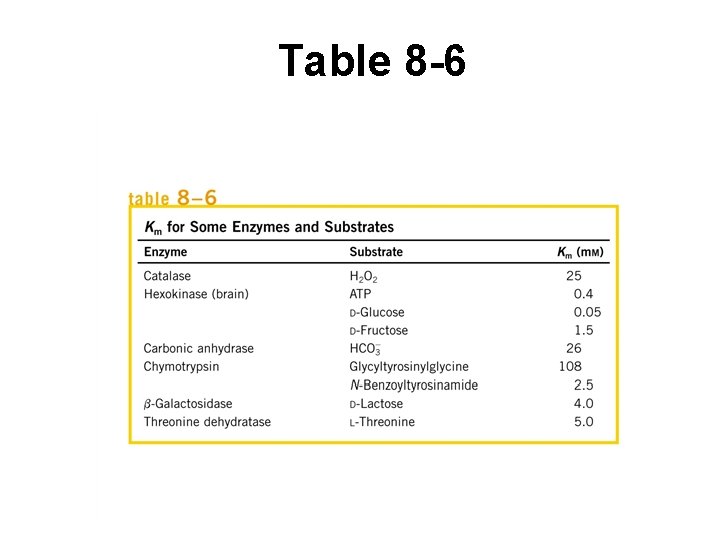
KM (Table 8 -6) • [S] at which ½ enz active sites filled • Related to rate constants • In living cells, value close to [S] for that E – Commonly enz active sites NOT saturated w/ S • May describe affinity of E for S ONLY if k-1 >>> k 2 – Right half of rxn equation negligible – KM = k-1 / k 1 – Describes rate form’n, breakdown of ES – Here, KM value indicates strength of binding E-S – In real life, system is more complex

Table 8 -6

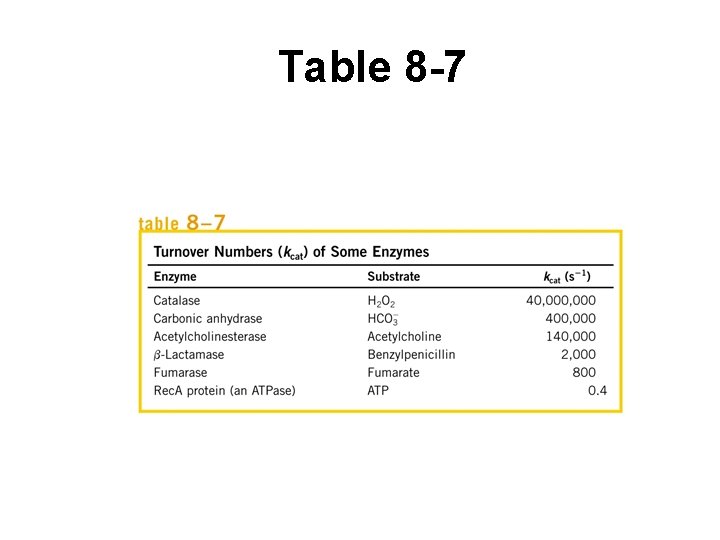
Other kinetics variables (Table 8 -7) • Turnover # – # S molecules converted P by 1 enz molecule per unit time – Use when enz is fully sat’d w/ S – Equals k 2 – Can calc from Vmax if know [ET]

Other kinetics variables (cont’d) • kcat – Max catalytic rate for E when S saturating – Equivalent to k of rate limiting step – For M-M ( E + S <==> E + P ), = k 2 – Can be complex – Book = turnover # kcat

Table 8 -7

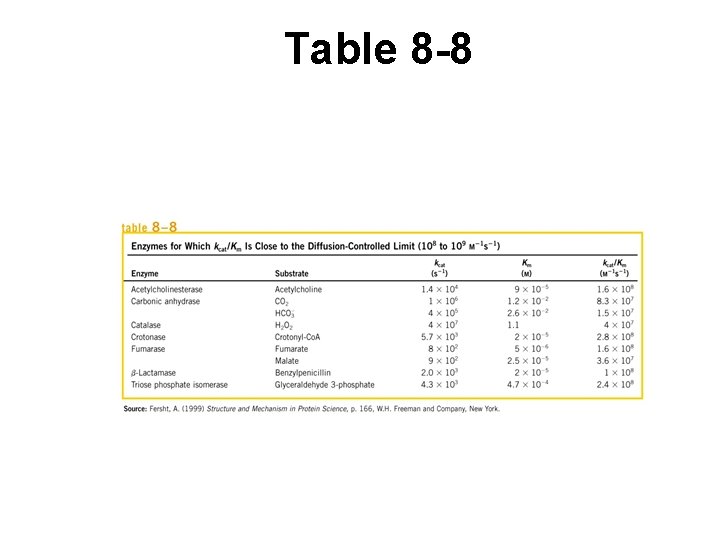
Comparisons of catalytic abilities • Optimum KM, kcat values for each E • Use ratio to compare catalytic efficiencies • Max efficiency at kcat / KM = 108 – 109 M-1 sec-1 – Velocity limited by E encounters w/ S – Called Diffusion Controlled Limit

Table 8 -8

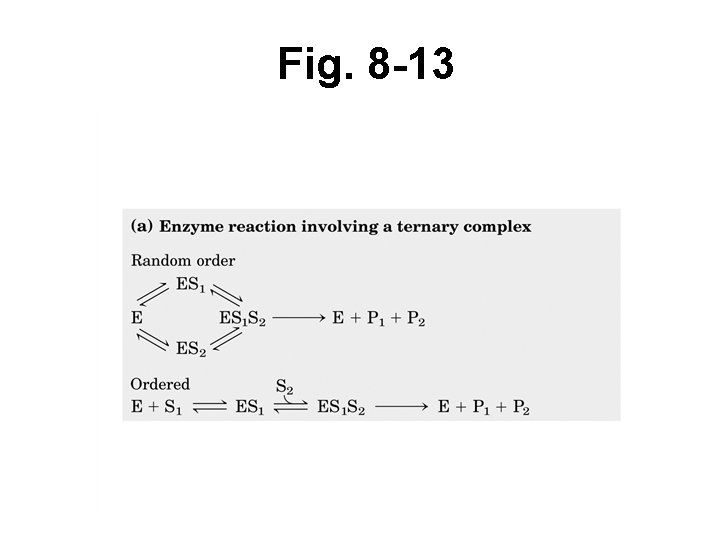
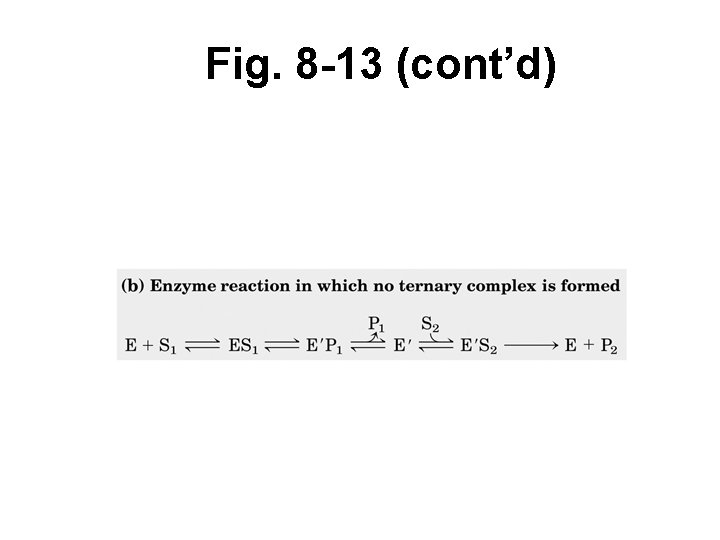
Kinetics when > 1 substrate • Random order = E can accept either S 1 or S 2 first • Ordered mechanism = E must accept S 1 first, before S 2 can bind • Double displacement (or ping-pong) = S 1 must bind and P 1 must be released before S 2 can bind and P 2 is released

Fig. 8 -13

Fig. 8 -13 (cont’d)

Inhibition • Used by cell to control catalysis in metabolic pathways • Used to alter catalysis by drugs, toxins • Used as tools to study mechanisms • Irreversible • Reversible – Includes competitive, noncompetitive, uncompetitive

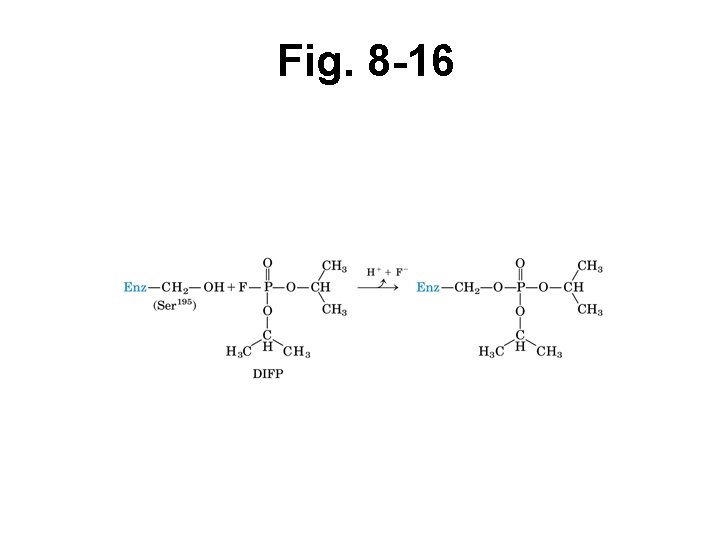
Irreversible inhibition • Inhibitor binds tightly to enz • Dissociates slowly or not at all • Book example: DIFP • Includes suicide substrate inhibitors

Fig. 8 -16

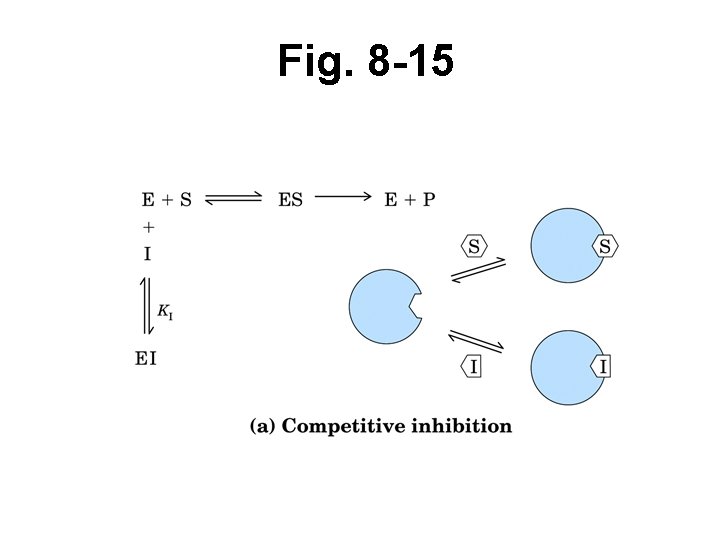
Reversible inhibition • Inhibitor may bind at active site or some distal site • Binding is reversible • Temporarily inhibits E, S binding or proper rxn • Can calculate KI • Competitive – “Appear as S” – Bind active site • So compete w/ S for active site – Overcome w/ incr’d [S] – Affects KM, not Vmax

Fig. 8 -15

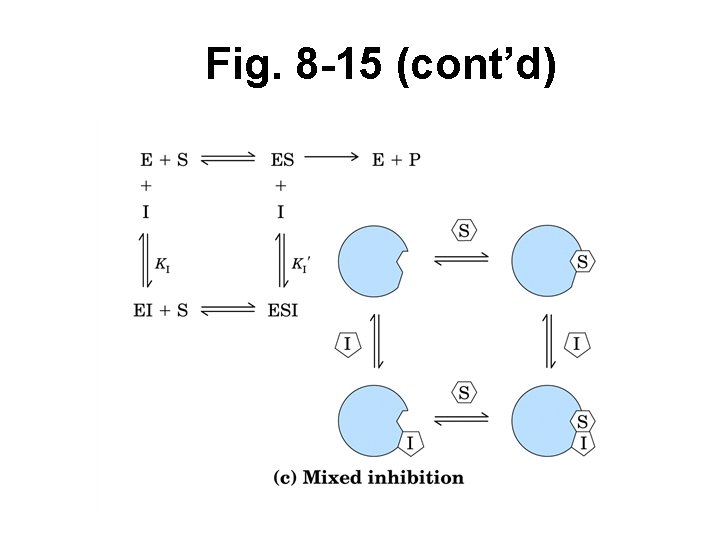
Reversible inhibition (cont’d) • Noncompetitive (Mixed) – When S bound or not – Bind at site away from active site – Causes conform’l change in E – E inactivated when I bound – Decr’d E avail for binding S, rxn catalysis – Not overcome w/ incr’d [S] – Affects both KM, Vmax – Common when S 1 + S 2

Fig. 8 -15 (cont’d)

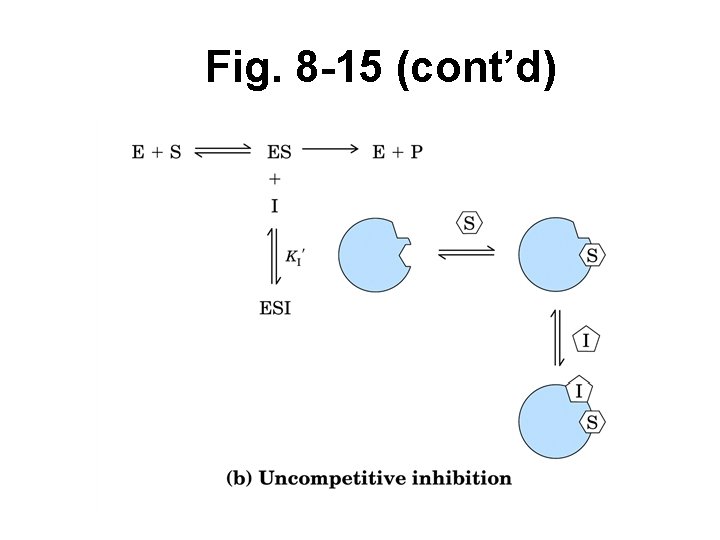
Reversible inhibition (cont’d) • Uncompetitive – Binds only when S already bound (so ES complex) – Bind at site away from active site – Causes conform’l change, E inactivated – Not overcome w/ incr’d [S] – Affects both KM, Vmax – Common when S 1 + S 2

Fig. 8 -15 (cont’d)

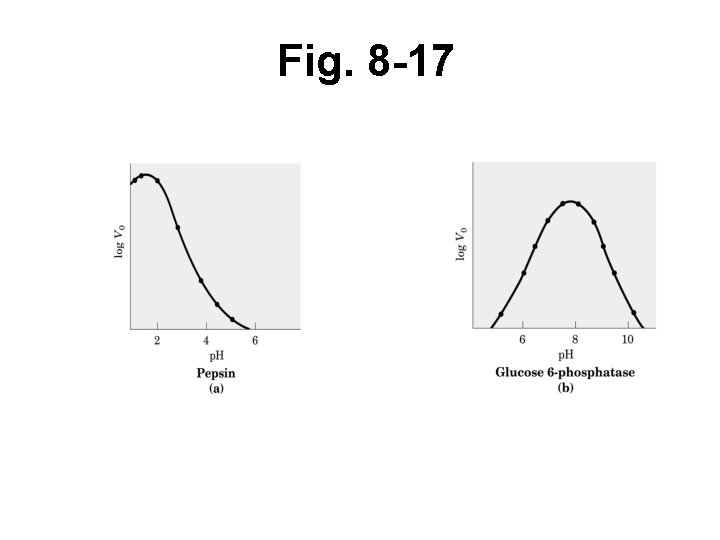
Effect of p. H on catalysis • Optimum p. H where maximal activity • Aa’s impt to catalysis must maintain particular ionization • Aa’s in other parts of enz impt to maintain folding, structure must also maintain partic. ionization • Can predict impt aa’s by activity changes at different p. H’s (use p. Ka info)

Fig. 8 -17