Enzyme Kinetics and Catalysis II 3242003 Kinetics of


![For Michaelis -Menton kinetics k 2= kcat When [S] << KM very little ES For Michaelis -Menton kinetics k 2= kcat When [S] << KM very little ES](https://slidetodoc.com/presentation_image_h/ed9875ca425e6bd0d680b9cf1f0efd65/image-15.jpg)


- Slides: 36

Enzyme Kinetics and Catalysis II 3/24/2003

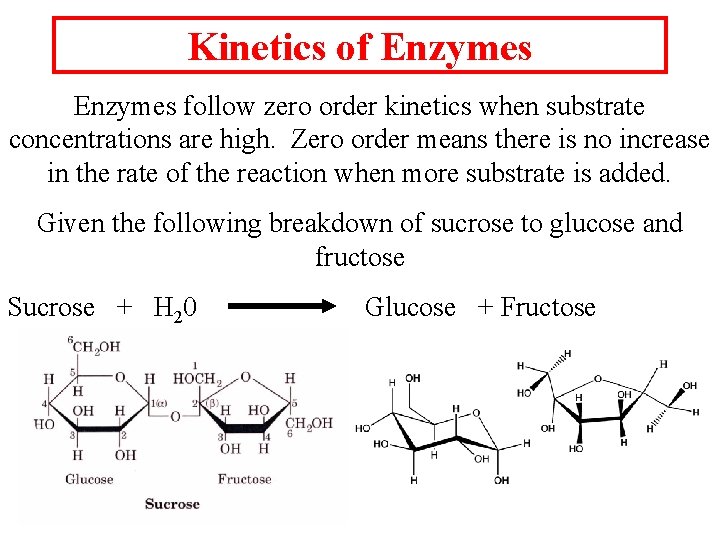
Kinetics of Enzymes follow zero order kinetics when substrate concentrations are high. Zero order means there is no increase in the rate of the reaction when more substrate is added. Given the following breakdown of sucrose to glucose and fructose Sucrose + H 20 Glucose + Fructose

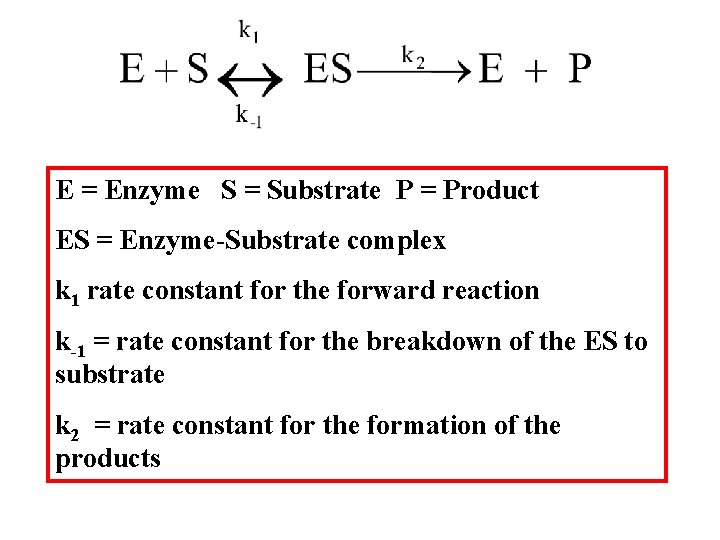
E = Enzyme S = Substrate P = Product ES = Enzyme-Substrate complex k 1 rate constant for the forward reaction k-1 = rate constant for the breakdown of the ES to substrate k 2 = rate constant for the formation of the products

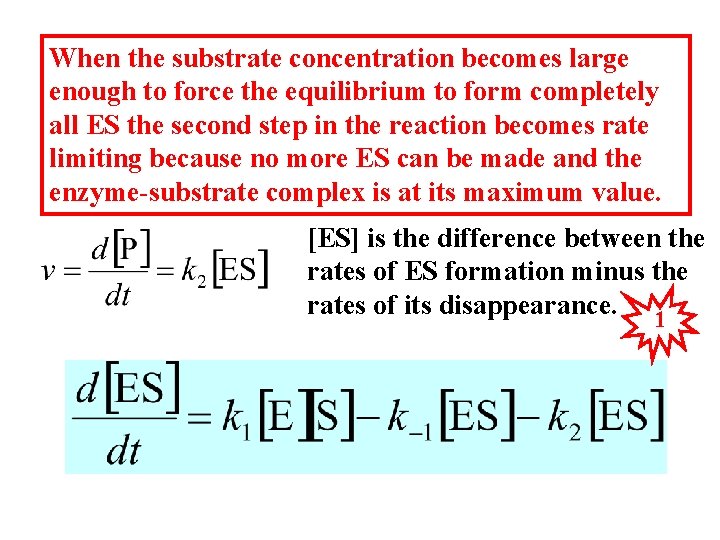
When the substrate concentration becomes large enough to force the equilibrium to form completely all ES the second step in the reaction becomes rate limiting because no more ES can be made and the enzyme-substrate complex is at its maximum value. [ES] is the difference between the rates of ES formation minus the rates of its disappearance. 1

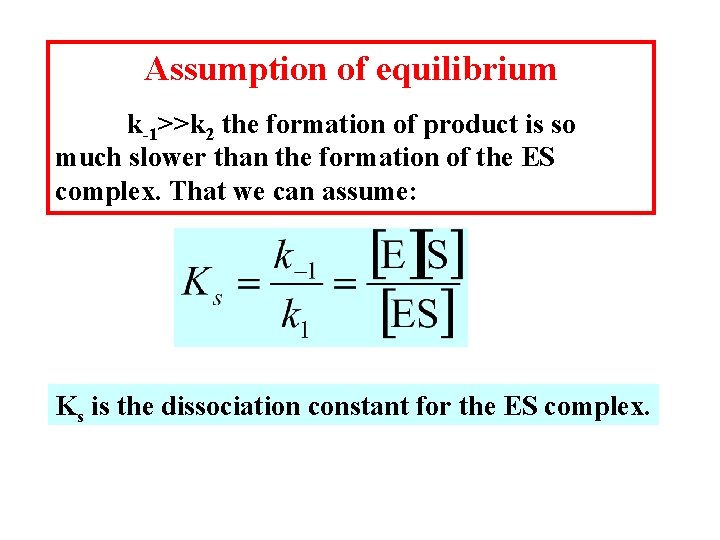
Assumption of equilibrium k-1>>k 2 the formation of product is so much slower than the formation of the ES complex. That we can assume: Ks is the dissociation constant for the ES complex.

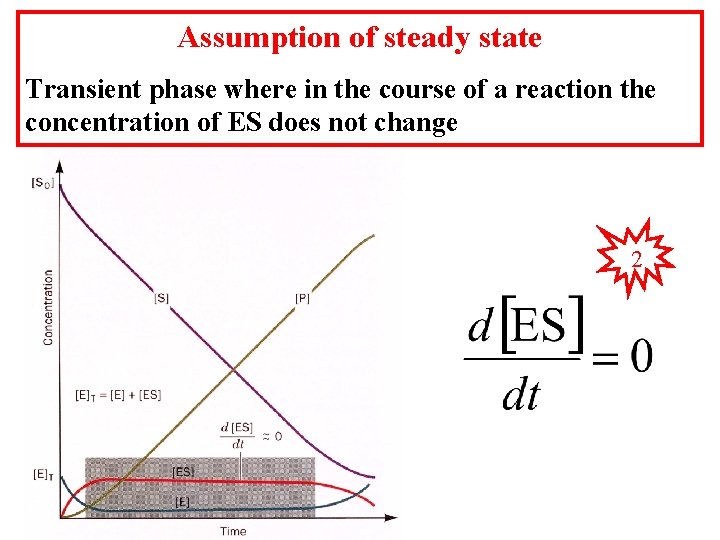
Assumption of steady state Transient phase where in the course of a reaction the concentration of ES does not change 2

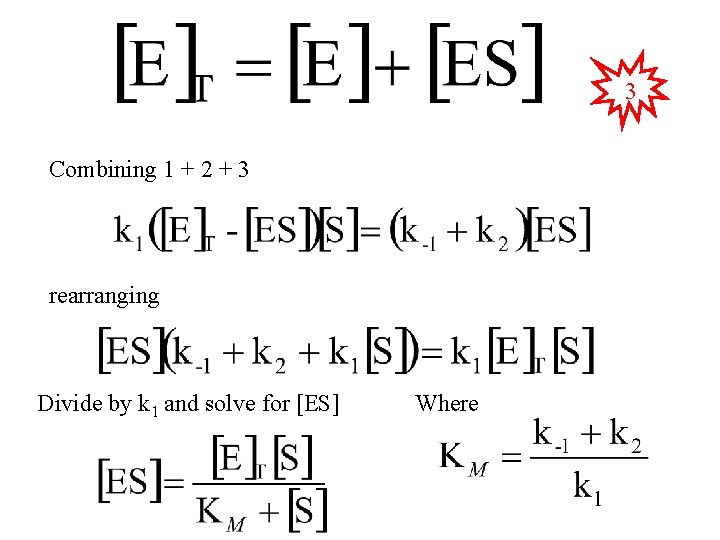
3 Combining 1 + 2 + 3 rearranging Divide by k 1 and solve for [ES] Where

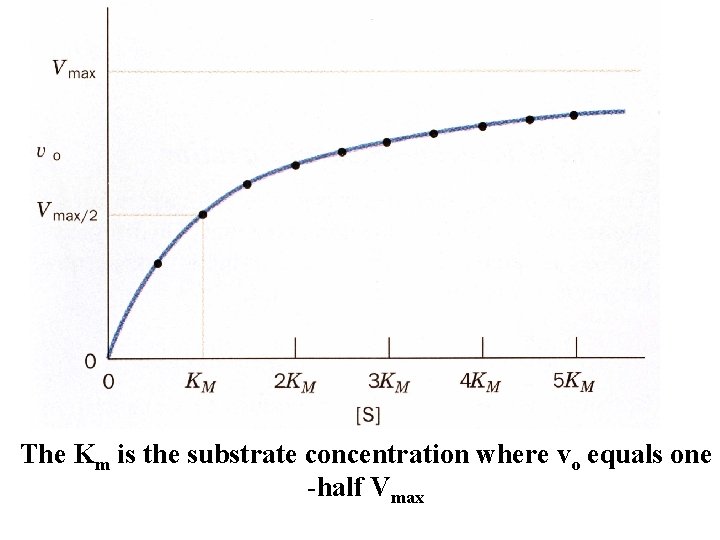
vo is the initial velocity when the reaction is just starting out. And is the maximum velocity The Michaelis - Menten equation

The Km is the substrate concentration where vo equals one -half Vmax

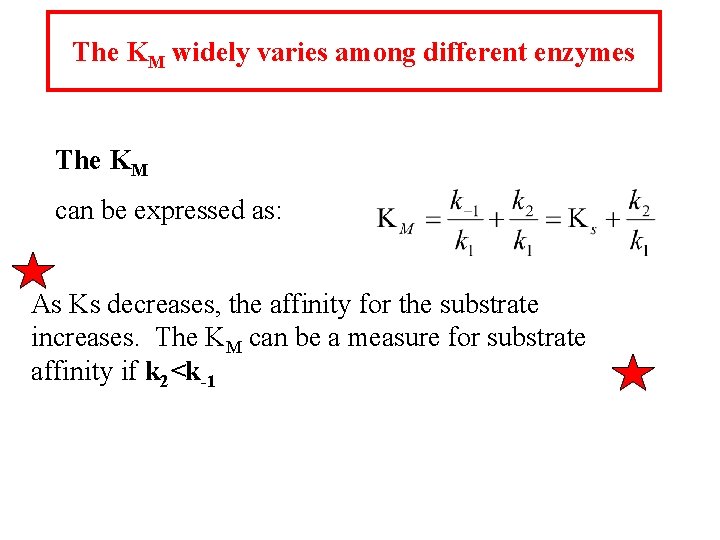
The KM widely varies among different enzymes The KM can be expressed as: As Ks decreases, the affinity for the substrate increases. The KM can be a measure for substrate affinity if k 2<k-1

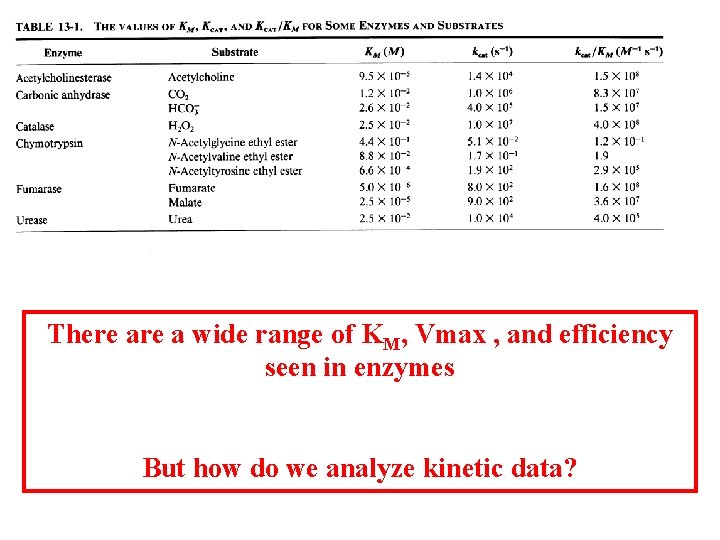
There a wide range of KM, Vmax , and efficiency seen in enzymes But how do we analyze kinetic data?

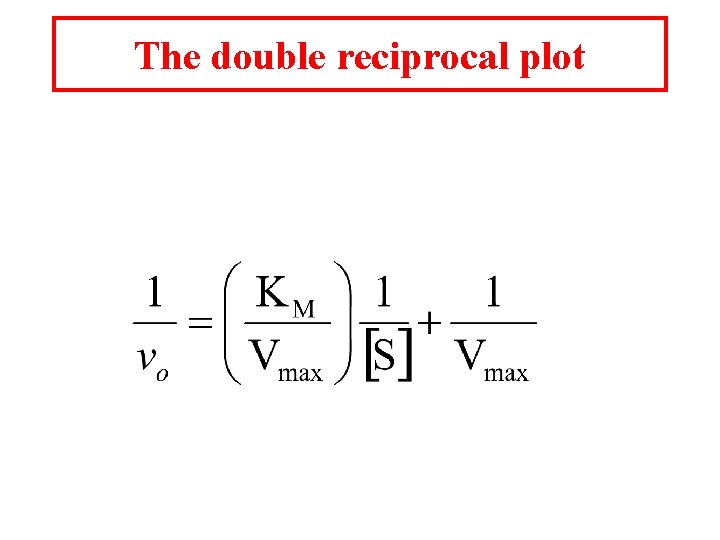
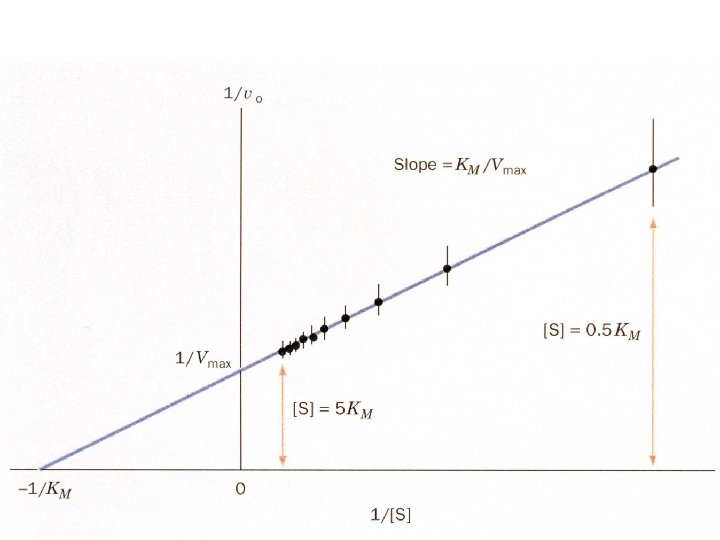
The double reciprocal plot


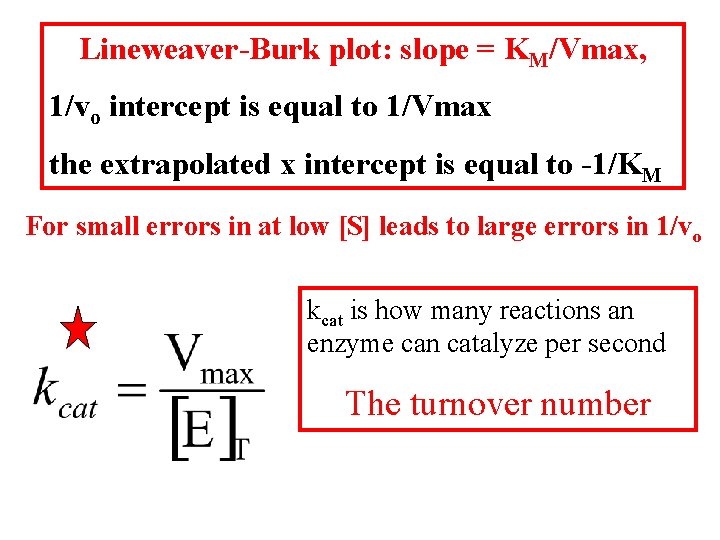
Lineweaver-Burk plot: slope = KM/Vmax, 1/vo intercept is equal to 1/Vmax the extrapolated x intercept is equal to -1/KM For small errors in at low [S] leads to large errors in 1/vo kcat is how many reactions an enzyme can catalyze per second The turnover number
![For Michaelis Menton kinetics k 2 kcat When S KM very little ES For Michaelis -Menton kinetics k 2= kcat When [S] << KM very little ES](https://slidetodoc.com/presentation_image_h/ed9875ca425e6bd0d680b9cf1f0efd65/image-15.jpg)
For Michaelis -Menton kinetics k 2= kcat When [S] << KM very little ES is formed and [E] = [E]T and Kcat/KM is a measure of catalytic efficiency

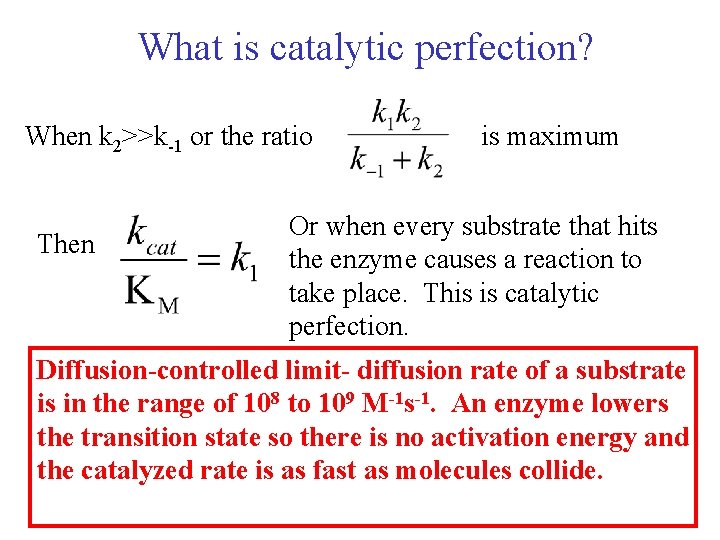
What is catalytic perfection? When k 2>>k-1 or the ratio Then is maximum Or when every substrate that hits the enzyme causes a reaction to take place. This is catalytic perfection. Diffusion-controlled limit- diffusion rate of a substrate is in the range of 108 to 109 M-1 s-1. An enzyme lowers the transition state so there is no activation energy and the catalyzed rate is as fast as molecules collide.

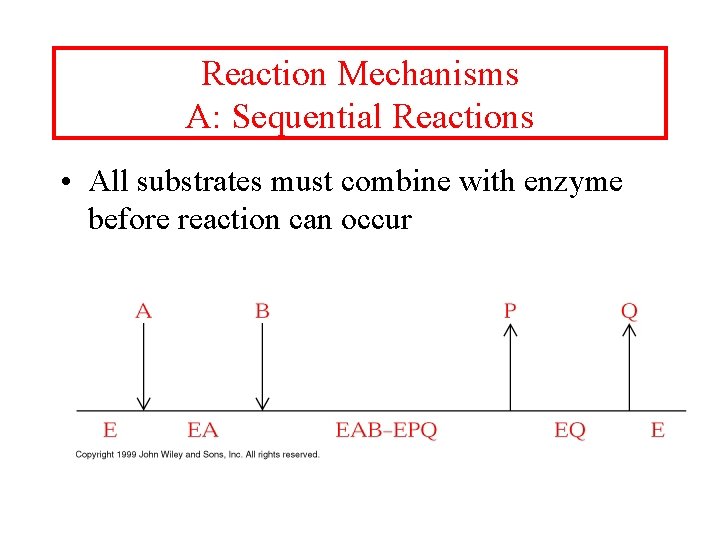
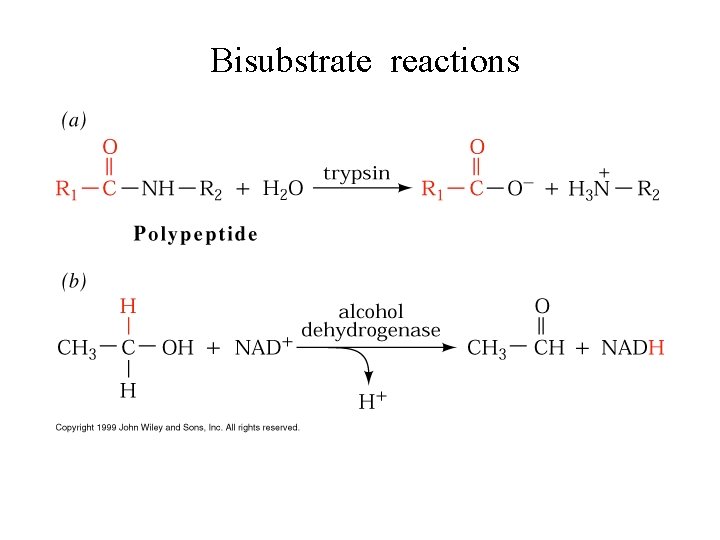
Reaction Mechanisms A: Sequential Reactions • All substrates must combine with enzyme before reaction can occur

Bisubstrate reactions

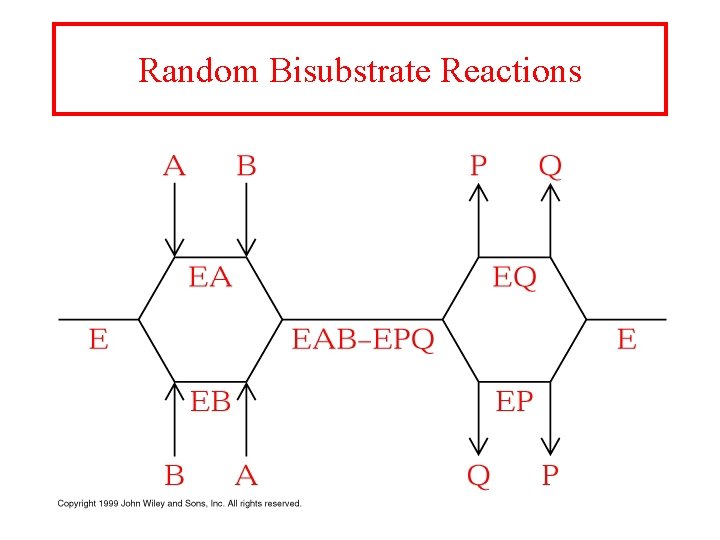
Random Bisubstrate Reactions

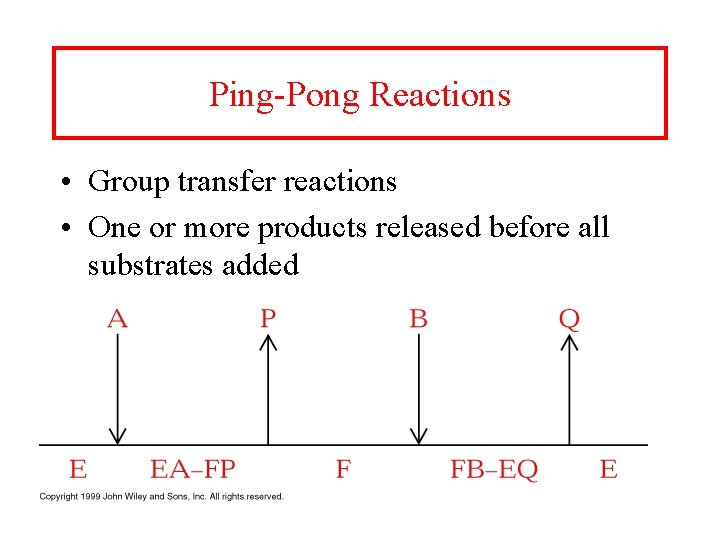
Ping-Pong Reactions • Group transfer reactions • One or more products released before all substrates added

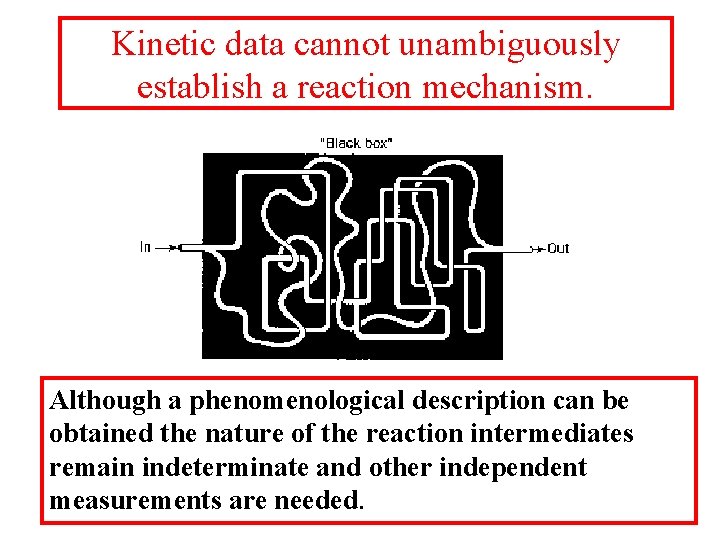
Kinetic data cannot unambiguously establish a reaction mechanism. Although a phenomenological description can be obtained the nature of the reaction intermediates remain indeterminate and other independent measurements are needed.

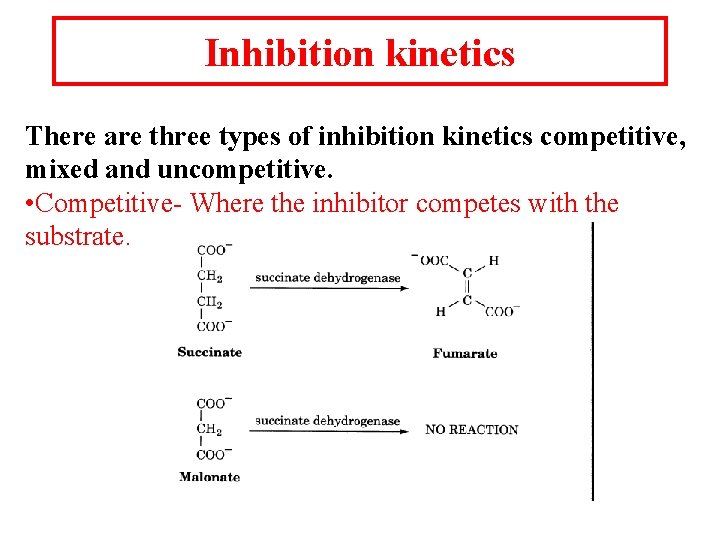
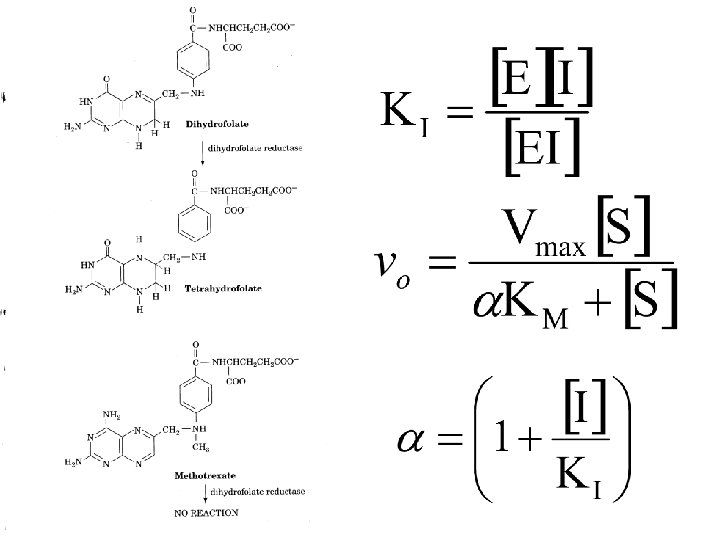
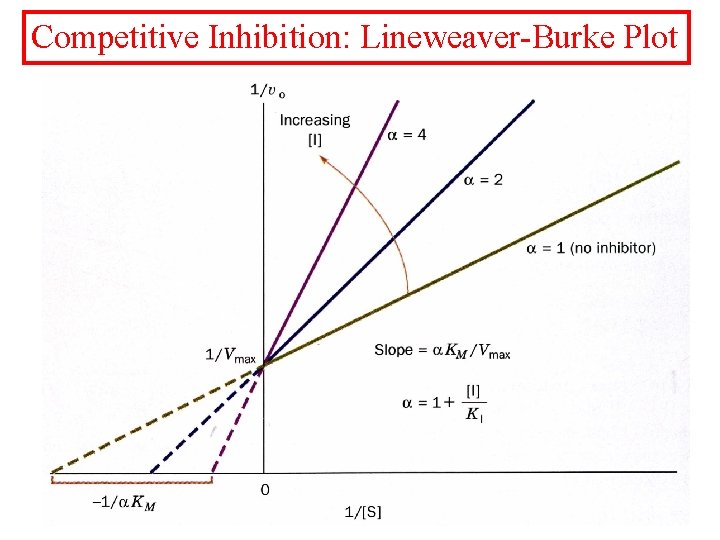
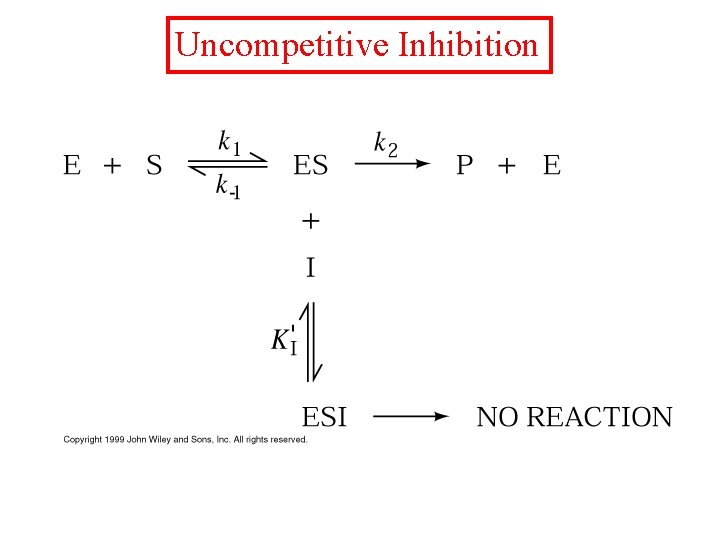
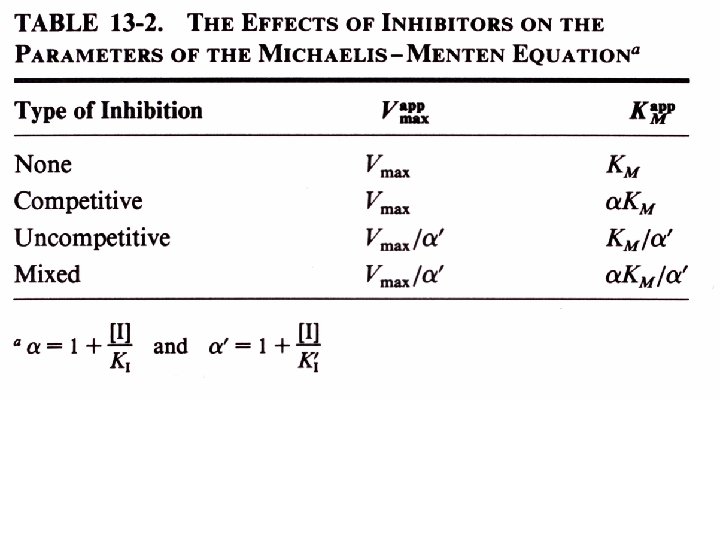
Inhibition kinetics There are three types of inhibition kinetics competitive, mixed and uncompetitive. • Competitive- Where the inhibitor competes with the substrate.

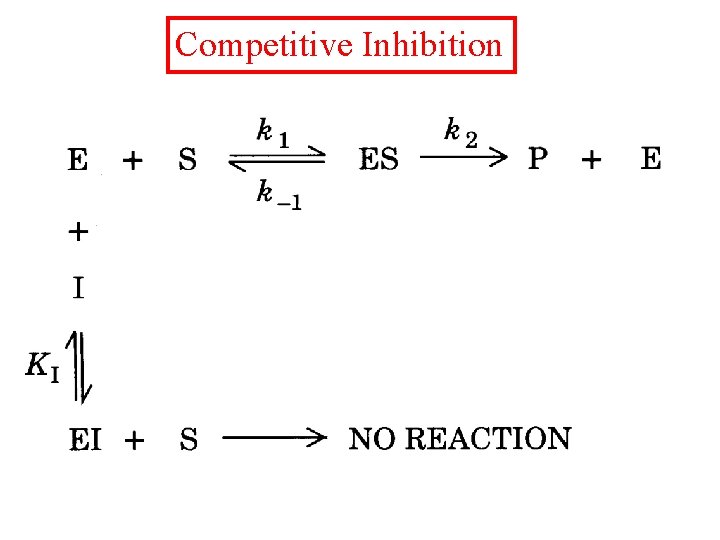
Competitive Inhibition



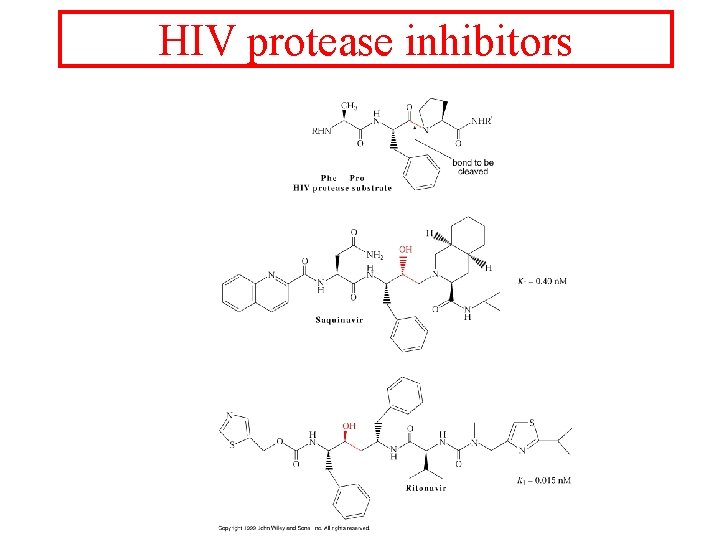
HIV protease inhibitors

Competitive Inhibition: Lineweaver-Burke Plot

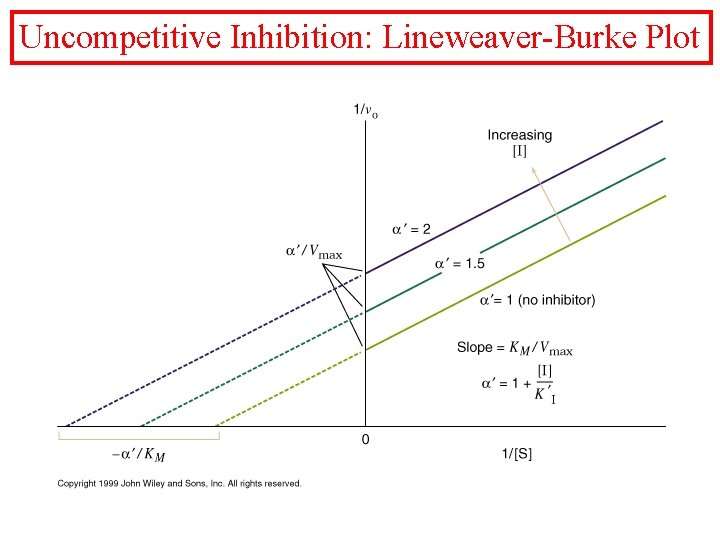
Uncompetitive Inhibition

Uncompetitive Inhibition: Lineweaver-Burke Plot

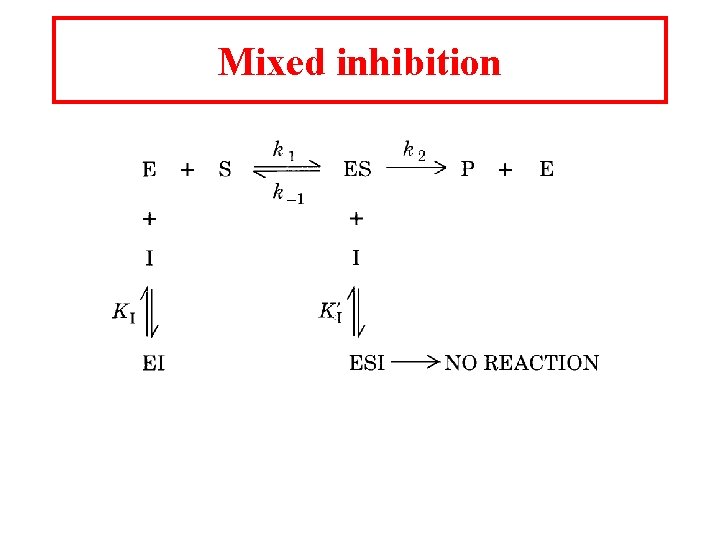
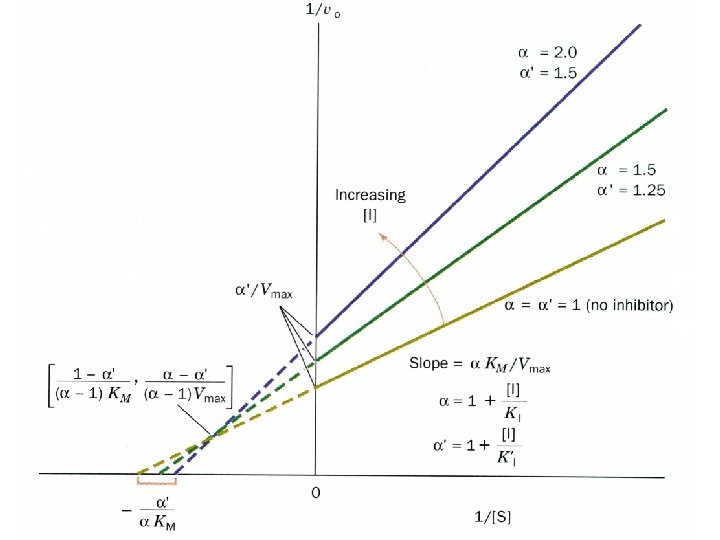
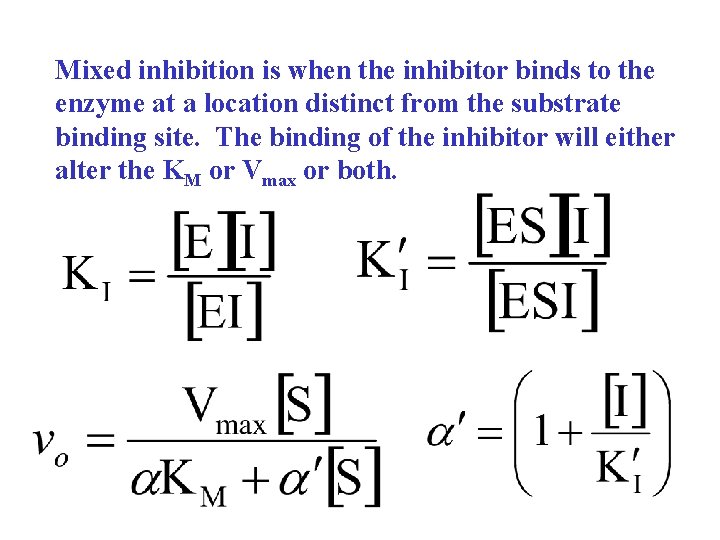
Mixed inhibition


Mixed inhibition is when the inhibitor binds to the enzyme at a location distinct from the substrate binding site. The binding of the inhibitor will either alter the KM or Vmax or both.


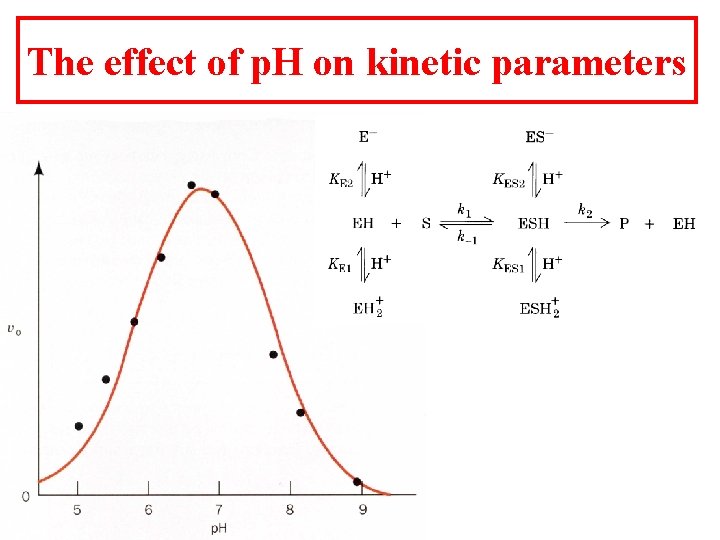
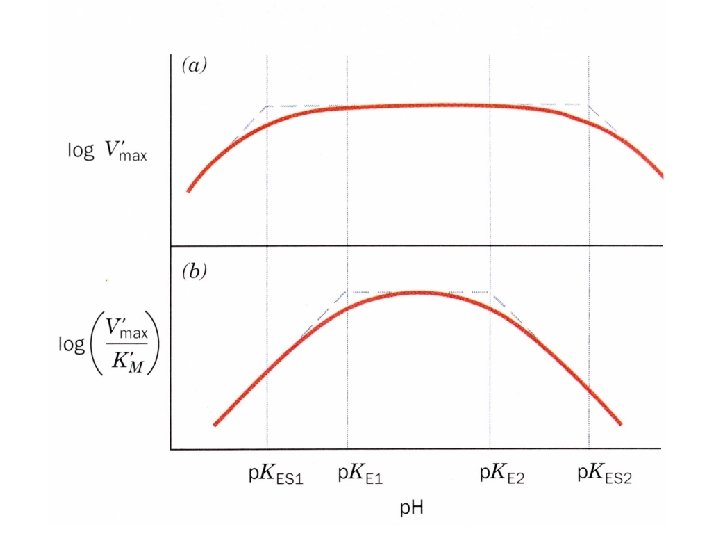
The effect of p. H on kinetic parameters


 Mixed inhibitor km and vmax
Mixed inhibitor km and vmax Competitive inhibition
Competitive inhibition Enzyme kinetics
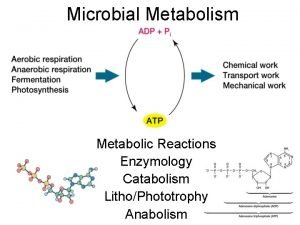
Enzyme kinetics Energy catalysis and biosynthesis
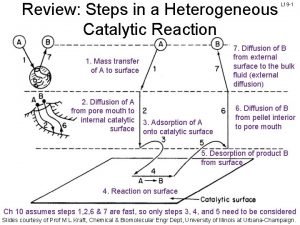
Energy catalysis and biosynthesis 7 steps of heterogeneous catalysis
7 steps of heterogeneous catalysis Apoenzyme
Apoenzyme Catalysis by approximation
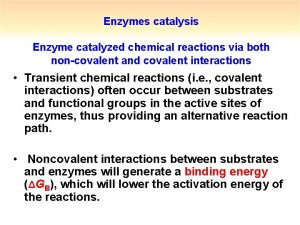
Catalysis by approximation What is covalent catalysis
What is covalent catalysis What is covalent catalysis
What is covalent catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Specific acid base catalysis
Specific acid base catalysis Langmuir-hinshelwood mechanism heterogeneous catalysis
Langmuir-hinshelwood mechanism heterogeneous catalysis Erzeng xue
Erzeng xue Site:slidetodoc.com
Site:slidetodoc.com Hydroxide catalysis bonding
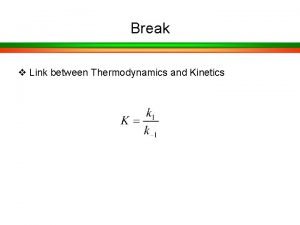
Hydroxide catalysis bonding Kinetics and equilibrium
Kinetics and equilibrium Antagonistic effect
Antagonistic effect Difference between zero and first order kinetics
Difference between zero and first order kinetics Planar kinetics of a rigid body: force and acceleration
Planar kinetics of a rigid body: force and acceleration Kinetics of a particle force and acceleration
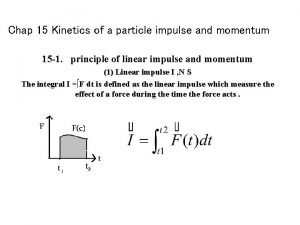
Kinetics of a particle force and acceleration Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Abbas et al
Abbas et al Kinematics of rigid bodies problems and solutions
Kinematics of rigid bodies problems and solutions Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy Cell kinetics and fermenter design
Cell kinetics and fermenter design Enzymes affect reactions in living cells by changing the
Enzymes affect reactions in living cells by changing the Define enzyme
Define enzyme Activators for enzymes
Activators for enzymes Molecularity of reaction
Molecularity of reaction First order drug elimination
First order drug elimination Plane movement
Plane movement Collision theory of kinetics
Collision theory of kinetics Chemistry grade 11 unit 4
Chemistry grade 11 unit 4 Kinetics flotation chemicals
Kinetics flotation chemicals Order of kinetics
Order of kinetics Chemical kinetics definition
Chemical kinetics definition