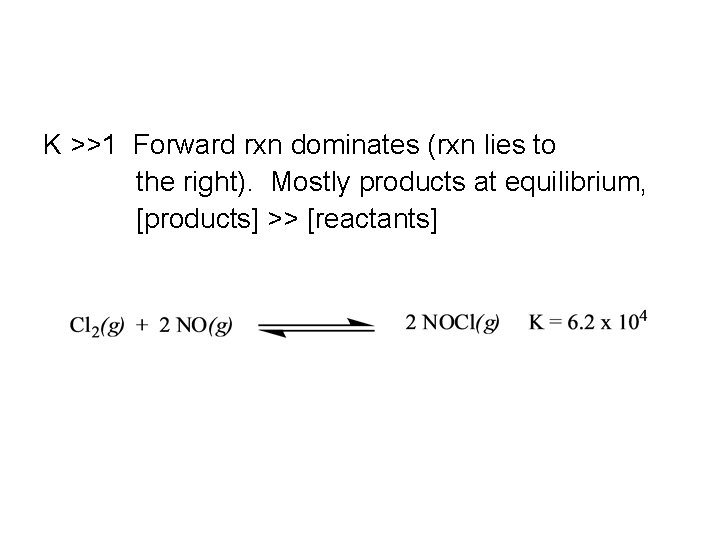
K 1 Forward rxn dominates rxn lies to
![K 1 Forward and reverse rxn occur to roughly the same extent, [products] [reactants] K 1 Forward and reverse rxn occur to roughly the same extent, [products] [reactants]](https://slidetodoc.com/presentation_image_h2/6c5d0d7bb4d8ea94a26a7c5be46c7a49/image-7.jpg)



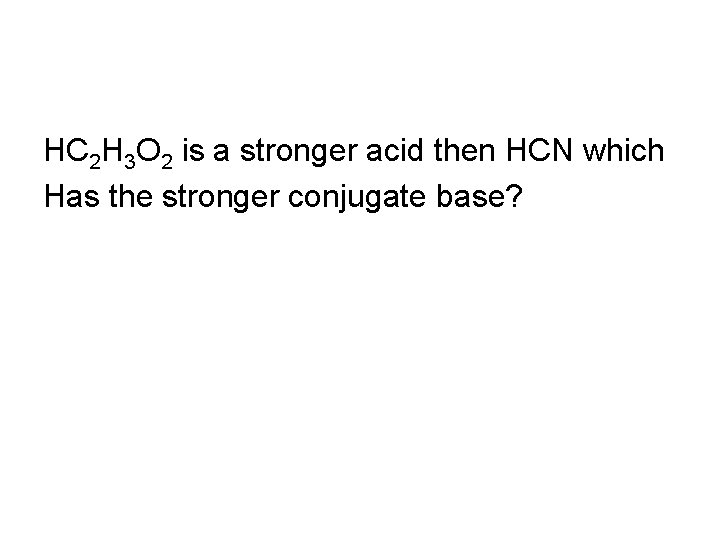



















- Slides: 46





K >>1 Forward rxn dominates (rxn lies to the right). Mostly products at equilibrium, [products] >> [reactants]

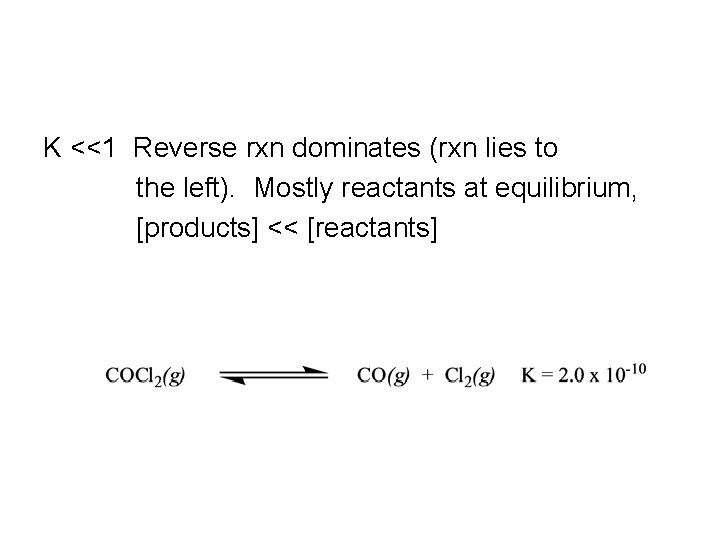
K <<1 Reverse rxn dominates (rxn lies to the left). Mostly reactants at equilibrium, [products] << [reactants]
![K 1 Forward and reverse rxn occur to roughly the same extent products reactants K 1 Forward and reverse rxn occur to roughly the same extent, [products] [reactants]](https://slidetodoc.com/presentation_image_h2/6c5d0d7bb4d8ea94a26a7c5be46c7a49/image-7.jpg)
K 1 Forward and reverse rxn occur to roughly the same extent, [products] [reactants]

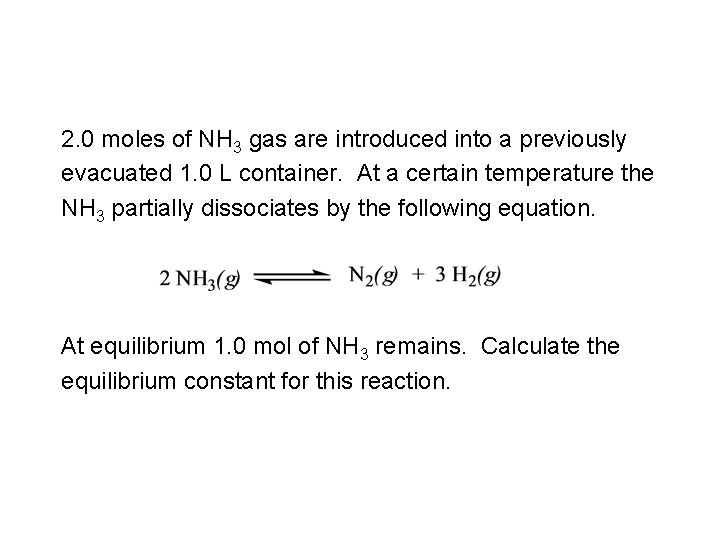
2. 0 moles of NH 3 gas are introduced into a previously evacuated 1. 0 L container. At a certain temperature the NH 3 partially dissociates by the following equation. At equilibrium 1. 0 mol of NH 3 remains. Calculate the equilibrium constant for this reaction.

Reaction Quotient (Q) Q = K: The rxn is at equilibrium. No shift. Q < K: The rxn shifts right to produce products to increase Q. Q > K: The rxn shifts left to produce reactants to decrease Q.

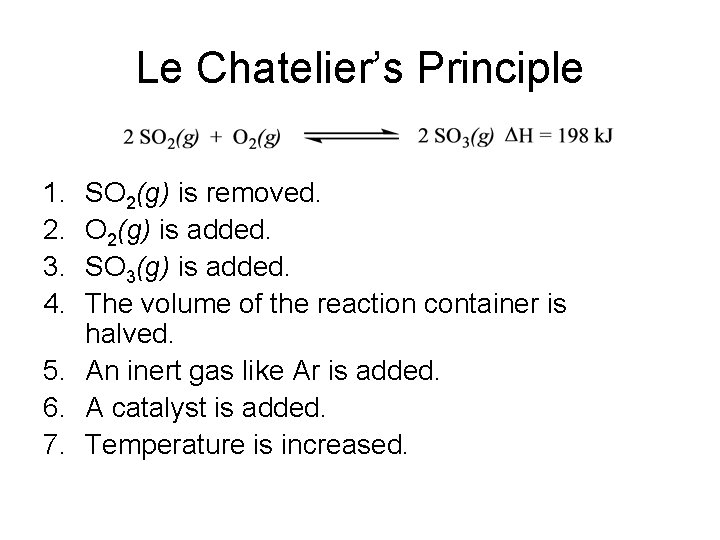
Le Chatelier’s Principle If a change (stress) is imposed on a system at equilibrium, the position of the equilibrium will shift in a direction that tends to reduce that change (stress).

Le Chatelier’s Principle 1. 2. 3. 4. SO 2(g) is removed. O 2(g) is added. SO 3(g) is added. The volume of the reaction container is halved. 5. An inert gas like Ar is added. 6. A catalyst is added. 7. Temperature is increased.

Le Chatelier’s Principle 1. 2. 3. 4. CO 2(g) is added. Ca. CO 3(s) is added. The volume is increased. The temperature is decreased.

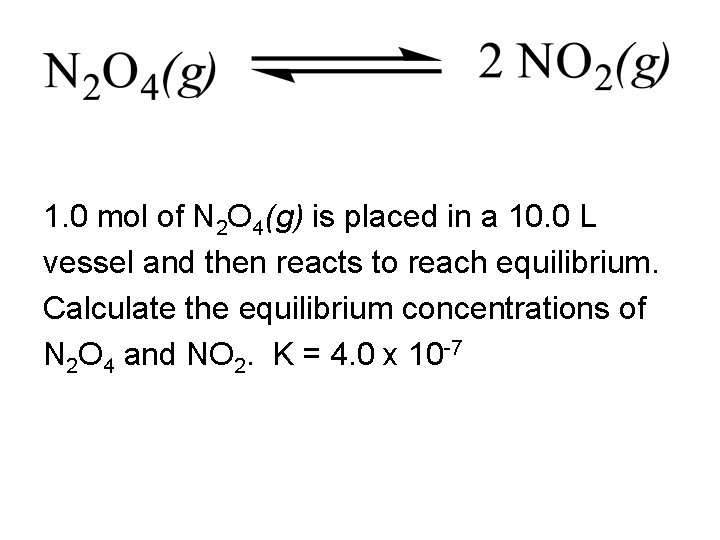
1. 0 mol of N 2 O 4(g) is placed in a 10. 0 L vessel and then reacts to reach equilibrium. Calculate the equilibrium concentrations of N 2 O 4 and NO 2. K = 4. 0 x 10 -7

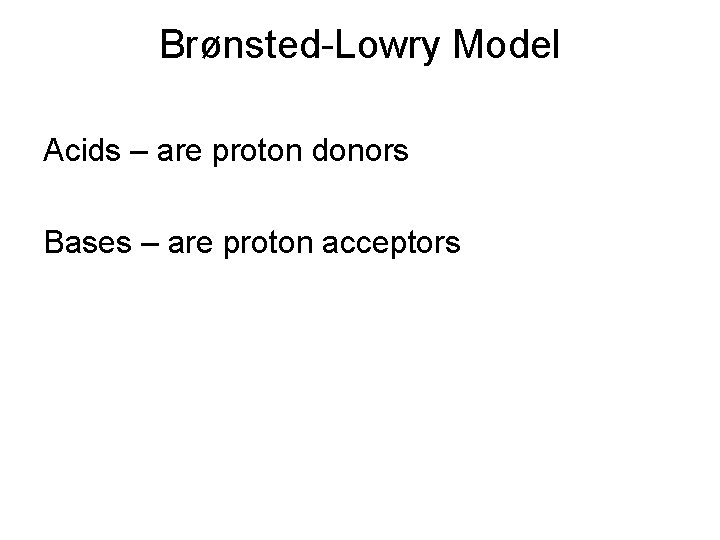
Brønsted-Lowry Model Acids – are proton donors Bases – are proton acceptors
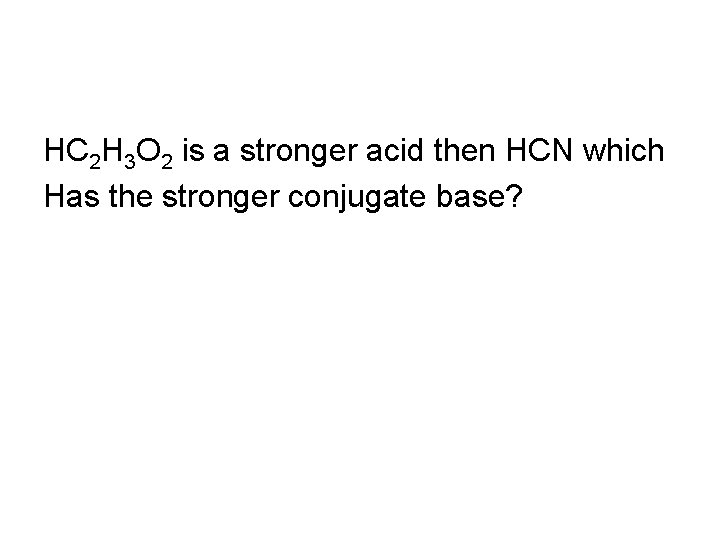
HC 2 H 3 O 2 is a stronger acid then HCN which Has the stronger conjugate base?

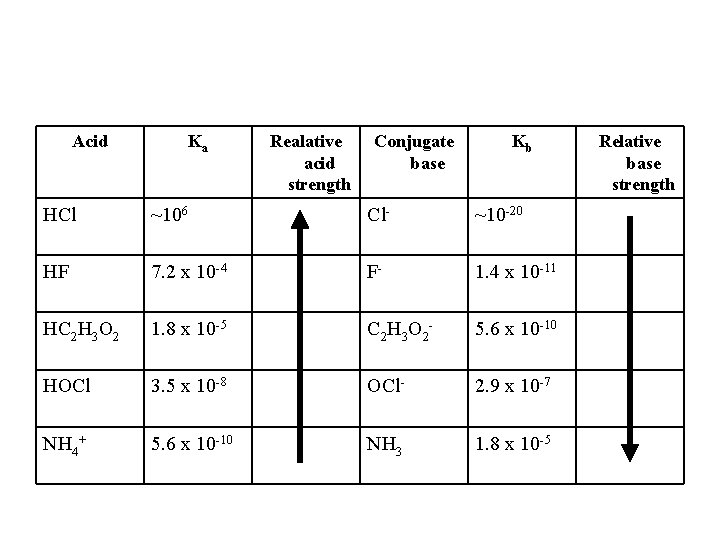
Comments on the Conjugates of Acids and Bases. • The weaker the acid the stronger its conjugate base. • The weaker the base the stronger its conjugate acid. • The conjugate base of a weak acid is a WEAK base. • The conjugate base of a strong acid is worthless. • The conjugate acid of a weak base is a WEAK acid.

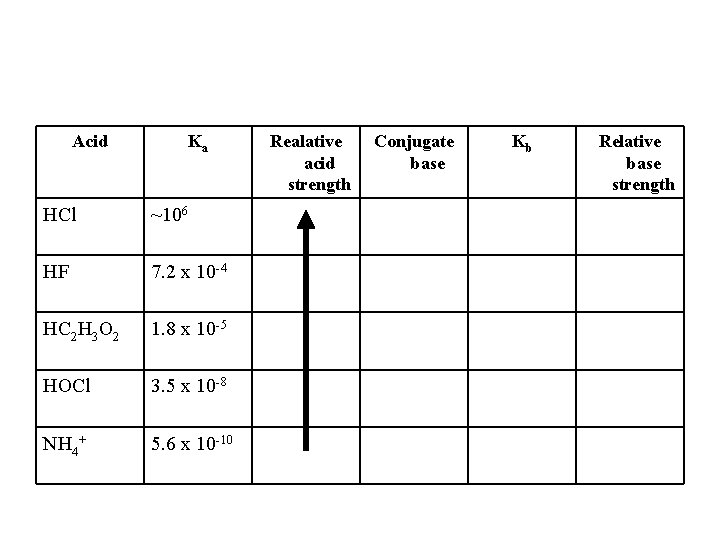
Acid Ka HCl ~106 HF 7. 2 x 10 -4 HC 2 H 3 O 2 1. 8 x 10 -5 HOCl 3. 5 x 10 -8 NH 4+ 5. 6 x 10 -10 Realative acid strength Conjugate base Kb Relative base strength

Acid Ka Realative acid strength Conjugate base Kb HCl ~106 Cl- ~10 -20 HF 7. 2 x 10 -4 F- 1. 4 x 10 -11 HC 2 H 3 O 2 1. 8 x 10 -5 C 2 H 3 O 2 - 5. 6 x 10 -10 HOCl 3. 5 x 10 -8 OCl- 2. 9 x 10 -7 NH 4+ 5. 6 x 10 -10 NH 3 1. 8 x 10 -5 Relative base strength

Stuff you should now know. 1. 2. 3. 4. 5. 6. 7. 8. Ka value is directly related to acid strength. Weak acids vs. strong acids (Ka’s and % dissociation. Conjugate acid-base pairs. Ka. Kb=Kw Kb value is directly related to base strength. How to write out Ka and Kb rxns and expressions. The weaker the acid the stronger the conjugate base (and vice versa). Conjugate bases of strong acids have no basic properties whatsoever! (Kb << Kw)

Calculate the p. H of a 0. 10 M HBr solution.

Calculate the p. H of a 0. 10 M HOCl solution. Ka. HOCl = 3. 5 x 10 -8

Calculate the p. H of a 0. 10 M Na. F. Ka. HF = 7. 2 x 10 -4

Calculate the p. H of a 0. 10 M Ca(OH)2 solution.

Calculate the p. H of a solution containing 0. 10 M HOCl and 0. 02 M Na. OCl. Ka. HOCl = 3. 5 x 10 -8

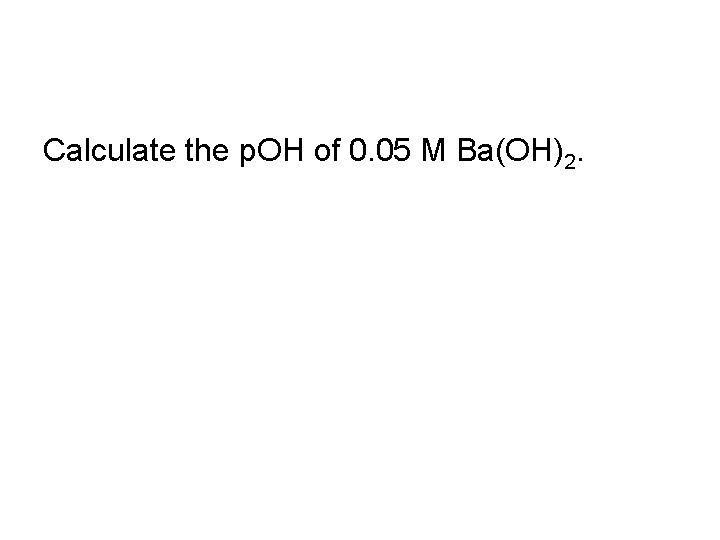
Calculate the p. OH of 0. 05 M Ba(OH)2.

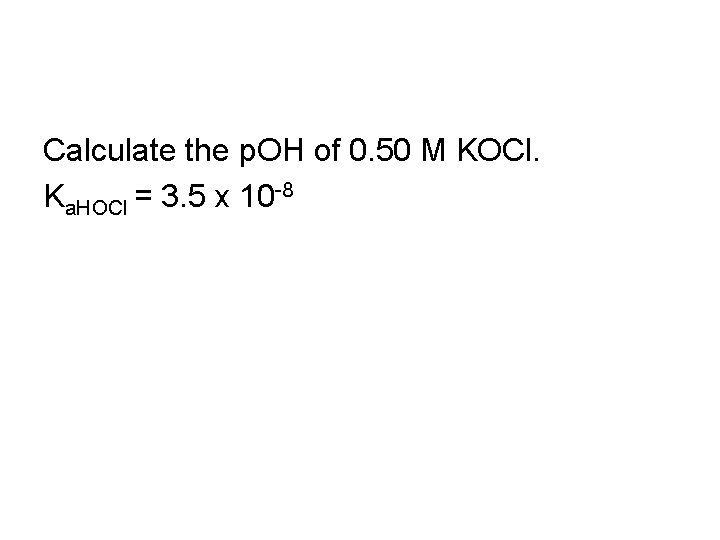
Calculate the p. OH of 0. 50 M KOCl. Ka. HOCl = 3. 5 x 10 -8

Calculate the p. OH of 1. 00 M HI.

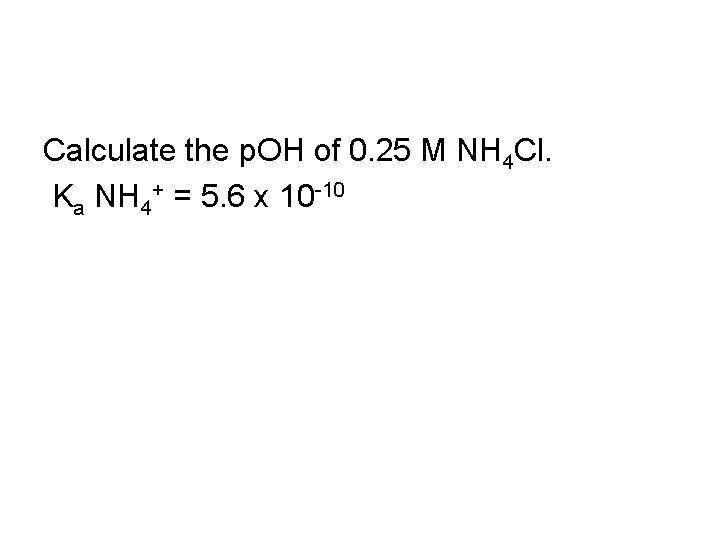
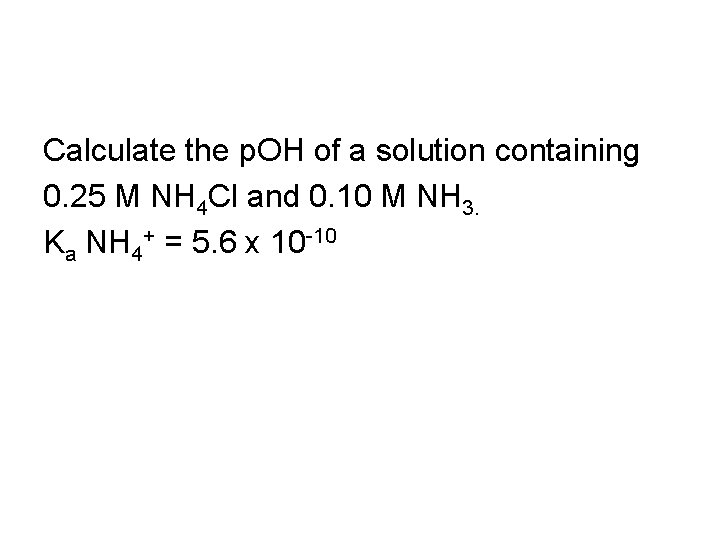
Calculate the p. OH of 0. 25 M NH 4 Cl. Ka NH 4+ = 5. 6 x 10 -10

Calculate the p. OH of a solution containing 0. 25 M NH 4 Cl and 0. 10 M NH 3. Ka NH 4+ = 5. 6 x 10 -10

Calculate the p. H of 1. 6 x 10 -13 M HNO 3.

A solution of 8. 00 M HCOOH is 0. 47% Ionized. What is the Ka for the acid? p. H?

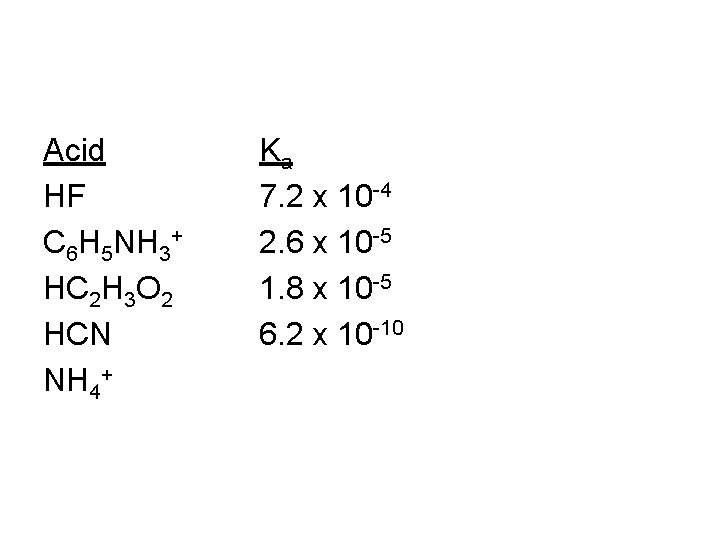
Acid HF C 6 H 5 NH 3+ HC 2 H 3 O 2 HCN NH 4+ Ka 7. 2 x 10 -4 2. 6 x 10 -5 1. 8 x 10 -5 6. 2 x 10 -10

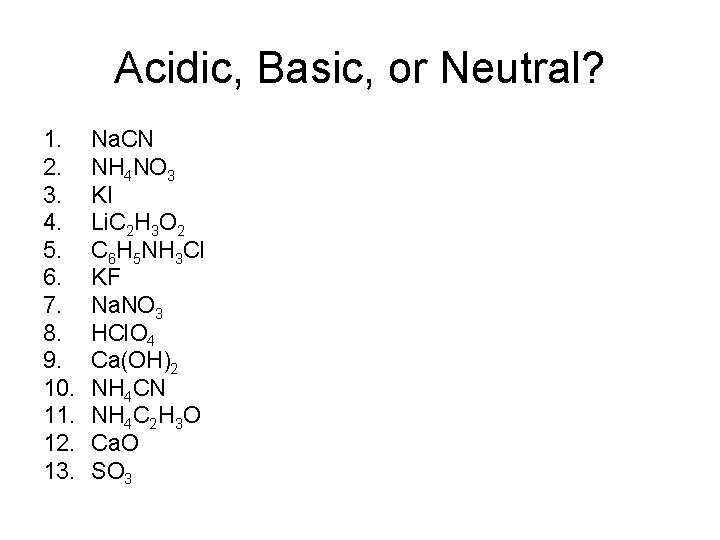
Acidic, Basic, or Neutral? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Na. CN NH 4 NO 3 KI Li. C 2 H 3 O 2 C 6 H 5 NH 3 Cl KF Na. NO 3 HCl. O 4 Ca(OH)2 NH 4 CN NH 4 C 2 H 3 O Ca. O SO 3

Acidic, Basic, or Neutral? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Na. CN NH 4 NO 3 KI Li. C 2 H 3 O 2 C 6 H 5 NH 3 Cl KF Na. NO 3 HCl. O 4 Ca(OH)2 NH 4 CN NH 4 C 2 H 3 O Ca. O SO 3 Na+ - worthless, CN- - weak base, basic NO 3 - - worthless, NH 4+ - weak acid, acidic K+ - worthless, I- - worthless, neutral Li+ - worthless, C 2 H 3 O 2 - - weak base, basic Cl- - worthless, C 6 H 5 NH 3+ - weak acid, acidic K+ - worthless, F- - weak base, basic Na+ - worthless, NO 3 - - worthless, neutral HCl. O 4 – strong acid, acidic Ca(OH)2 – strong base, basic Ka. NH < Kb. CN - basic Ka. NH = Kb. C H O - neutral metal oxide - basic nonmetal oxide - acidic - 4 4 2 3 -

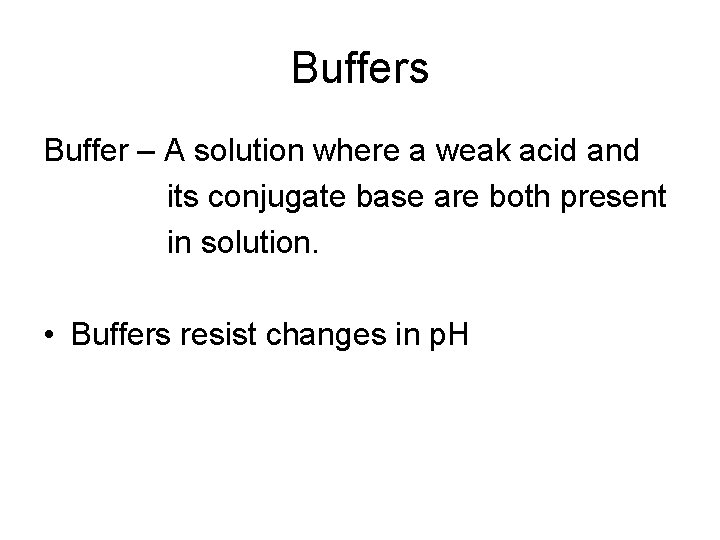
Buffers Buffer – A solution where a weak acid and its conjugate base are both present in solution. • Buffers resist changes in p. H

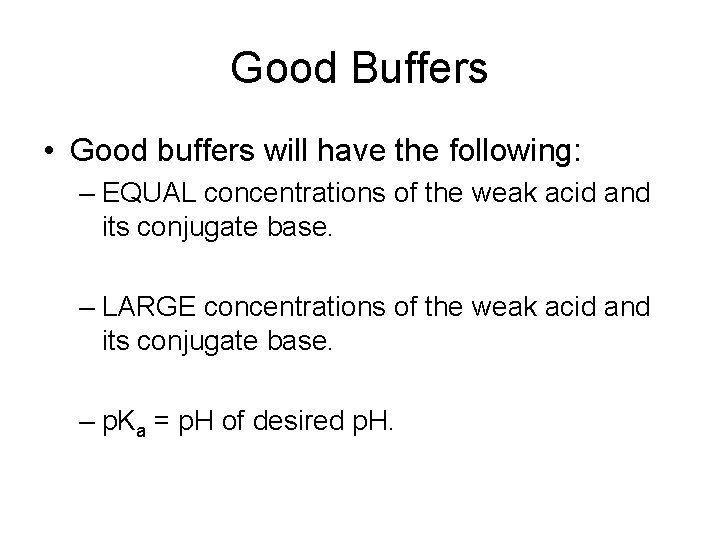
Good Buffers • Good buffers will have the following: – EQUAL concentrations of the weak acid and its conjugate base. – LARGE concentrations of the weak acid and its conjugate base. – p. Ka = p. H of desired p. H.

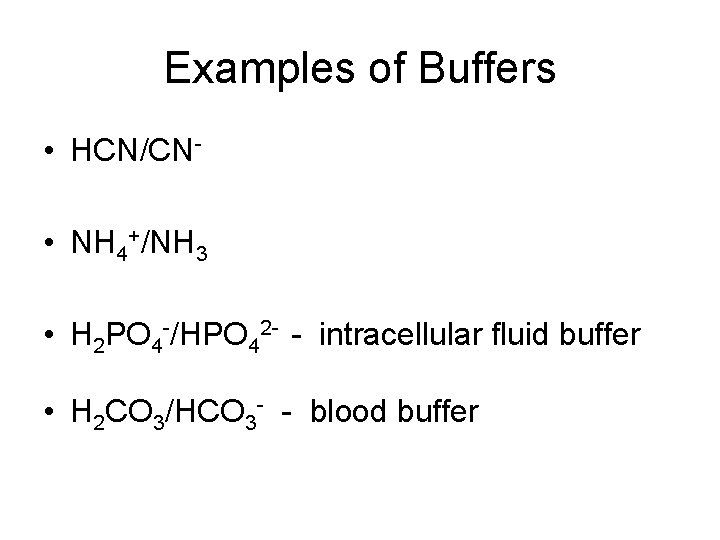
Examples of Buffers • HCN/CN • NH 4+/NH 3 • H 2 PO 4 -/HPO 42 - - intracellular fluid buffer • H 2 CO 3/HCO 3 - - blood buffer

Calculate the p. H of a solution that is 1. 00 M HNO 2 and 1. 00 M Na. NO 2. Ka. HNO = 4. 0 x 10 -4 2

Calculate the p. H when 0. 10 mol of HCl is Added to a 1. 00 L solution containing 1. 00 M HNO 2 and 1. 00 M Na. NO 2. Ka. HNO = 4. 0 x 10 -4 2

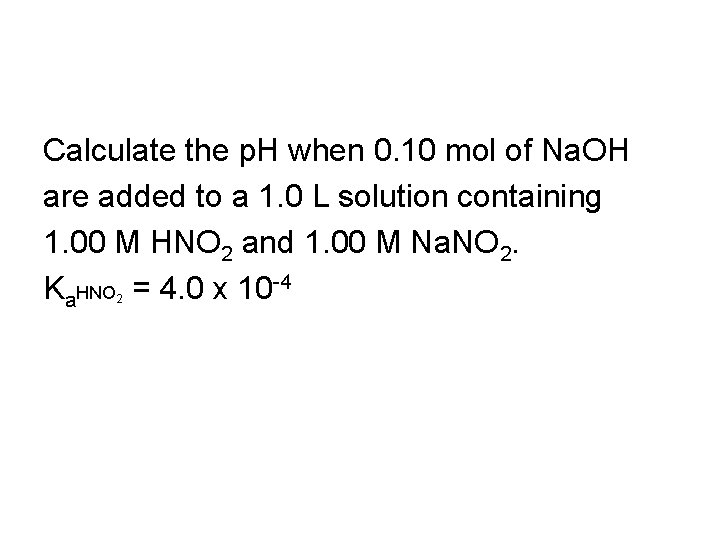
Calculate the p. H when 0. 10 mol of Na. OH are added to a 1. 0 L solution containing 1. 00 M HNO 2 and 1. 00 M Na. NO 2. Ka. HNO = 4. 0 x 10 -4 2

Calculate the p. H of a solution formed by Mixing 500. 0 m. L of 0. 100 M NH 3 and 500. 0 m. L of 0. 0500 M HCl. Kb. NH = 1. 8 x 10 -5 3

You want to prepare a HOCl buffer of p. H 8. 00. You want to make a 500. m. L solution and use all of the 0. 75 mol of HOCl you have on hand. How many mol of KOCl must you add? Ka. HOCl = 3. 5 x 10 -8

Calculate the p. H of a solution formed by mixing 500. m. L of 1. 50 M HCN with 250. m. L of 1. 00 M Na. OH. Ka. HCN = 6. 2 x 10 -10

• Total Points in course: 800 • Points to be decided next week: ~415

Proposed Study Plan • • Thursday: HE III Material (finish Lon Capa) Friday: HE I Material Saturday: HE II Material Sunday: HE III Material Monday: HE III Material Tuesday: He III Material Wednesday: HE I, II Material Thursday: HE I, III Material

A 100. m. L solution of 0. 10 M HF is titrated by 0. 10 M Na. OH. Calculate the p. H when 0. 0, 25. 0, 50. 0, 100. 0, and 125. 0 m. L of Na. OH have been added. Ka. HF = 7. 4 x 10 -4