Edgenuity unit 4 notes Evidence of Chemical Reactions



















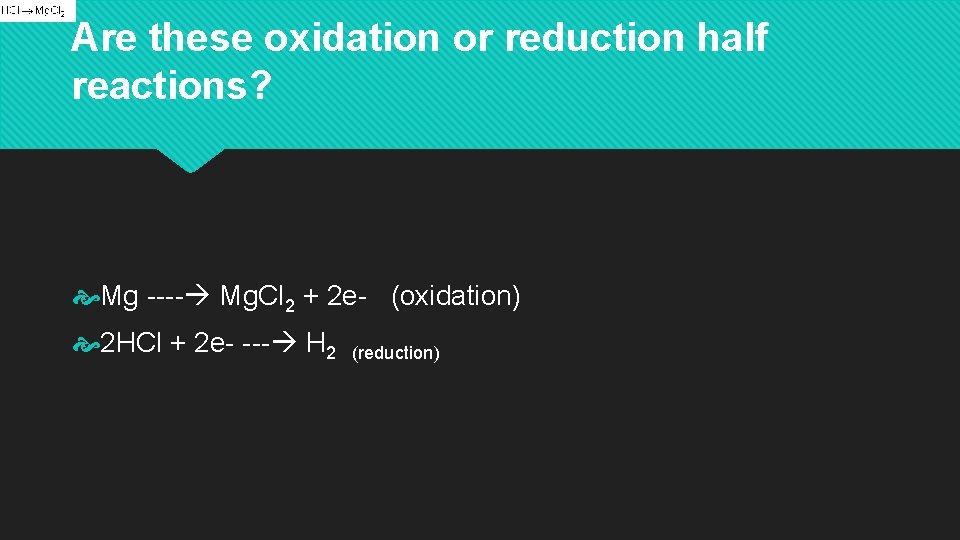



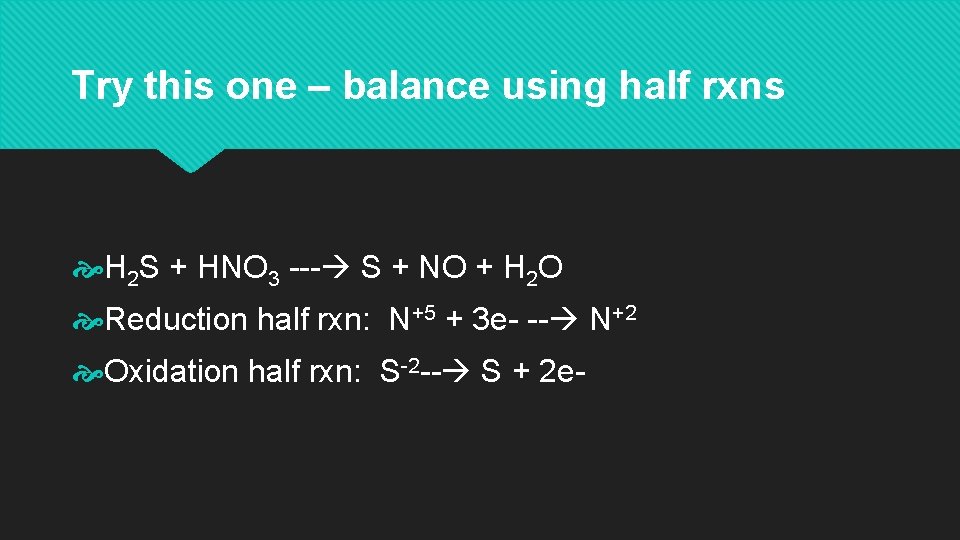










- Slides: 50

Edgenuity unit 4 notes

Evidence of Chemical Reactions Change in identity of a substance (cooking pancakes) Every chemical change is a chemical reaction Chemical reactions A rearrangement of the atoms in the reactants (what you start with in a reaction) Results in a new substance

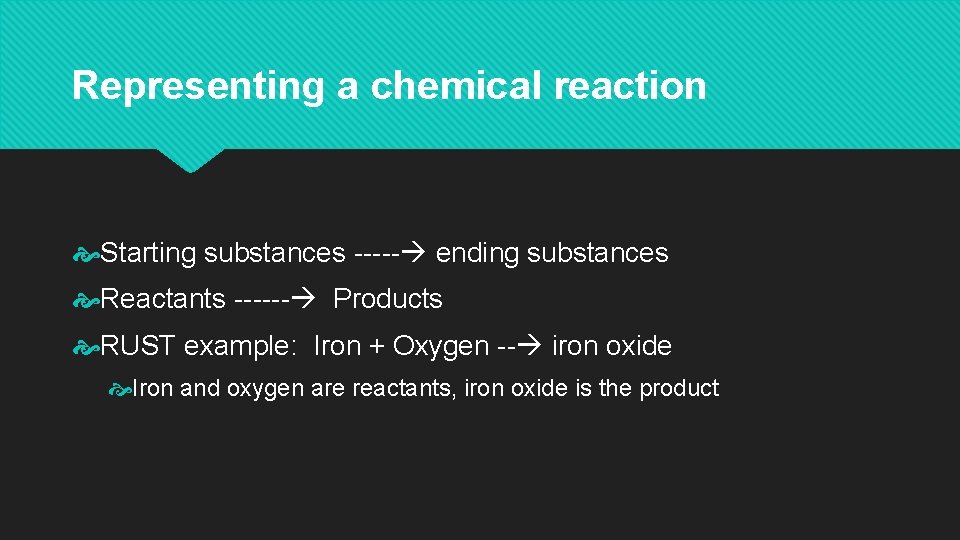
Representing a chemical reaction Starting substances ----- ending substances Reactants ------ Products RUST example: Iron + Oxygen -- iron oxide Iron and oxygen are reactants, iron oxide is the product

Methane + oxygen --- carbon dioxide + water What are the reactants? Products?

Rate of a chemical reaction Rates of reactions can vary Fireworks – fast chemical reaction Some reactions are SLOW – acid rain affecting a limestone statue

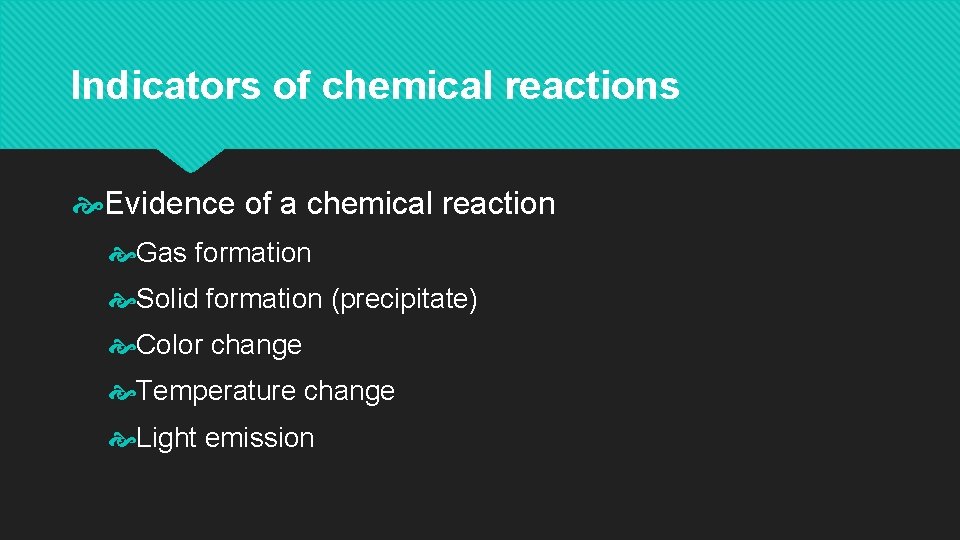
Indicators of chemical reactions Evidence of a chemical reaction Gas formation Solid formation (precipitate) Color change Temperature change Light emission

Gas or solid formation Mixing baking soda and vinegar – bubbles or a gas is formed Solid is formed from two liquids – a precipitate Color changes Energy change Endothermic reactions – absorb energy from the surroundings (temp is decreased) Exothermic reactions – release energy into the surroundings (temp is increased, light is emitted)

Indicators of a chemical reaction are not always absolute proof of a chemical reaction Some physical changes are accompanied by gas (mentos and diet coke) or solid formation (water freezing), or temperature change (water boiling), or color change (food coloring) Generally, more indicators mean it’s more likely that a reaction has taken place

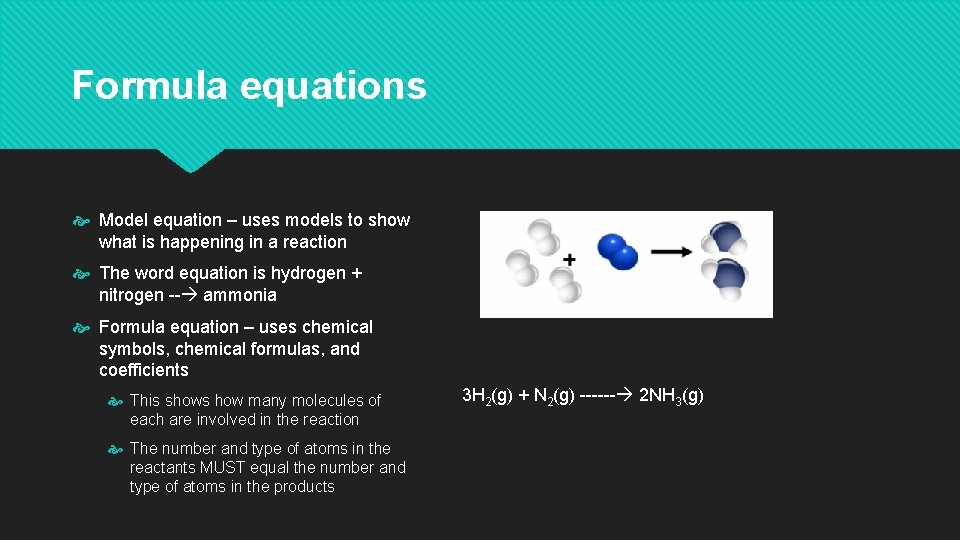
Word equations Use words to describe the reaction Reactants on the left, then the arrow, then products on the right The arrow represents the word yield or product Chemical equation – a group of chemical symbols and formulas that represent the reactants and products in a chemical reaction Hydrogen + nitrogen --- ammonia (this is a word equation)

Formula equations Model equation – uses models to show what is happening in a reaction The word equation is hydrogen + nitrogen -- ammonia Formula equation – uses chemical symbols, chemical formulas, and coefficients This shows how many molecules of each are involved in the reaction The number and type of atoms in the reactants MUST equal the number and type of atoms in the products 3 H 2(g) + N 2(g) ------ 2 NH 3(g)

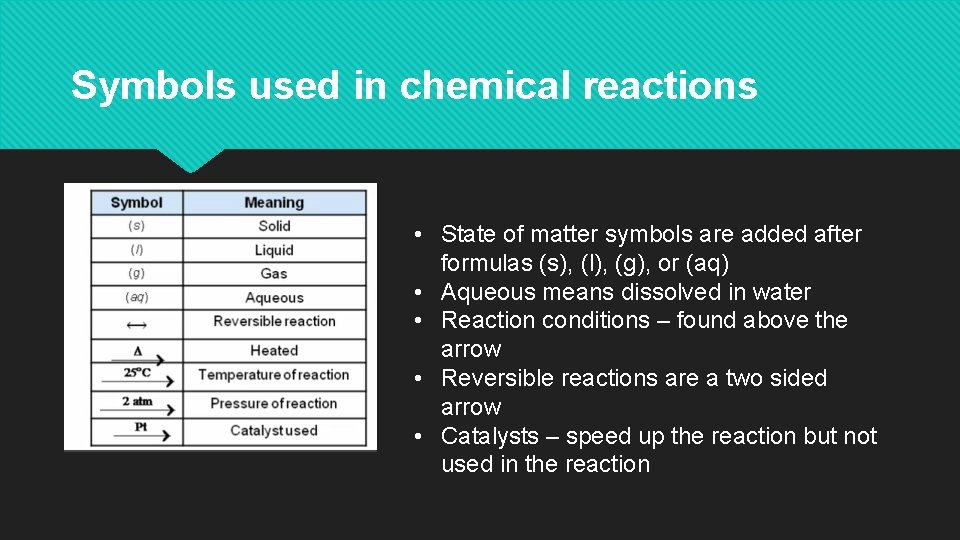
Symbols used in chemical reactions • State of matter symbols are added after formulas (s), (l), (g), or (aq) • Aqueous means dissolved in water • Reaction conditions – found above the arrow • Reversible reactions are a two sided arrow • Catalysts – speed up the reaction but not used in the reaction

Writing and balancing Chemical Equations Law of conservation of mass: Matter cannot be created or destroyed. The number and type of atoms in the products has to equal the number and type of atoms in the reactants. Coefficient – number placed in front of a chemical formula in an equation

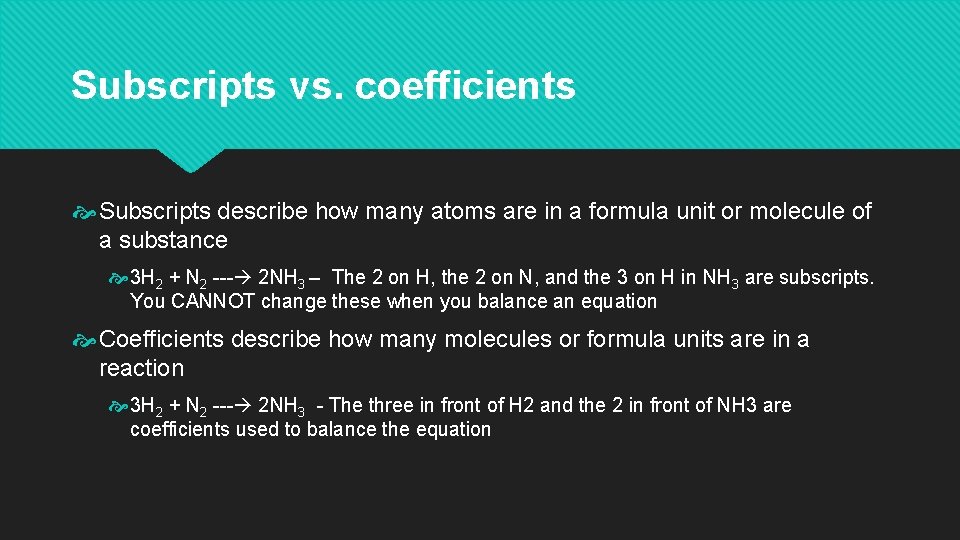
Subscripts vs. coefficients Subscripts describe how many atoms are in a formula unit or molecule of a substance 3 H 2 + N 2 --- 2 NH 3 – The 2 on H, the 2 on N, and the 3 on H in NH 3 are subscripts. You CANNOT change these when you balance an equation Coefficients describe how many molecules or formula units are in a reaction 3 H 2 + N 2 --- 2 NH 3 - The three in front of H 2 and the 2 in front of NH 3 are coefficients used to balance the equation

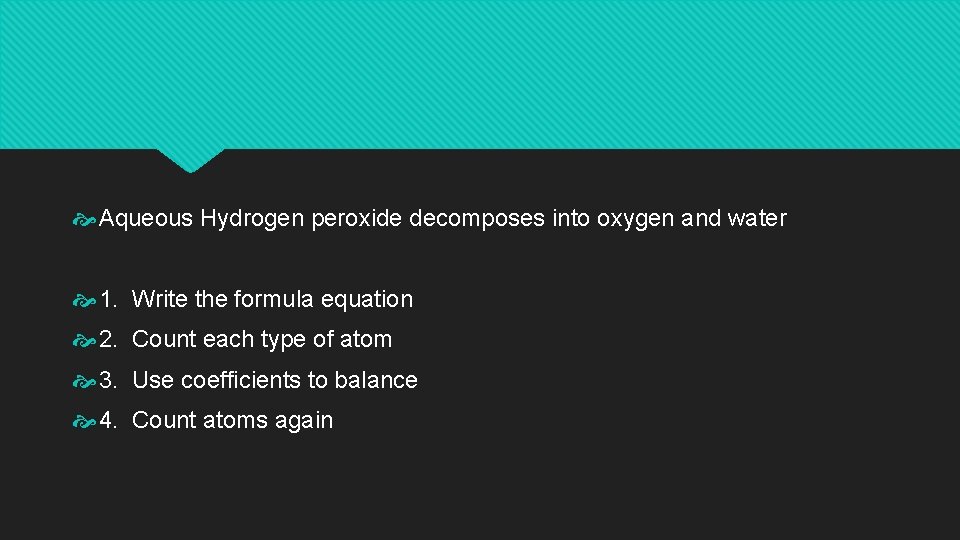
Aqueous Hydrogen peroxide decomposes into oxygen and water 1. Write the formula equation 2. Count each type of atom 3. Use coefficients to balance 4. Count atoms again

Examples: H 2 + O 2 --- H 2 O Reactants: 2 H atoms, 2 O atoms Products: 2 H atoms, 1 O atom Add coefficients to balance it. You must multiply the number of each atom by the coefficient. __H 2 + __O 2 ---- __H 2 O

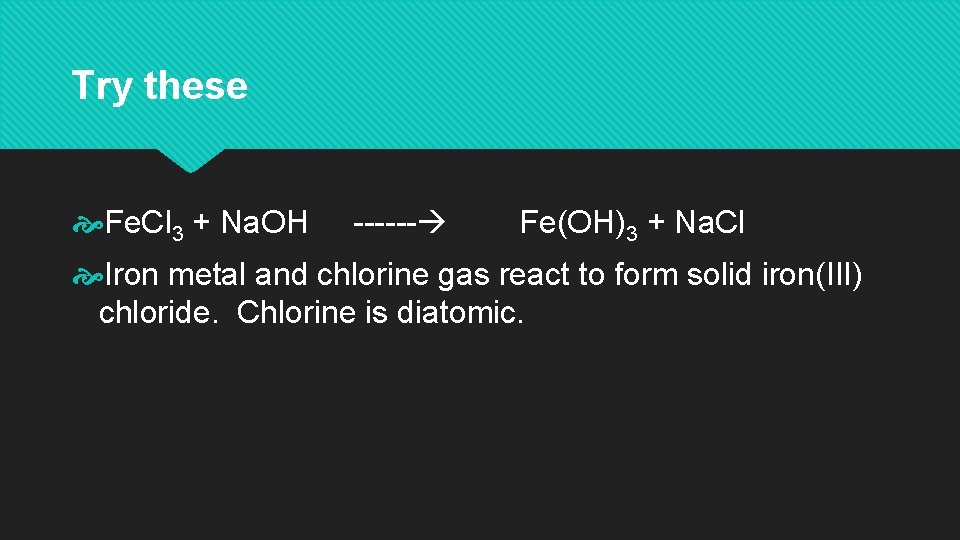
Try these Fe. Cl 3 + Na. OH ------ Fe(OH)3 + Na. Cl Iron metal and chlorine gas react to form solid iron(III) chloride. Chlorine is diatomic.

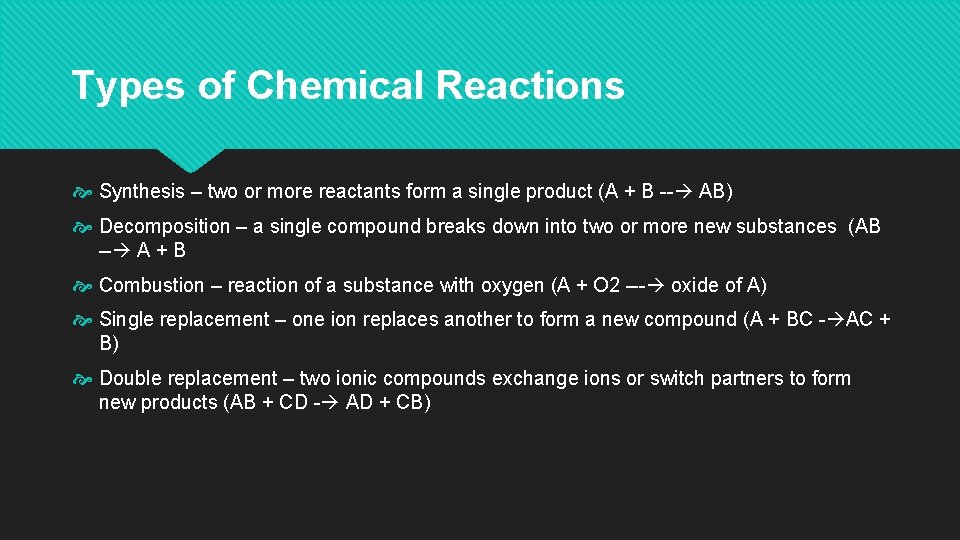
Types of Chemical Reactions Synthesis – two or more reactants form a single product (A + B -- AB) Decomposition – a single compound breaks down into two or more new substances (AB -- A + B Combustion – reaction of a substance with oxygen (A + O 2 --- oxide of A) Single replacement – one ion replaces another to form a new compound (A + BC - AC + B) Double replacement – two ionic compounds exchange ions or switch partners to form new products (AB + CD - AD + CB)

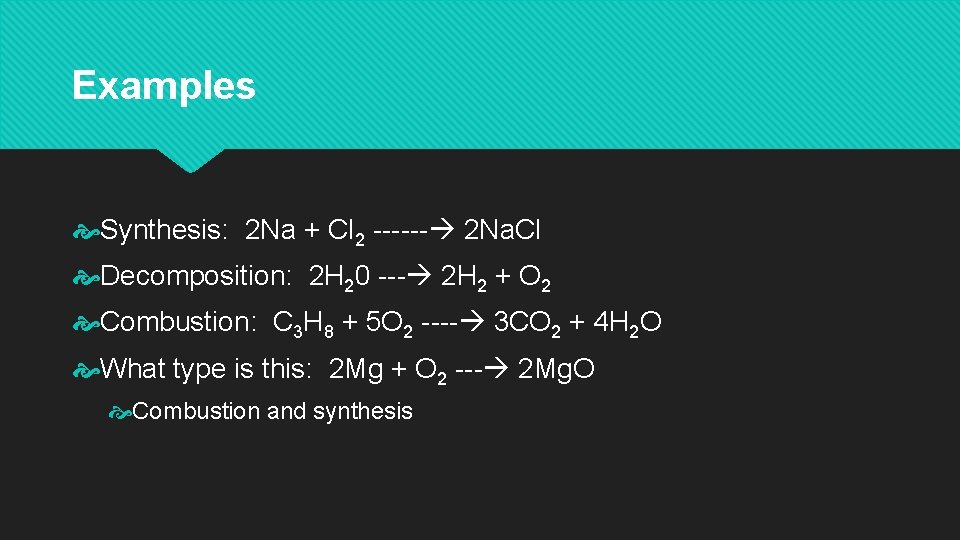
Examples Synthesis: 2 Na + Cl 2 ------ 2 Na. Cl Decomposition: 2 H 20 --- 2 H 2 + O 2 Combustion: C 3 H 8 + 5 O 2 ---- 3 CO 2 + 4 H 2 O What type is this: 2 Mg + O 2 --- 2 Mg. O Combustion and synthesis

More examples Single replacement: Fe 2 O 3 + 2 Al ---- Al 2 O 3 + 2 Fe. The aluminum replaced the iron. You’re left with a compound an element. Single replacement: Mg + 2 H 2 O ---- Mg(OH)2 + H 2. The magnesium replaced the hydrogen. Left with an element and a compound Double replacement: Pb(NO 3)2 + 2 KI -- Pb. I 2 + 2 KNO 3. The ions switched partners. Reactants and products are ionic compounds

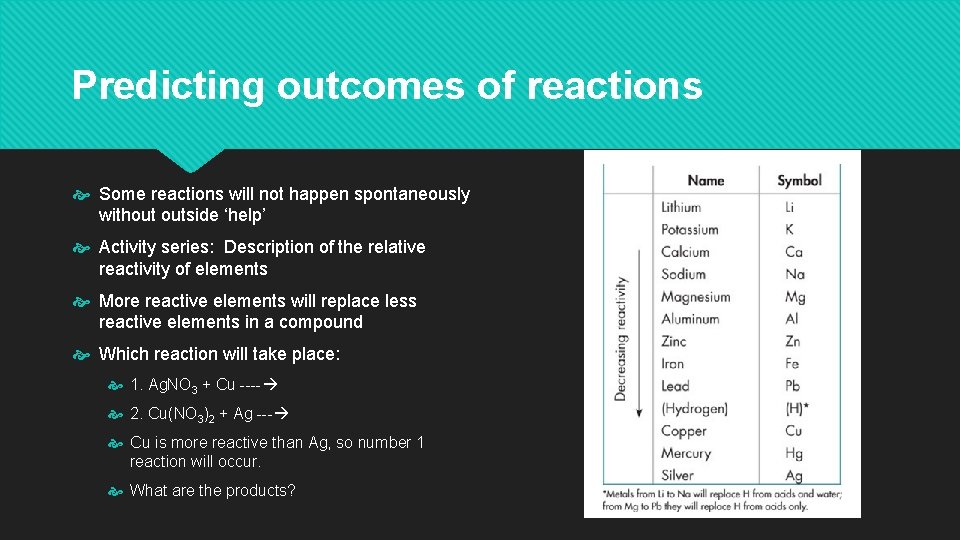
Predicting outcomes of reactions Some reactions will not happen spontaneously without outside ‘help’ Activity series: Description of the relative reactivity of elements More reactive elements will replace less reactive elements in a compound Which reaction will take place: 1. Ag. NO 3 + Cu ---- 2. Cu(NO 3)2 + Ag --- Cu is more reactive than Ag, so number 1 reaction will occur. What are the products?

Oxidation numbers Indicates how many electrons an atom has gained, lost, or shared to form a chemical bond Is the same as the charge of an ion – remember you can predict the charges based on the number of valence electrons. If it has 1, 2, or 3 valence, it will lose those to get to 8 valence electrons and form cations (+ charge). If it has 5, 6, 7 valence electrons, it will gain electrons to get to 8 valence electrons and form anions (- charge). The sum of oxidation numbers must be zero in neutral compounds (just like balancing charges when forming ionic compounds)

Rules for assigning oxidation numbers Pure element is zero. Ex: Na, H 2 Monatomic ion is the ion’s charge. Fluorine is -1. Oxygen is -2, except in peroxides (then it’s -1) Hydrogen is +1, except in hydrides (then it’s -1) Elements in groups 1 and 2 are +1 and +2. Ex: Na is +1, Ca is +2

The sum of oxidation numbers equals the total charge on the compound Example: 2 SO 2 + O 2 -- 2 SO 3 You can determine the charges and the electrons involved in this redox reaction – see whiteboard or slide 2 of the instruction in U 4 L 5

Practice assigning oxidation numbers What are the oxidation numbers for the atoms in these reactions? Mg(s) + 2 HCl(aq) → Mg. Cl 2(aq) + H 2(g) CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g)

Oxidation and Reduction Oxidation – loss of electrons, oxidation number increases Reduction – gain of electrons, oxidation number decreases LEO says GER. Loss Electrons Oxidation, Gain Electrons Reduction OIL RIG – oxidation is Loss, Reduction is Gain

Practice Identify the following as oxidation or reduction Cu 2+ + e− → Cu+ Fe → Fe 3+ + 3 e− F + e − → F− 2 l− → l 2 + 2 e− 2 H+ + 2 e− → H 2

Oxidation Reduction reactions Reactions in which electrons are transferred from one substance to another substance. Called redox reactions Reducing agent – the substance that is oxidized, or losing electrons Oxidizing agent – the substance that is reduced, or gaining electrons 4 Fe + 3 O 2 -- 2 Fe 2 O 3 Iron is oxidized and is the reducing agent Oxygen is reduced and is the oxidizing agent.

Practice 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) Identify the atoms that are oxidized and reduced. Identify the oxidizing agent and the reducing agent

Identifying Redox Reactions If a reaction involves the exchange of electrons, it is a redox reaction 3 NO --- N 2 O + NO 2 Some reactions don’t involve the exchange of electrons – these are not redox reactions. Mg(OH)2 + HCl ----- Mg. Cl 2 + H 2 O Determine oxidation numbers of each element. If they are the same in the reactants and products, it is NOT a redox reaction

Practice – are these redox rxns? Fe. S(s) + 2 HCl(aq) → H 2 S(g) + Fe. Cl 2(g) Ag. NO 3(aq) + Na. Cl(aq) → Ag. Cl(s) + Na. NO 3 2 C 3 H 6(g) + 9 O 2(g) → 6 CO 2(g) + 6 H 2 O(g)

Examples of oxidation reduction reactions Combustion Photosynthesis Many reactions of metals (rust, silver tarnish) Many biochemical reactions including metabolic reactions

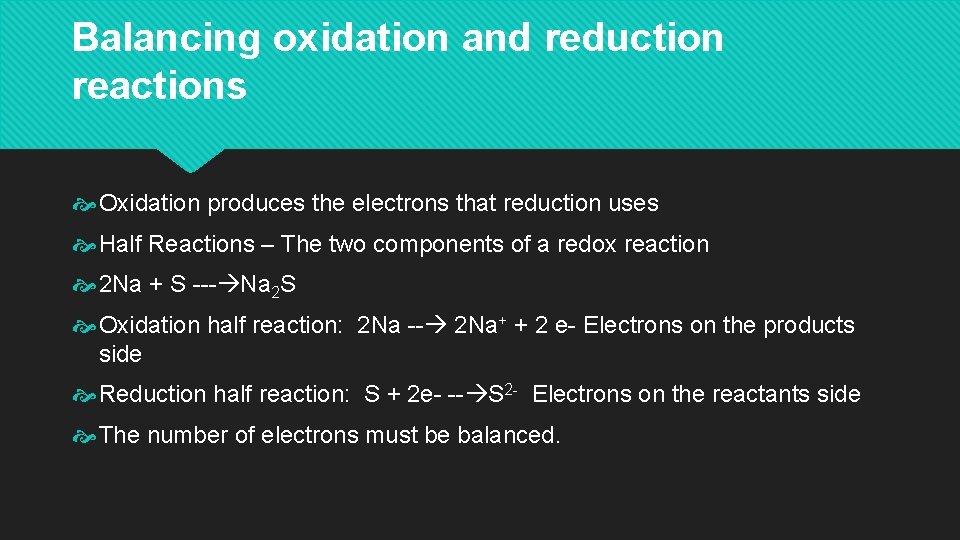
Balancing oxidation and reduction reactions Oxidation produces the electrons that reduction uses Half Reactions – The two components of a redox reaction 2 Na + S --- Na 2 S Oxidation half reaction: 2 Na -- 2 Na+ + 2 e- Electrons on the products side Reduction half reaction: S + 2 e- -- S 2 - Electrons on the reactants side The number of electrons must be balanced.
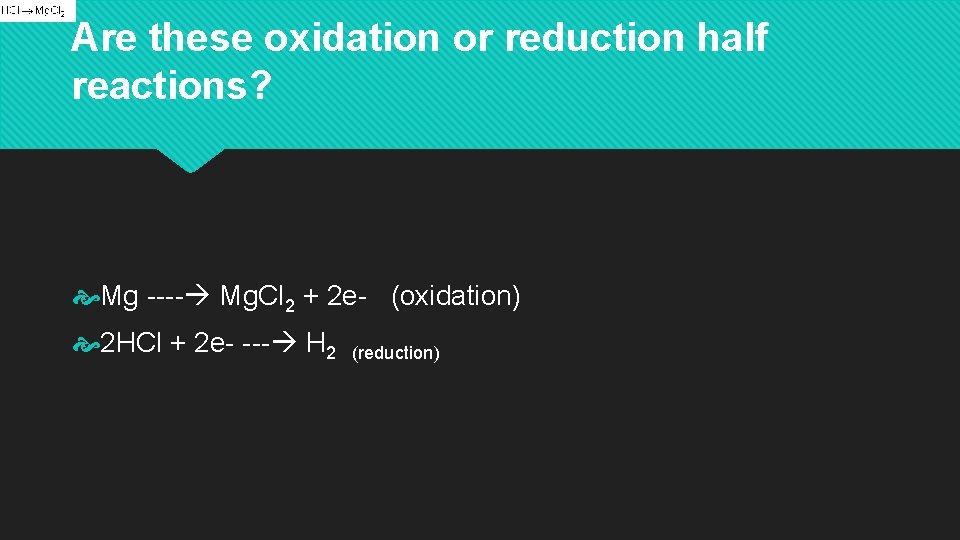
Are these oxidation or reduction half reactions? Mg ---- Mg. Cl 2 + 2 e- (oxidation) 2 HCl + 2 e- --- H 2 (reduction)

Practice Which element’s oxidation number does not change? Mg + 2 HCl --- Mg. Cl 2 + H 2 C + O 2 --- CO 2 – write the half reactions. Determine which elements were oxidized or reduced,

practice How many electrons would be exchanged in this redox reaction? Fe + O 2 -- Fe 2 O 3

Balancing oxidation reduction reactions Oxidation state method Ag + O 2 --- Ag 2 O Write the half reaction for silver: 2 Ag --- 2 Ag+2 + 2 e Write the half reaction for oxygen: O 2 + 4 e- -- 2 O 2 The electrons aren’t balanced, need to multiply the silver equation by 2. 2(2 Ag --- 2 Ag+2 + 2 e-) = 4 Ag ---- 4 Ag+2 + 4 e The balanced equation is: 4 Ag + O 2 --- 2 Ag 2 O
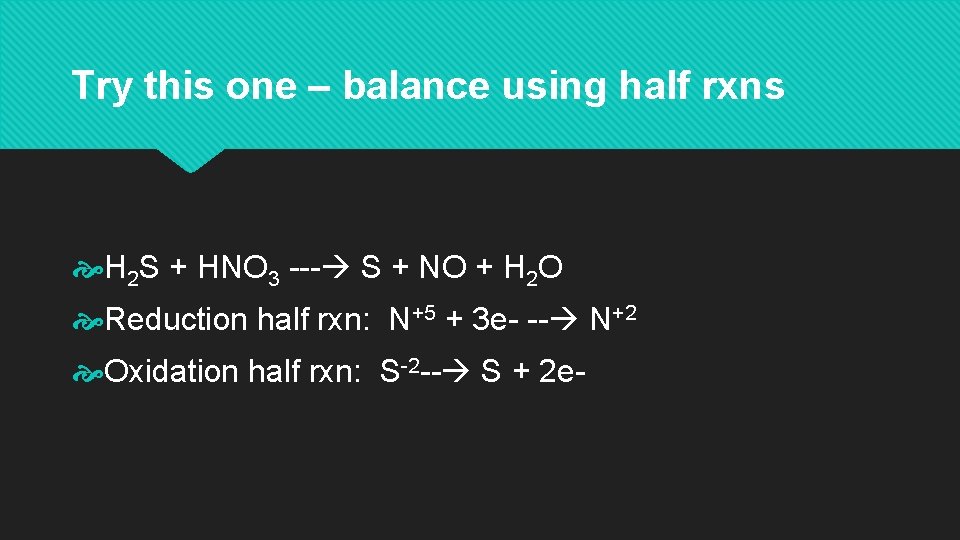
Try this one – balance using half rxns H 2 S + HNO 3 --- S + NO + H 2 O Reduction half rxn: N+5 + 3 e- -- N+2 Oxidation half rxn: S-2 -- S + 2 e-

HNO 3 + H 3 PO 3 ---- NO + H 3 PO 4 + H 2 O Assign oxidation numbers to each element – see whiteboard OR slide 14 in U 4 L 6 Determine which elements changed oxidation states – Nitrogen and Phosphorus Write your half reactions. Oxidation: P 3+ -- P 5+ + 2 e- Reduction: N 5+ + 3 e- -- N 2+ Make sure they have the same number of each type of atom on both sides and the same total charge on both sides Balance the electrons in the half reactions. Combine the half reactions, cancel the electrons Return to overall equation and add the coefficients Check for conservation of mass and unbalanced charges

Try this one Al + Ag. NO 3 --- Ag + Al(NO 3)3 1. Determine oxidation states of each atom 2. Write the half reactions 3. Balance the electrons in the half reactions 4. Balance the final equation

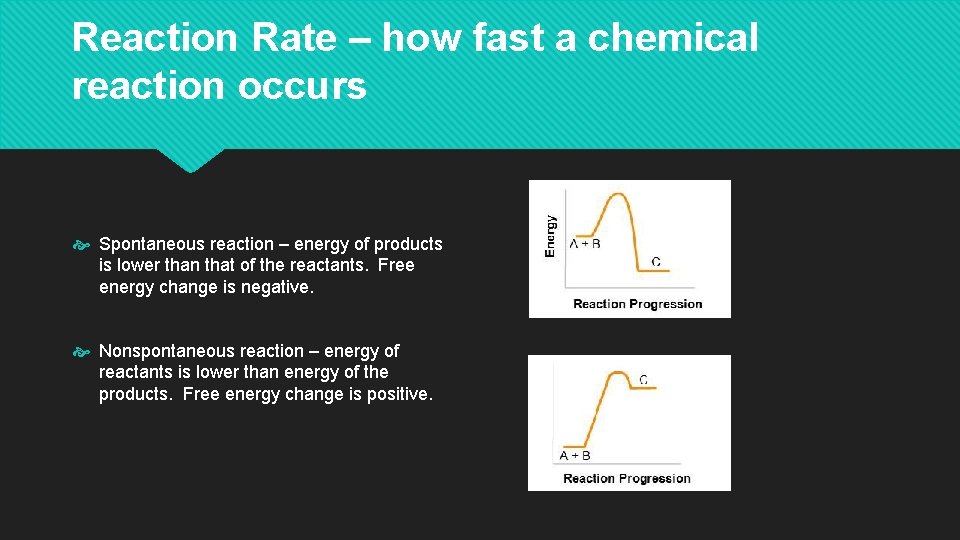
Reaction Rate – how fast a chemical reaction occurs Spontaneous reaction – energy of products is lower than that of the reactants. Free energy change is negative. Nonspontaneous reaction – energy of reactants is lower than energy of the products. Free energy change is positive.

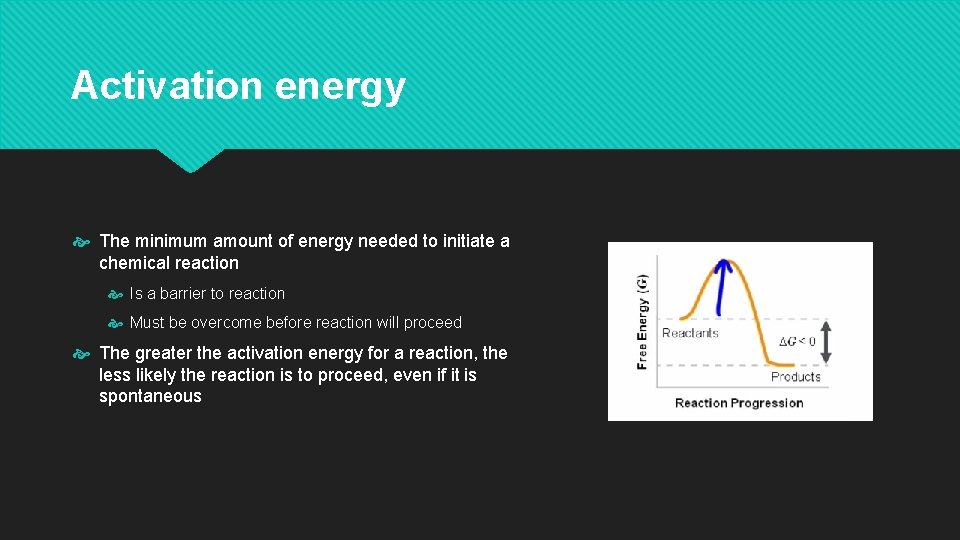
Activation energy The minimum amount of energy needed to initiate a chemical reaction Is a barrier to reaction Must be overcome before reaction will proceed The greater the activation energy for a reaction, the less likely the reaction is to proceed, even if it is spontaneous

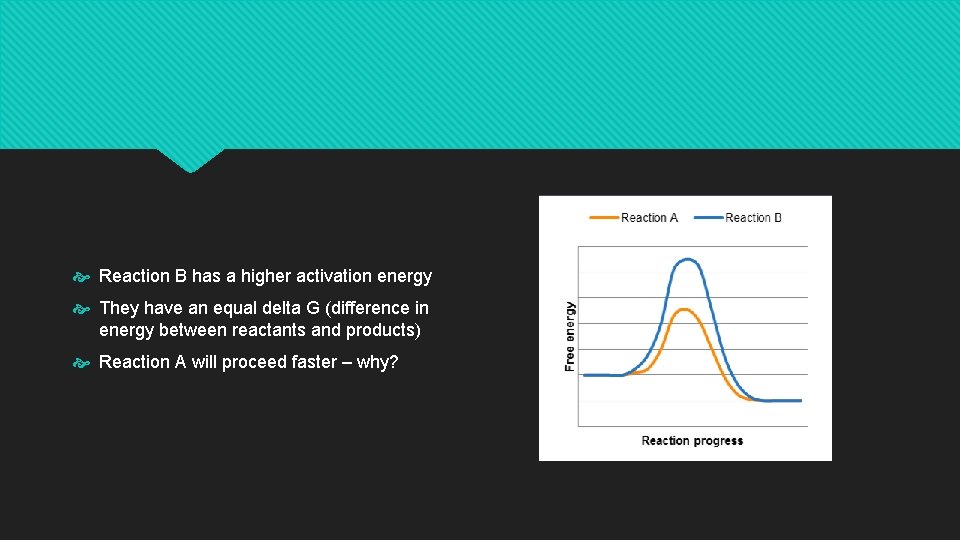
Reaction B has a higher activation energy They have an equal delta G (difference in energy between reactants and products) Reaction A will proceed faster – why?

Reaction rate The rate at which the reactants are converted into products Depend on activation energy High activation energy barriers result in low rates Low activation energy barriers, result in high rates Rate of a reaction is not related to whether it is spontaneous (delta Grxn)

Collision theory A model for chemical reactions that requires particle to collide in order to react. Every reaction begins with collision of molecules or particles. Not all collisions lead to reactions. The collisions must be effective and energetic enough for a reaction to occur

Effective collisions Collision must be energetic enough Energy comes from the kinetic energy of random collisions Collision should be in the correct orientation

Factors affecting reaction rate Factors that increase frequency or energy of collisions will increase reaction rate. Factors that decrease frequency or energy of collisions will decrease reaction rate

Temperature represents the average kinetic energy of a substance Increasing temperature increases speeds of particles, which can increase collisions which increases reaction rate

Concentration Higher concentration of reactants leads to more frequent collisions. More collisions means an increase in rate of reaction Higher concentration correlates to an increase of reaction rate

Pressure For gases, to increase reaction rate, add reactants or decrease the volume (particles in a smaller space will have more collisions) Both of these will increase the pressure. Increasing pressure increases reaction rate for gases IF the reactants contain more gas than the products

Surface area Increasing surface area increases the particles available for reaction – more collisions. Decreasing the particle size increases the surface area. Think sugar cube vs. spoonful of sugar. Spoonful of sugar has more surface area and smaller particles Increasing surface area increases reaction rate
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Unit 11 chemical reactions
Unit 11 chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Radical functions unit test
Radical functions unit test An example of redox reaction
An example of redox reaction Lesson 90 solid evidence precipitation reactions answer key
Lesson 90 solid evidence precipitation reactions answer key Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Types of chemical reactions redox
Types of chemical reactions redox Types of reactions
Types of reactions Types of reactions chemistry
Types of reactions chemistry What are the 5 types of chemical reactions
What are the 5 types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Ouchterlony
Ouchterlony Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Combination reaction example
Combination reaction example Toxic reactions chemical equations lesson 68 answers
Toxic reactions chemical equations lesson 68 answers Four types of chemical reactions
Four types of chemical reactions What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Chemical reactions in everyday life
Chemical reactions in everyday life Five chemical
Five chemical Reactants and products
Reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Solubility rules
Solubility rules Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Equilibrium of chemical reactions
Equilibrium of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Four types of chemical reactions
Four types of chemical reactions Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes What are the 5 types of chemical reactions
What are the 5 types of chemical reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Chemical reactions of esters
Chemical reactions of esters