Determining Empirical and Molecular Formulas What is an



- Slides: 36

Determining Empirical and Molecular Formulas

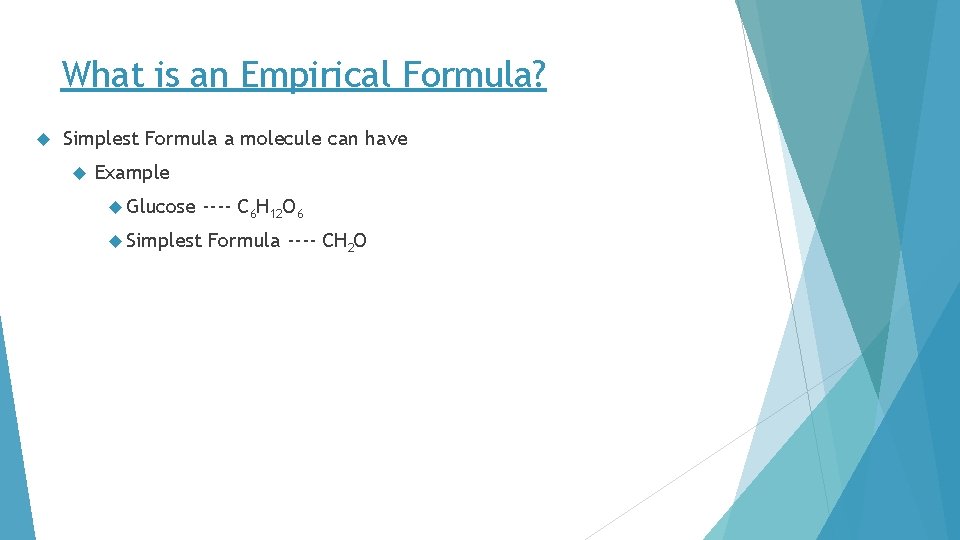
What is an Empirical Formula? Simplest Formula a molecule can have Example Glucose Simplest ---- C 6 H 12 O 6 Formula ---- CH 2 O

3 ways to Determine an Empirical Formula From Analysis From Percent Sample From Combustion Analysis

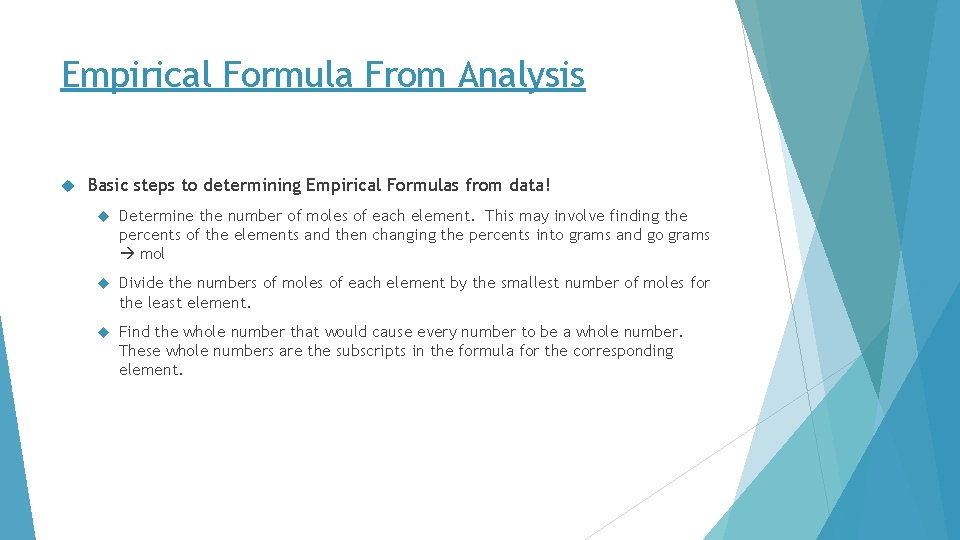
Empirical Formula From Analysis Basic steps to determining Empirical Formulas from data! Determine the number of moles of each element. This may involve finding the percents of the elements and then changing the percents into grams and go grams mol Divide the numbers of moles of each element by the smallest number of moles for the least element. Find the whole number that would cause every number to be a whole number. These whole numbers are the subscripts in the formula for the corresponding element.

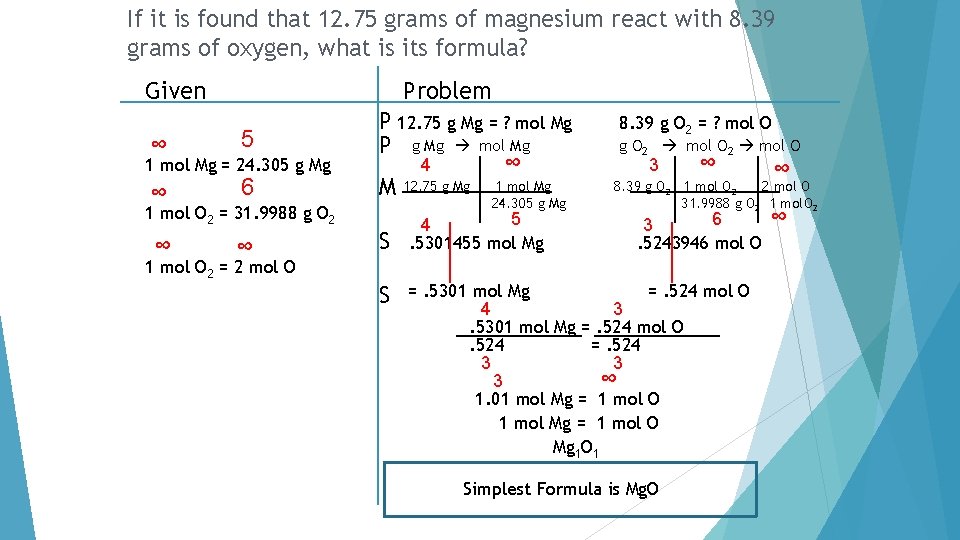
If it is found that 12. 75 grams of magnesium react with 8. 39 grams of oxygen, what is its formula?

If it is found that 12. 75 grams of magnesium react with 8. 39 grams of oxygen, what is its formula? Given Problem ∞ 5 ∞ 6 1 mol Mg = 24. 305 g Mg P 12. 75 g Mg = ? mol Mg P g Mg mol Mg ∞ 4 M 12. 75 g Mg 1 mol Mg 24. 305 g Mg 1 mol O 2 = 31. 9988 g O 2 ∞ ∞ S 1 mol O 2 = 2 mol O S 5 4. 5301455 mol Mg 8. 39 g O 2 = ? mol O g O 2 mol O 3 ∞ ∞ 8. 39 g O 2 1 mol O 2 2 mol O 31. 9988 g O 2 1 mol. O 2 6 3. 5243946 mol O =. 5301 mol Mg =. 524 mol O 4 3. 5301 mol Mg =. 524 mol O. 524 =. 524 3 3 ∞ 3 1. 01 mol Mg = 1 mol O Mg 1 O 1 Simplest Formula is Mg. O ∞

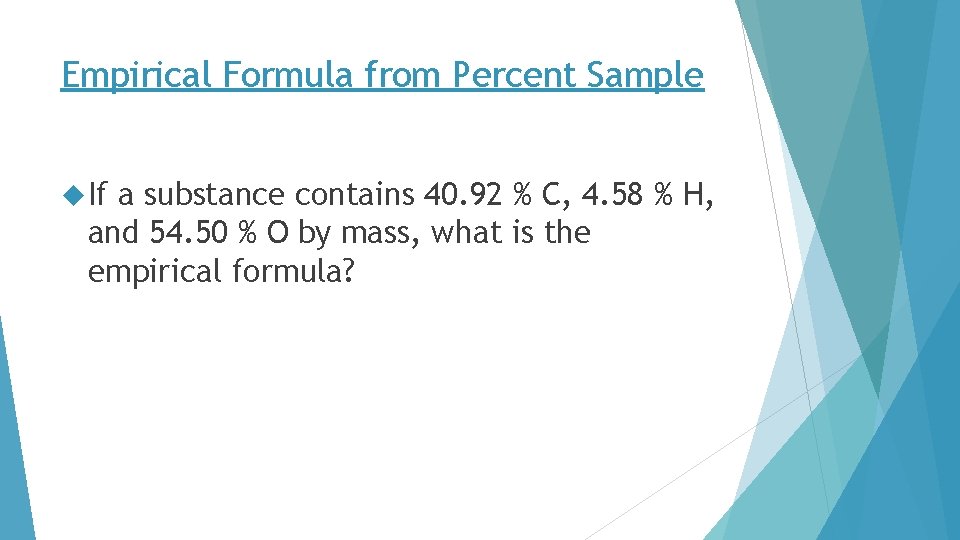
Empirical Formula from Percent Sample If a substance contains 40. 92 % C, 4. 58 % H, and 54. 50 % O by mass, what is the empirical formula?

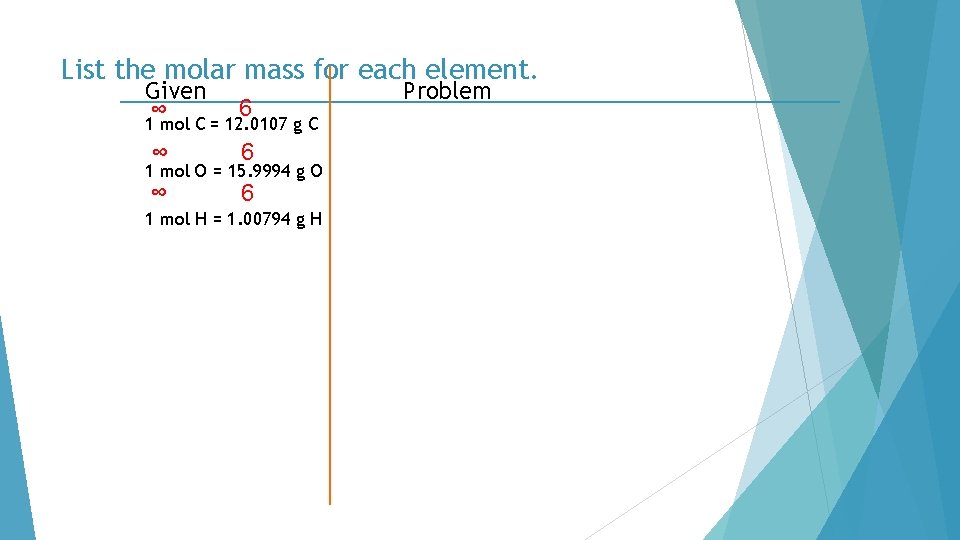
List the molar mass for each element. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H Problem

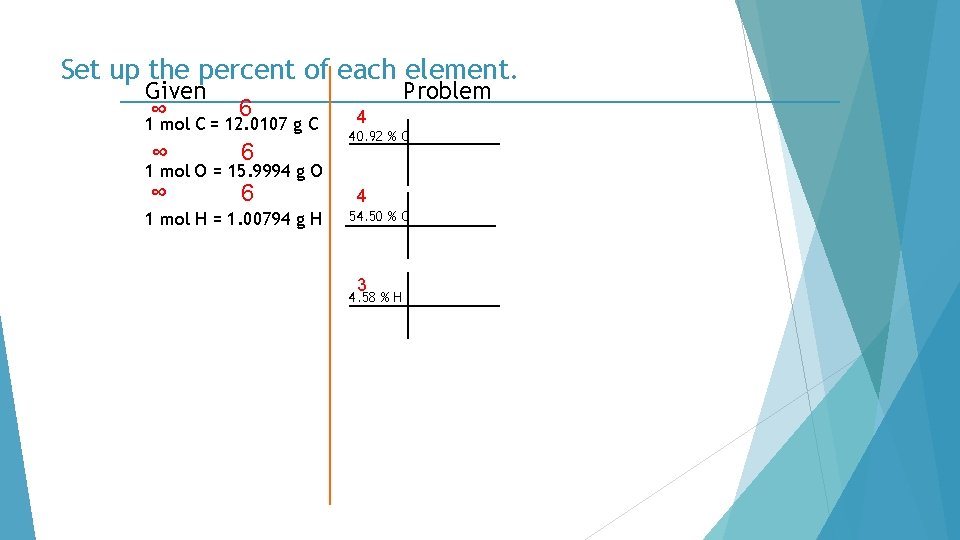
Set up the percent of each element. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 Problem 4 40. 92 % C 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 4 54. 50 % O 3 4. 58 % H

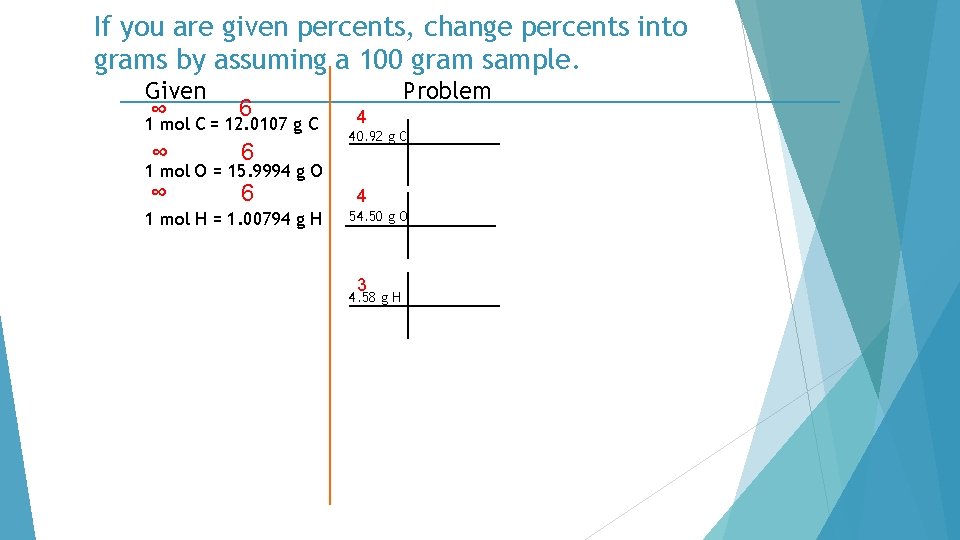
If you are given percents, change percents into grams by assuming a 100 gram sample. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 Problem 4 40. 92 g C 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 4 54. 50 g O 3 4. 58 g H

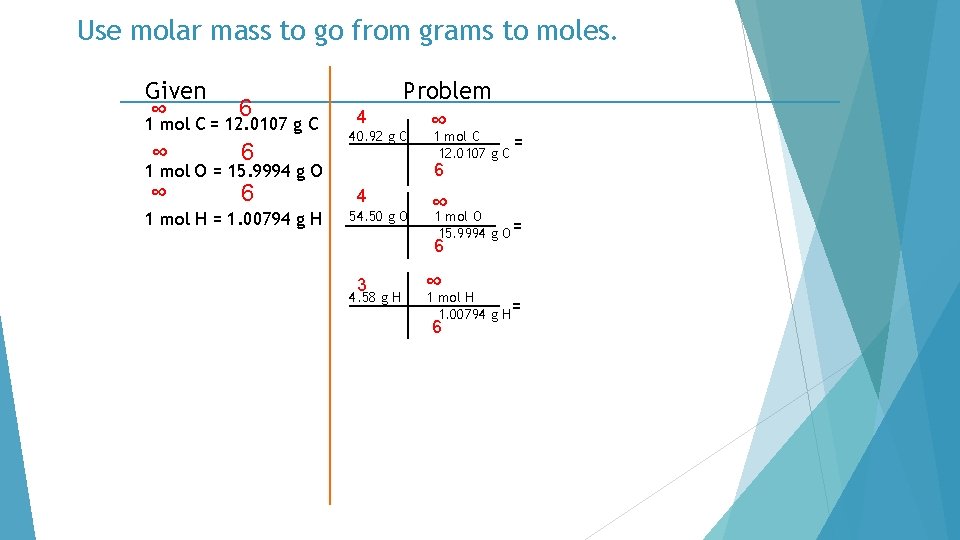
Use molar mass to go from grams to moles. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 4 Problem ∞ 40. 92 g C 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol C = 12. 0107 g C 4 54. 50 g O ∞ 1 mol O 15. 9994 g O = 6 3 4. 58 g H ∞ 1 mol H 1. 00794 g H= 6

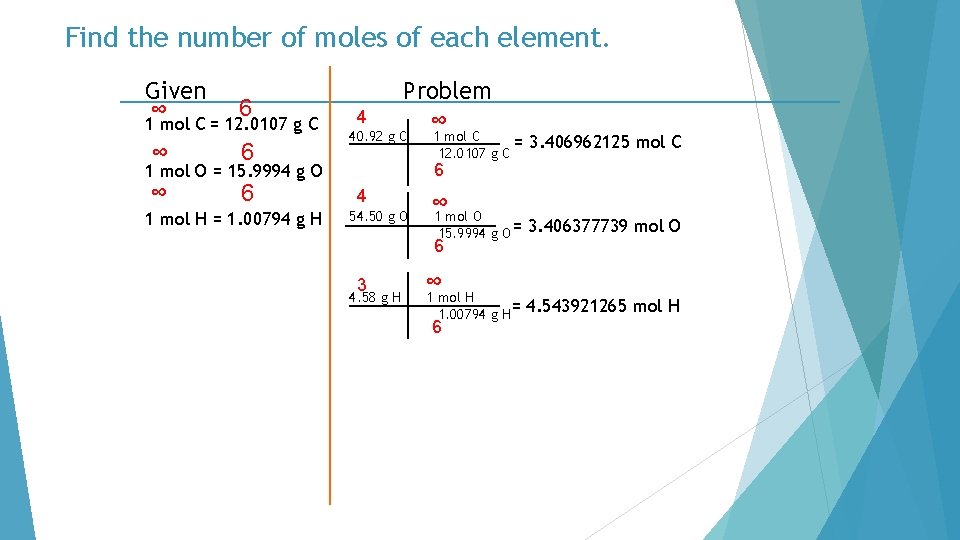
Find the number of moles of each element. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 4 Problem ∞ 40. 92 g C 3. 406962125 mol C 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol C = 12. 0107 g C 4 54. 50 g O ∞ 1 mol O 15. 9994 g O = 6 3 4. 58 g H ∞ 1 mol H 1. 00794 g H= 6 3. 406377739 mol O 4. 543921265 mol H

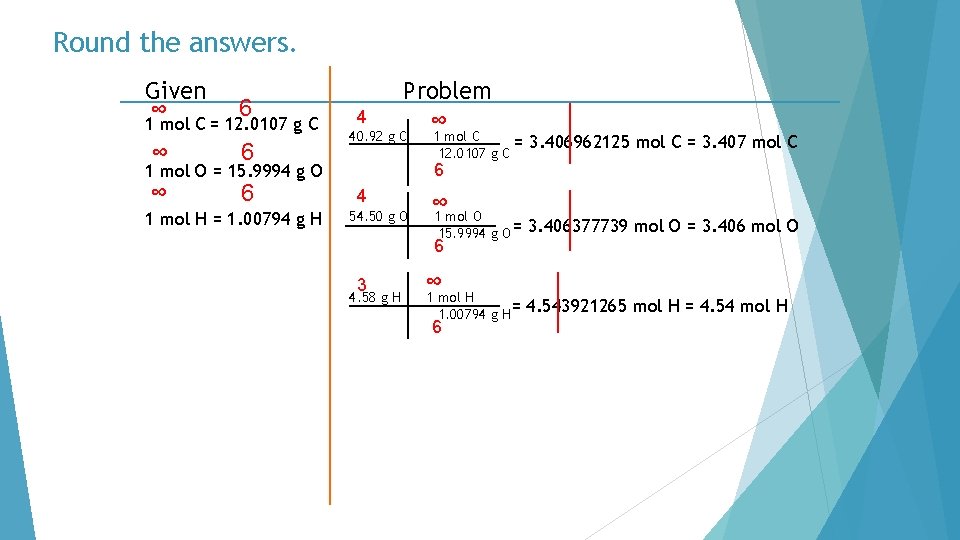
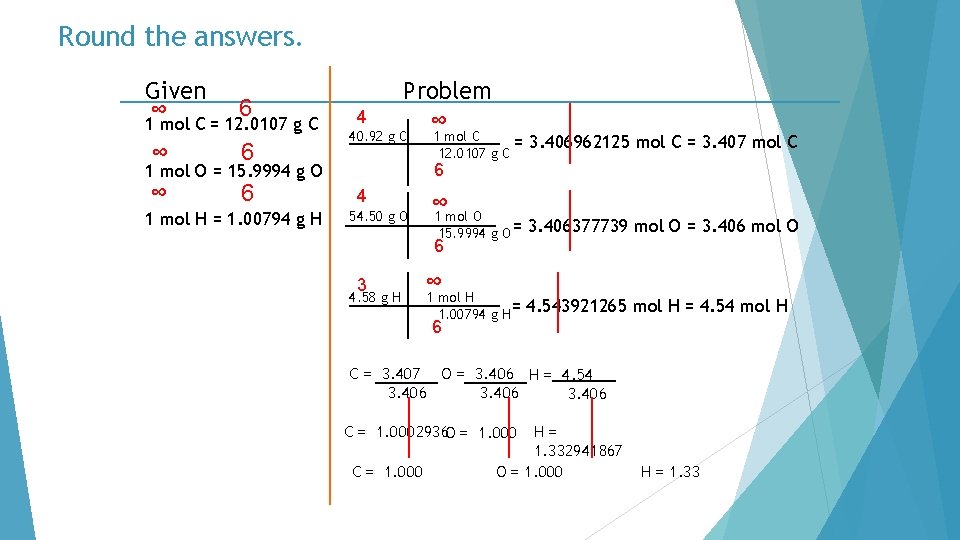
Round the answers. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 4 Problem ∞ 40. 92 g C 3. 406962125 mol C = 3. 407 mol C 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol C = 12. 0107 g C 4 54. 50 g O ∞ 1 mol O 15. 9994 g O = 6 3 4. 58 g H ∞ 1 mol H 1. 00794 g H= 6 3. 406377739 mol O = 3. 406 mol O 4. 543921265 mol H = 4. 54 mol H

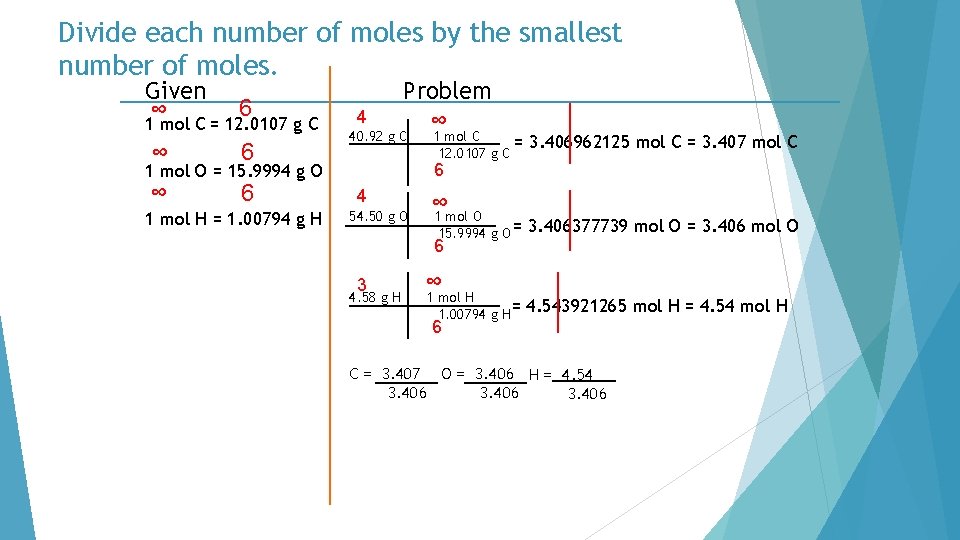
Divide each number of moles by the smallest number of moles. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 4 Problem ∞ 40. 92 g C 3. 406962125 mol C = 3. 407 mol C 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol C = 12. 0107 g C 4 54. 50 g O ∞ 1 mol O 15. 9994 g O = 6 3 4. 58 g H ∞ 1 mol H 1. 00794 g H= 3. 406377739 mol O = 3. 406 mol O 4. 543921265 mol H = 4. 54 mol H 6 C = 3. 407 O = 3. 406 H = 4. 54 3. 406

Round the answers. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 4 Problem ∞ 40. 92 g C 3. 406962125 mol C = 3. 407 mol C 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol C = 12. 0107 g C 4 54. 50 g O ∞ 1 mol O 15. 9994 g O = 6 3 4. 58 g H ∞ 1 mol H 1. 00794 g H= 3. 406377739 mol O = 3. 406 mol O 4. 543921265 mol H = 4. 54 mol H 6 C = 3. 407 O = 3. 406 H = 4. 54 3. 406 C = 1. 0002936 O = 1. 000 C = 1. 000 H= 1. 332941867 O = 1. 000 H = 1. 33

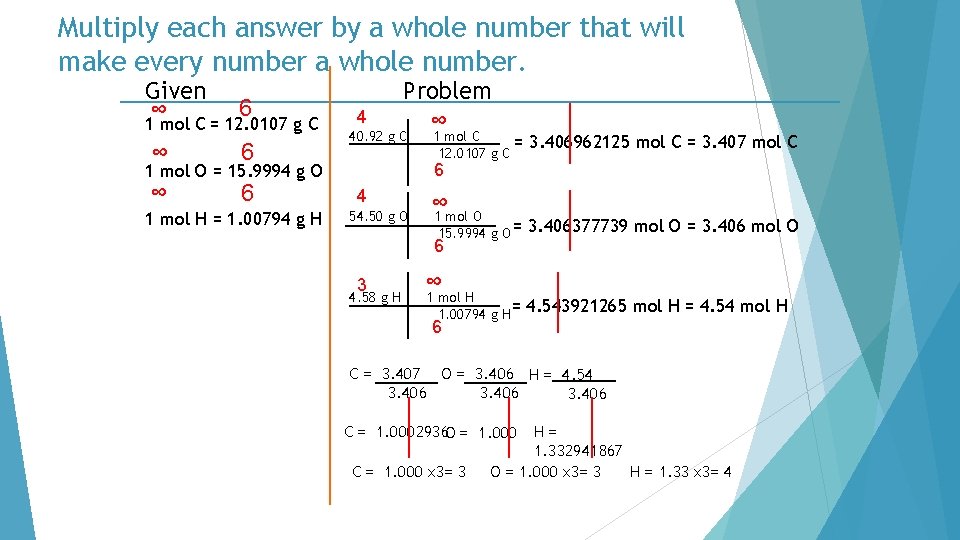
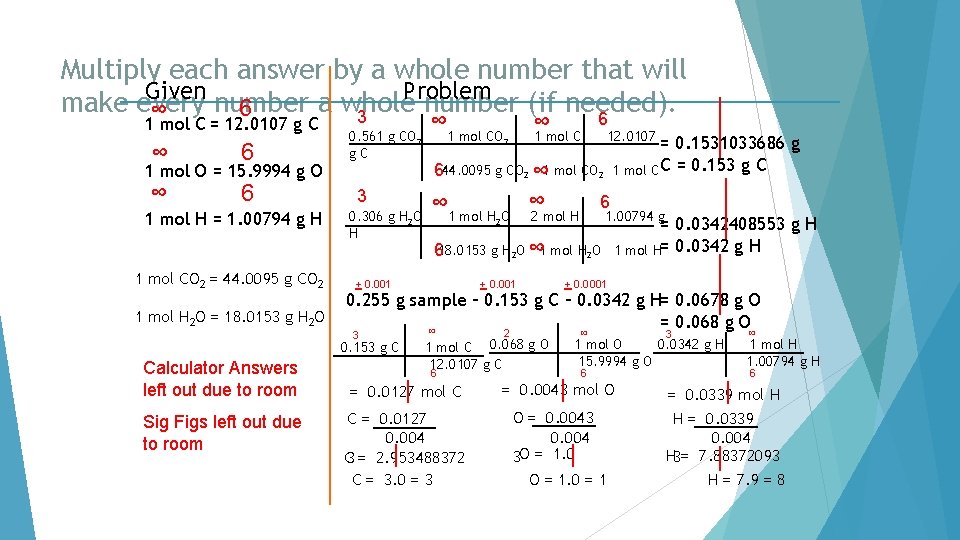
Multiply each answer by a whole number that will make every number a whole number. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 4 Problem ∞ 40. 92 g C 3. 406962125 mol C = 3. 407 mol C 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol C = 12. 0107 g C 4 54. 50 g O ∞ 1 mol O 15. 9994 g O = 6 3 4. 58 g H ∞ 1 mol H 1. 00794 g H= 3. 406377739 mol O = 3. 406 mol O 4. 543921265 mol H = 4. 54 mol H 6 C = 3. 407 O = 3. 406 H = 4. 54 3. 406 C = 1. 0002936 O = 1. 000 C = 1. 000 x 3= 3 H= 1. 332941867 O = 1. 000 x 3= 3 H = 1. 33 x 3= 4

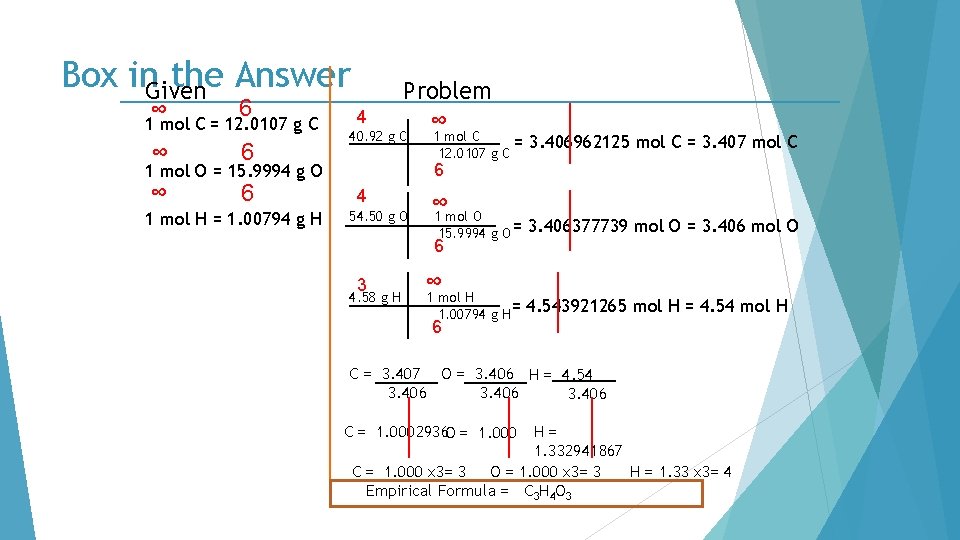
Box in. Given the Answer ∞ 6 1 mol C = 12. 0107 g C ∞ 6 4 Problem ∞ 40. 92 g C 3. 406962125 mol C = 3. 407 mol C 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol C = 12. 0107 g C 4 54. 50 g O ∞ 1 mol O 15. 9994 g O = 6 3 4. 58 g H ∞ 1 mol H 1. 00794 g H= 3. 406377739 mol O = 3. 406 mol O 4. 543921265 mol H = 4. 54 mol H 6 C = 3. 407 O = 3. 406 H = 4. 54 3. 406 C = 1. 0002936 O = 1. 000 H= 1. 332941867 C = 1. 000 x 3= 3 O = 1. 000 x 3= 3 H = 1. 33 x 3= 4 Empirical Formula = C 3 H 4 O 3

Combustion Analysis Technique to determine the empirical formula of a substance that contain primarily carbon and hydrogen. After the substance is burned, the products are collected. Carbon dioxide and water contain the carbon and hydrogen. Same concept of other analysis problem, but one more initial step to find out the amount of Oxygen if it is present in the sample. May get a little more involved if there are four elements in the sample, but not more difficult.

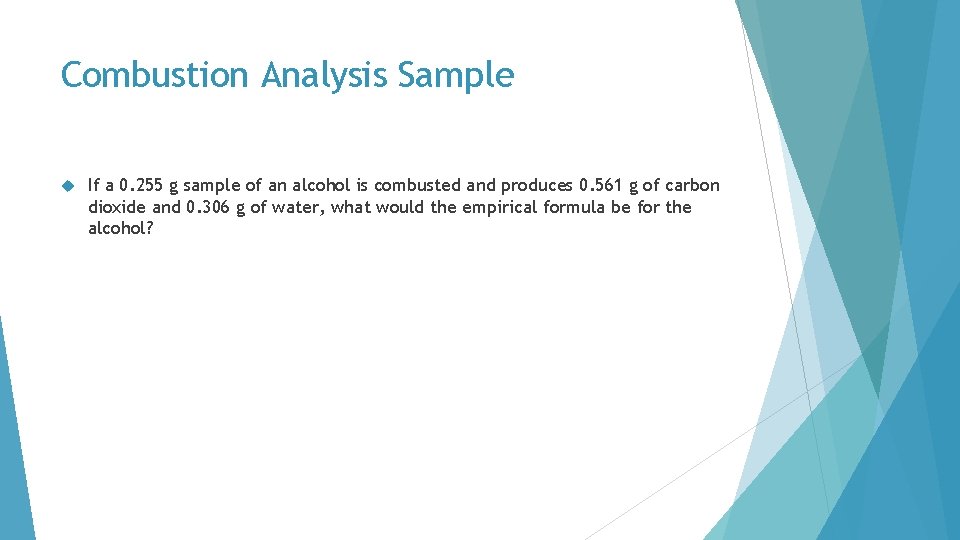
Combustion Analysis Sample If a 0. 255 g sample of an alcohol is combusted and produces 0. 561 g of carbon dioxide and 0. 306 g of water, what would the empirical formula be for the alcohol?

Steps to remember! Find the mass of Carbon and Hydrogen from the products. These should be in only one product each. Subtract those masses from the sample to find mass of Oxygen in products will also come from Oxygen as a reactant. Find the moles of each element Divide by least amount of moles. Get whole numbers for subscripts (multiply by same number if necessary)

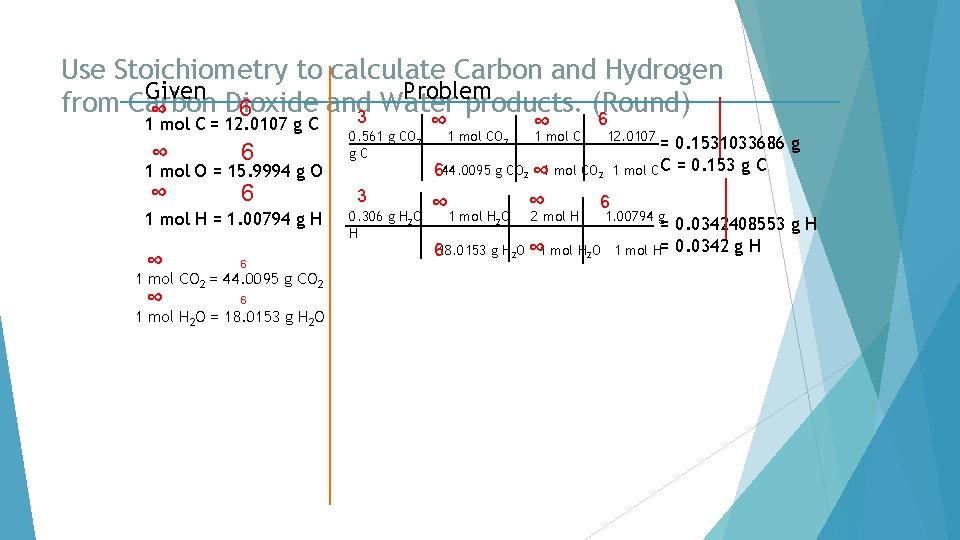
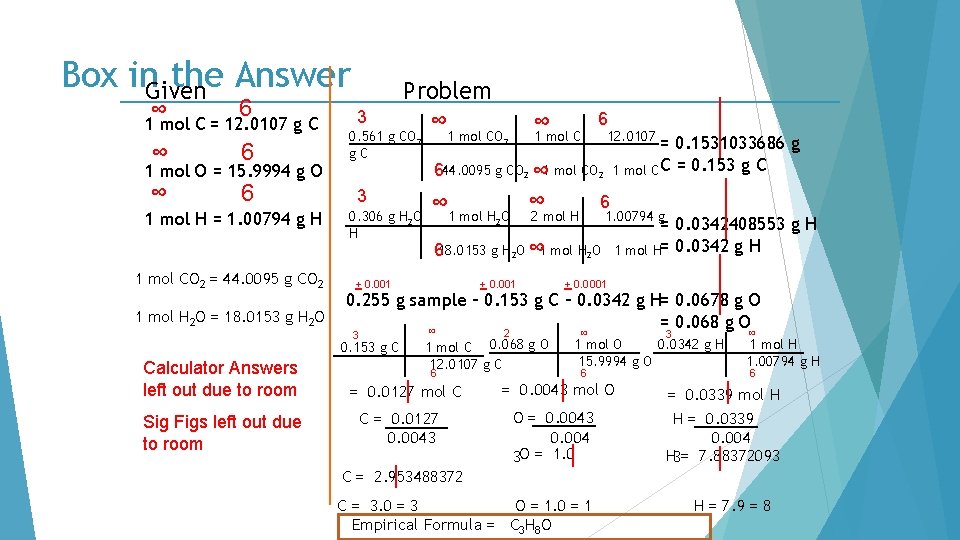
Use Stoichiometry to calculate Carbon and Hydrogen Given Problem from Carbon Dioxide and Water products. (Round) 6 ∞ 3 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H ∞ 6 1 mol CO 2 = 44. 0095 g CO 2 1 mol H 2 O = 18. 0153 g H 2 O 0. 561 g CO 2 g. C 3 0. 306 g H 2 O H ∞ 1 mol CO 2 ∞ 1 mol C 12. 0107 = 0. 1531033686 g 1 mol C C = 0. 153 g C 644. 0095 g CO 2 ∞ 1 mol H O ∞ 2 mol H 6 2 618. 0153 g H 2 O ∞ 1 mol H 2 O 1. 00794 g = 0. 0342408553 g H 1 mol H= 0. 0342 g H

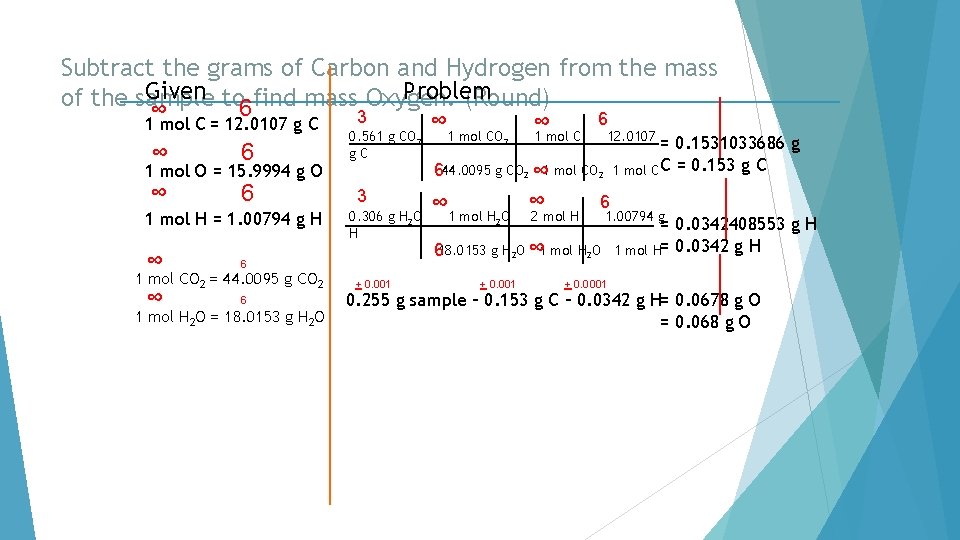
Subtract the grams of Carbon and Hydrogen from the mass Given to find mass Oxygen. Problem of the sample (Round) 6 ∞ 3 6 ∞ 1 mol C = 12. 0107 g C ∞ 0. 561 g CO 1 mol C 12. 0107 = 0. 1531033686 g g. C 6 ∞ 644. 0095 g CO ∞ 1 mol CO 1 mol C C = 0. 153 g C 1 mol O = 15. 9994 g O ∞ 6 3 6 ∞ ∞ 2 2 2 1 mol H = 1. 00794 g H ∞ 6 1 mol CO 2 = 44. 0095 g CO 2 ∞ 0. 306 g H 2 O H 6 1 mol H 2 O = 18. 0153 g H 2 O + 0. 001 1 mol H 2 O 2 2 mol H 1. 00794 g 618. 0153 g H 2 O ∞ 1 mol H 2 O + 0. 001 + 0. 0001 = 0. 0342408553 g H 1 mol H= 0. 0342 g H 0. 255 g sample – 0. 153 g C – 0. 0342 g H= 0. 0678 g O = 0. 068 g O

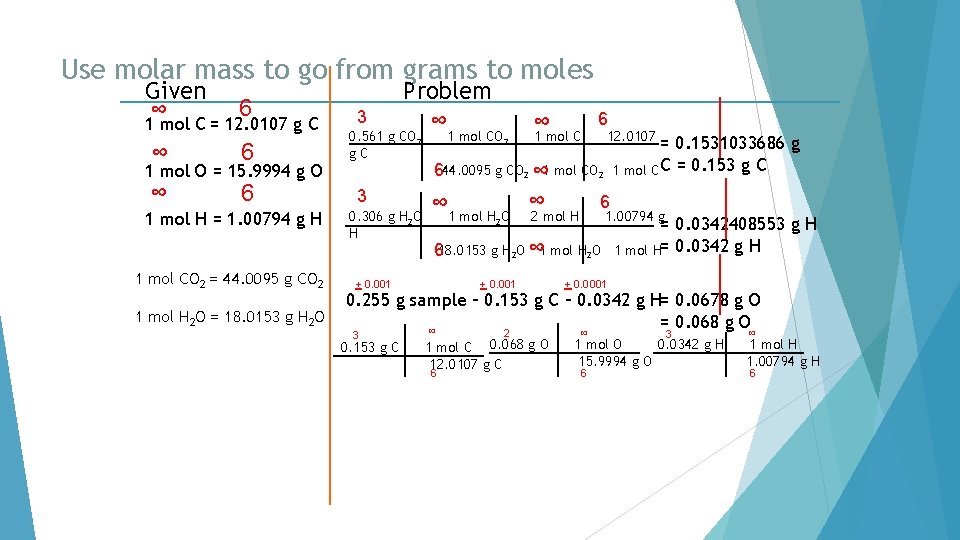
Use molar mass to go from grams to moles Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol CO 2 = 44. 0095 g CO 2 1 mol H 2 O = 18. 0153 g H 2 O 3 Problem ∞ 0. 561 g CO 2 g. C 3 0. 306 g H 2 O H 1 mol CO 2 ∞ 1 mol C 6 12. 0107 = 0. 1531033686 g 1 mol C C = 0. 153 g C 644. 0095 g CO 2 ∞ 1 mol H O ∞ 2 mol H 6 1. 00794 g 2 618. 0153 g H 2 O ∞ 1 mol H 2 O + 0. 001 = 0. 0342408553 g H 1 mol H= 0. 0342 g H + 0. 0001 0. 255 g sample – 0. 153 g C – 0. 0342 g H= 0. 0678 g O = 0. 068 g O ∞ 3 0. 153 g C 2 1 mol C 0. 068 g O 12. 0107 g C 6 ∞ 3 0. 0342 g H 1 mol O 15. 9994 g O 6 ∞ 1 mol H 1. 00794 g H 6

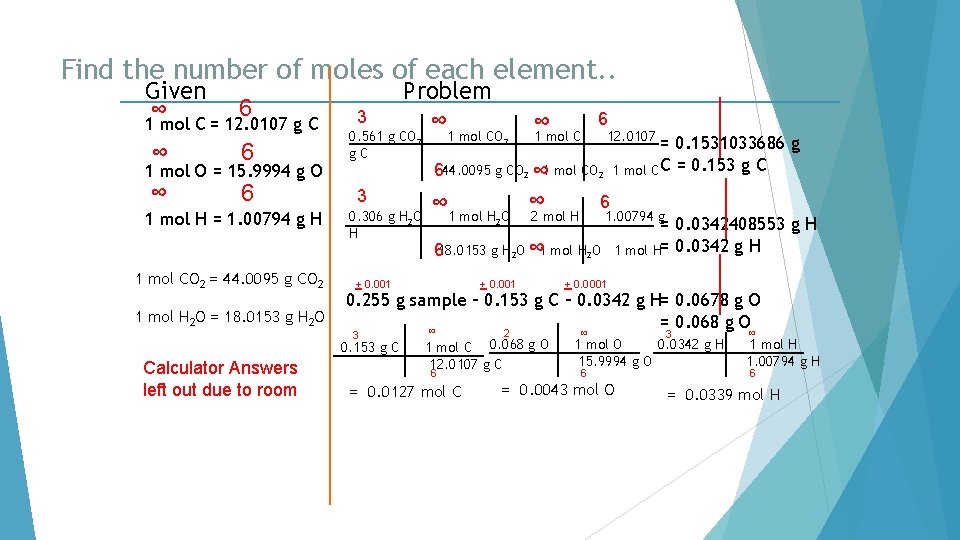
Find the number of moles of each element. . Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol CO 2 = 44. 0095 g CO 2 1 mol H 2 O = 18. 0153 g H 2 O 3 0. 561 g CO 2 g. C 3 0. 306 g H 2 O H 1 mol CO 2 ∞ 1 mol C 6 12. 0107 = 0. 1531033686 g 1 mol C C = 0. 153 g C 644. 0095 g CO 2 ∞ 1 mol H O ∞ 2 mol H 6 1. 00794 g 2 618. 0153 g H 2 O ∞ 1 mol H 2 O + 0. 001 = 0. 0342408553 g H 1 mol H= 0. 0342 g H + 0. 0001 0. 255 g sample – 0. 153 g C – 0. 0342 g H= 0. 0678 g O = 0. 068 g O ∞ 3 0. 153 g C Calculator Answers left out due to room Problem ∞ 2 1 mol C 0. 068 g O 12. 0107 g C 6 = 0. 0127 mol C ∞ 3 0. 0342 g H 1 mol O 15. 9994 g O 6 = 0. 0043 mol O ∞ 1 mol H 1. 00794 g H 6 = 0. 0339 mol H

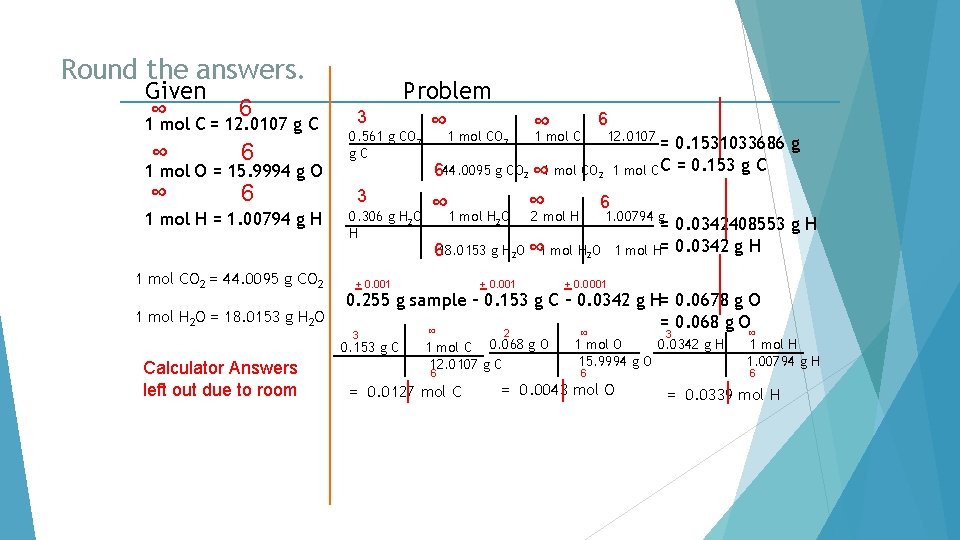
Round the answers. Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol CO 2 = 44. 0095 g CO 2 1 mol H 2 O = 18. 0153 g H 2 O 3 0. 561 g CO 2 g. C 3 0. 306 g H 2 O H 1 mol CO 2 ∞ 1 mol C 6 12. 0107 = 0. 1531033686 g 1 mol C C = 0. 153 g C 644. 0095 g CO 2 ∞ 1 mol H O ∞ 2 mol H 6 1. 00794 g 2 618. 0153 g H 2 O ∞ 1 mol H 2 O + 0. 001 = 0. 0342408553 g H 1 mol H= 0. 0342 g H + 0. 0001 0. 255 g sample – 0. 153 g C – 0. 0342 g H= 0. 0678 g O = 0. 068 g O ∞ 3 0. 153 g C Calculator Answers left out due to room Problem ∞ 2 1 mol C 0. 068 g O 12. 0107 g C 6 = 0. 0127 mol C ∞ 3 0. 0342 g H 1 mol O 15. 9994 g O 6 = 0. 0043 mol O ∞ 1 mol H 1. 00794 g H 6 = 0. 0339 mol H

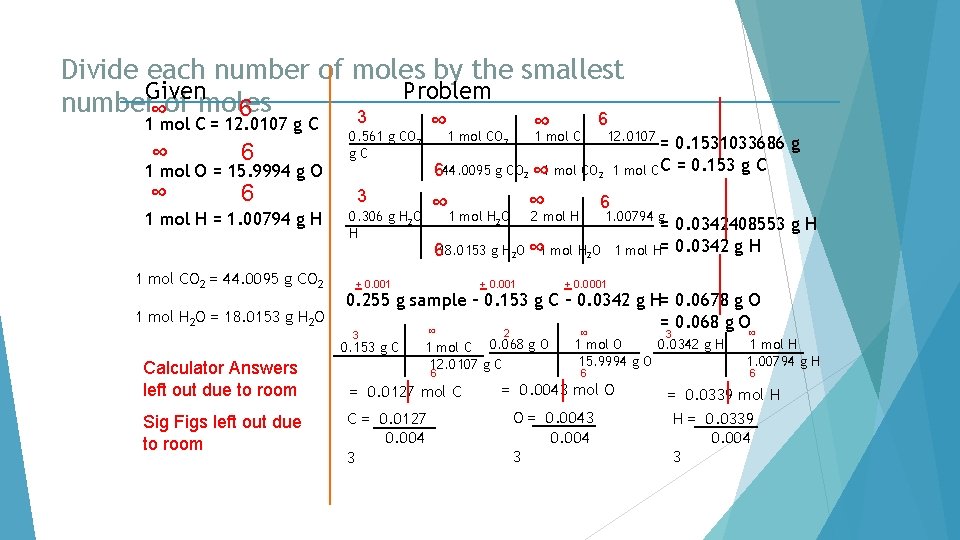
Divide each number of moles by the smallest Given Problem number∞of moles 6 3 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol CO 2 = 44. 0095 g CO 2 1 mol H 2 O = 18. 0153 g H 2 O Sig Figs left out due to room ∞ 1 mol CO 2 12. 0107 = 0. 1531033686 g 1 mol C C = 0. 153 g C 644. 0095 g CO 2 ∞ 1 mol CO 2 3 ∞ 1 mol H O ∞ 2 mol H 0. 306 g H 2 O H 6 1. 00794 g 2 618. 0153 g H 2 O ∞ 1 mol H 2 O + 0. 001 = 0. 0342408553 g H 1 mol H= 0. 0342 g H + 0. 0001 0. 255 g sample – 0. 153 g C – 0. 0342 g H= 0. 0678 g O = 0. 068 g O ∞ 3 0. 153 g C Calculator Answers left out due to room ∞ 0. 561 g CO 2 g. C 2 1 mol C 0. 068 g O 12. 0107 g C 6 = 0. 0127 mol C C = 0. 0127 0. 004 3 ∞ 3 0. 0342 g H 1 mol O 15. 9994 g O 6 = 0. 0043 mol O O = 0. 0043 0. 004 3 ∞ 1 mol H 1. 00794 g H 6 = 0. 0339 mol H H = 0. 0339 0. 004 3

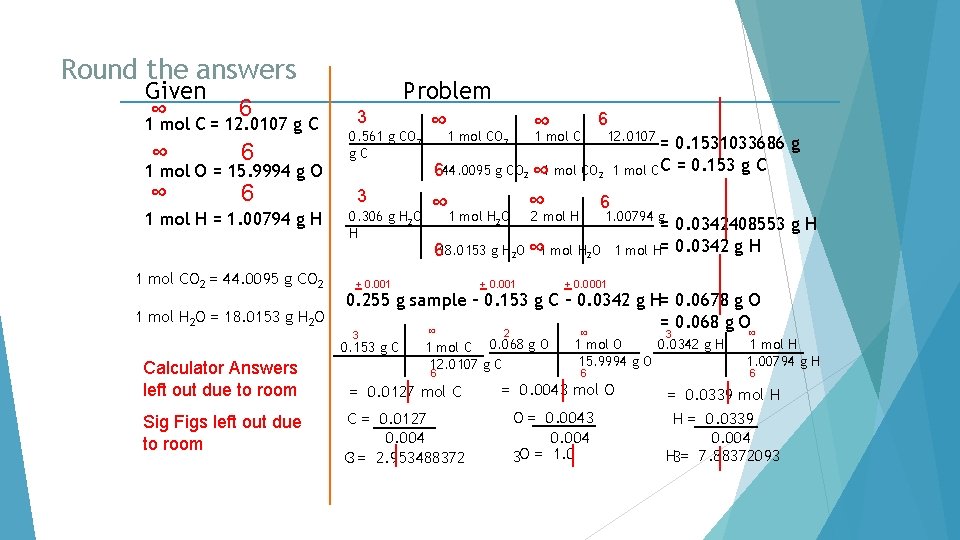
Round the answers Given ∞ 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol CO 2 = 44. 0095 g CO 2 1 mol H 2 O = 18. 0153 g H 2 O 3 0. 561 g CO 2 g. C 3 0. 306 g H 2 O H Sig Figs left out due to room ∞ 1 mol CO 2 6 12. 0107 = 0. 1531033686 g 1 mol C C = 0. 153 g C 644. 0095 g CO 2 ∞ 1 mol H O ∞ 2 mol H 6 1. 00794 g 2 618. 0153 g H 2 O ∞ 1 mol H 2 O + 0. 001 = 0. 0342408553 g H 1 mol H= 0. 0342 g H + 0. 0001 0. 255 g sample – 0. 153 g C – 0. 0342 g H= 0. 0678 g O = 0. 068 g O ∞ 3 0. 153 g C Calculator Answers left out due to room Problem ∞ 2 1 mol C 0. 068 g O 12. 0107 g C 6 = 0. 0127 mol C C = 0. 0127 0. 004 C 3 = 2. 953488372 ∞ 3 0. 0342 g H 1 mol O 15. 9994 g O 6 = 0. 0043 mol O O = 0. 0043 0. 004 3 O = 1. 0 ∞ 1 mol H 1. 00794 g H 6 = 0. 0339 mol H H = 0. 0339 0. 004 H 3= 7. 88372093

Multiply each answer by a whole number that will Given Problem make every number a whole number (if needed). 6 ∞ 3 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol CO 2 = 44. 0095 g CO 2 1 mol H 2 O = 18. 0153 g H 2 O 0. 561 g CO 2 g. C 3 0. 306 g H 2 O H Sig Figs left out due to room ∞ 1 mol CO 2 12. 0107 = 0. 1531033686 g 1 mol C C = 0. 153 g C 644. 0095 g CO 2 ∞ 1 mol H O ∞ 2 mol H 6 1. 00794 g 2 618. 0153 g H 2 O ∞ 1 mol H 2 O + 0. 001 = 0. 0342408553 g H 1 mol H= 0. 0342 g H + 0. 0001 0. 255 g sample – 0. 153 g C – 0. 0342 g H= 0. 0678 g O = 0. 068 g O ∞ 3 0. 153 g C Calculator Answers left out due to room ∞ 2 1 mol C 0. 068 g O 12. 0107 g C 6 ∞ 3 0. 0342 g H 1 mol O 15. 9994 g O 6 ∞ 1 mol H 1. 00794 g H 6 = 0. 0127 mol C = 0. 0043 mol O = 0. 0339 mol H C = 0. 0127 0. 004 C 3 = 2. 953488372 C = 3. 0 = 3 O = 0. 0043 0. 004 3 O = 1. 0 = 1 H = 0. 0339 0. 004 H 3= 7. 88372093 H = 7. 9 = 8

Box in. Given the Answer ∞ 6 1 mol C = 12. 0107 g C ∞ 6 1 mol O = 15. 9994 g O 1 mol H = 1. 00794 g H 1 mol CO 2 = 44. 0095 g CO 2 1 mol H 2 O = 18. 0153 g H 2 O 3 0. 561 g CO 2 g. C 3 0. 306 g H 2 O H Sig Figs left out due to room ∞ 1 mol CO 2 6 12. 0107 = 0. 1531033686 g 1 mol C C = 0. 153 g C 644. 0095 g CO 2 ∞ 1 mol H O ∞ 2 mol H 6 1. 00794 g 2 618. 0153 g H 2 O ∞ 1 mol H 2 O + 0. 001 = 0. 0342408553 g H 1 mol H= 0. 0342 g H + 0. 0001 0. 255 g sample – 0. 153 g C – 0. 0342 g H= 0. 0678 g O = 0. 068 g O ∞ 3 0. 153 g C Calculator Answers left out due to room Problem ∞ 2 1 mol C 0. 068 g O 12. 0107 g C 6 = 0. 0127 mol C C = 0. 0127 0. 0043 ∞ 3 0. 0342 g H 1 mol O 15. 9994 g O 6 = 0. 0043 mol O O = 0. 0043 0. 004 3 O = 1. 0 ∞ 1 mol H 1. 00794 g H 6 = 0. 0339 mol H H = 0. 0339 0. 004 H 3= 7. 88372093 C = 2. 953488372 C = 3. 0 = 3 O = 1. 0 = 1 Empirical Formula = C 3 H 8 O H = 7. 9 = 8

Molecular Formulas from Empirical Formulas You can calculate the molecular formula from the empirical formula if the formula mass (molecular weight) or molar mass is also known. The formula mass will be a multiple of the empirical formula mass. First find the empirical formula mass. May get a little more involved if you have to find the empirical formula first.

Molecular Formulas from Empirical Formulas Mesitylene, a hydrocarbon found in crude oil, has an empirical formula of C 3 H 4 and an experimentally determined formula weight of 121 amu. Determine the molecular formula.

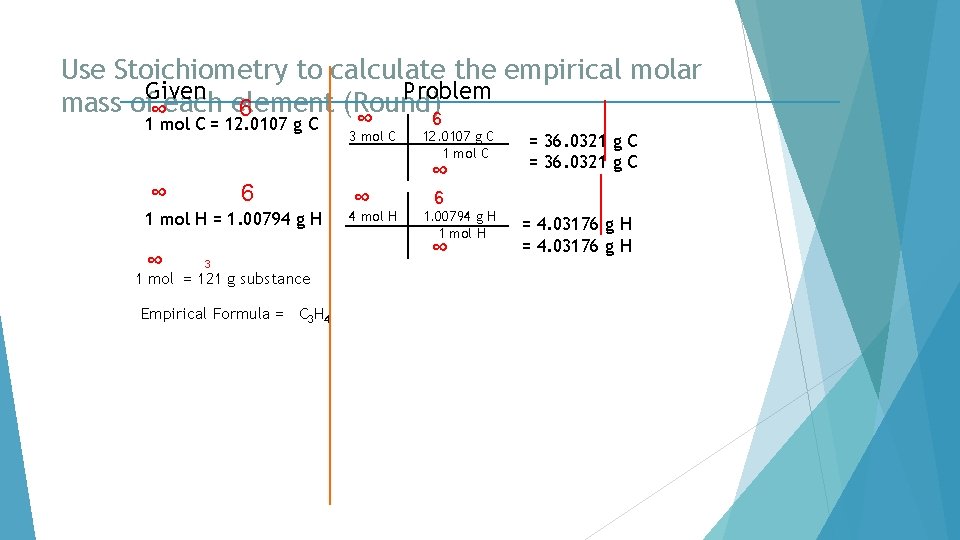
Use Stoichiometry to calculate the empirical molar Given Problem mass of∞each element (Round)6 6 1 mol C = 12. 0107 g C ∞ 6 1 mol H = 1. 00794 g H ∞ 3 1 mol = 121 g substance Empirical Formula = C 3 H 4 ∞ 3 mol C 12. 0107 g C 1 mol C ∞ ∞ 4 mol H = 36. 0321 g C 6 1. 00794 g H 1 mol H ∞ = 4. 03176 g H

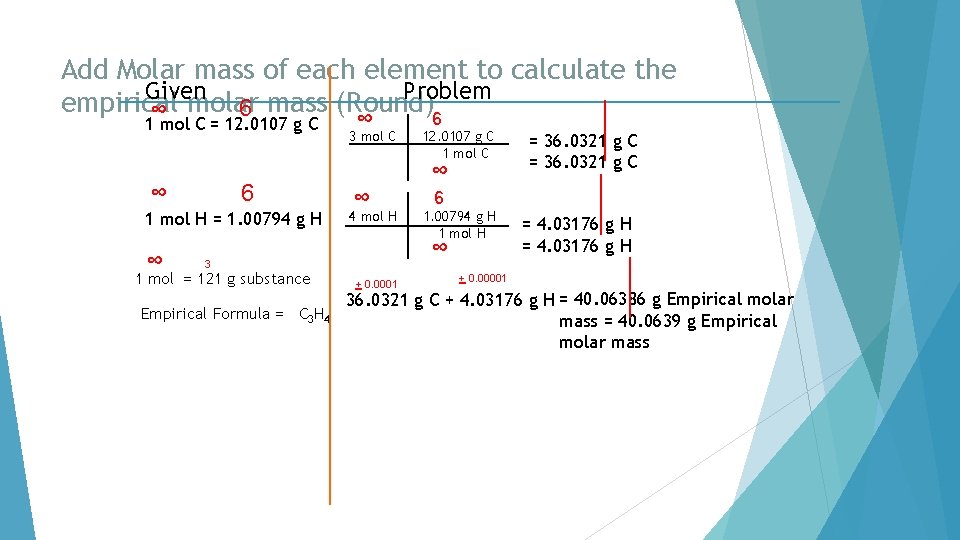
Add Molar mass of each element to calculate the Given Problem empirical 6 mass (Round)6 ∞ molar 1 mol C = 12. 0107 g C ∞ 6 1 mol H = 1. 00794 g H ∞ ∞ 3 mol C ∞ ∞ 4 mol H Empirical Formula = C 3 H 4 + 0. 0001 = 36. 0321 g C 6 1. 00794 g H 1 mol H ∞ 3 1 mol = 121 g substance 12. 0107 g C 1 mol C = 4. 03176 g H + 0. 00001 36. 0321 g C + 4. 03176 g H = 40. 06386 g Empirical molar mass = 40. 0639 g Empirical molar mass

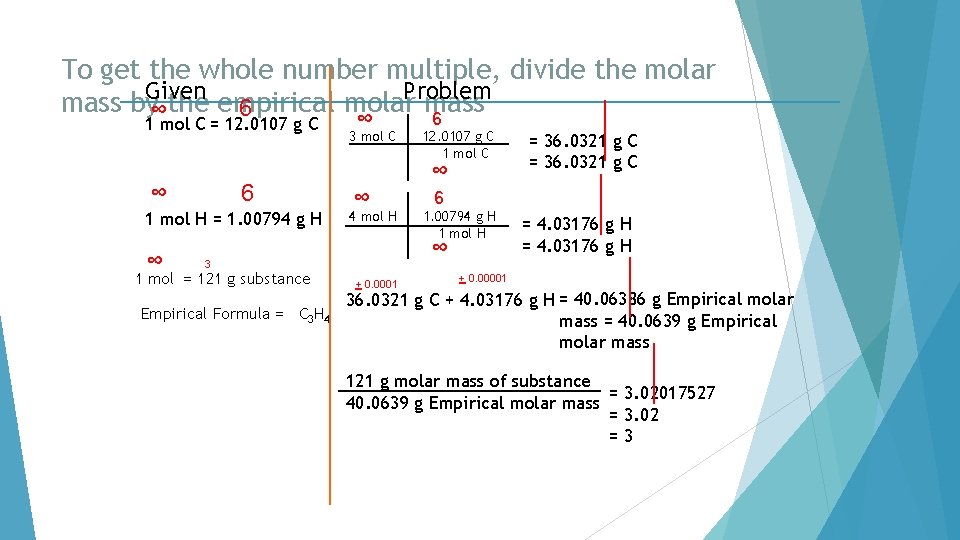
To get the whole number multiple, divide the molar Given Problem mass by∞the empirical molar mass 6 6 1 mol C = 12. 0107 g C ∞ 6 1 mol H = 1. 00794 g H ∞ ∞ 3 mol C ∞ ∞ 4 mol H Empirical Formula = C 3 H 4 + 0. 0001 = 36. 0321 g C 6 1. 00794 g H 1 mol H ∞ 3 1 mol = 121 g substance 12. 0107 g C 1 mol C = 4. 03176 g H + 0. 00001 36. 0321 g C + 4. 03176 g H = 40. 06386 g Empirical molar mass = 40. 0639 g Empirical molar mass 121 g molar mass of substance = 3. 02017527 40. 0639 g Empirical molar mass = 3. 02 =3

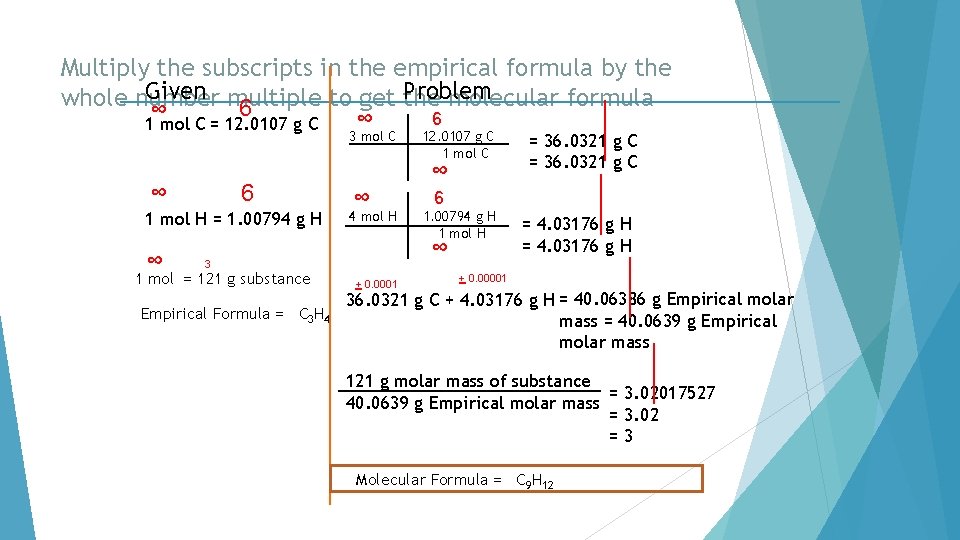
Multiply the subscripts in the empirical formula by the Given multiple to get the Problem whole number molecular formula 6 ∞ 1 mol C = 12. 0107 g C 3 mol C ∞ 6 1 mol H = 1. 00794 g H ∞ ∞ ∞ 4 mol H Empirical Formula = C 3 H 4 + 0. 0001 = 36. 0321 g C 6 1. 00794 g H 1 mol H ∞ 3 1 mol = 121 g substance 12. 0107 g C 1 mol C = 4. 03176 g H + 0. 00001 36. 0321 g C + 4. 03176 g H = 40. 06386 g Empirical molar mass = 40. 0639 g Empirical molar mass 121 g molar mass of substance = 3. 02017527 40. 0639 g Empirical molar mass = 3. 02 =3 Molecular Formula = C 9 H 12

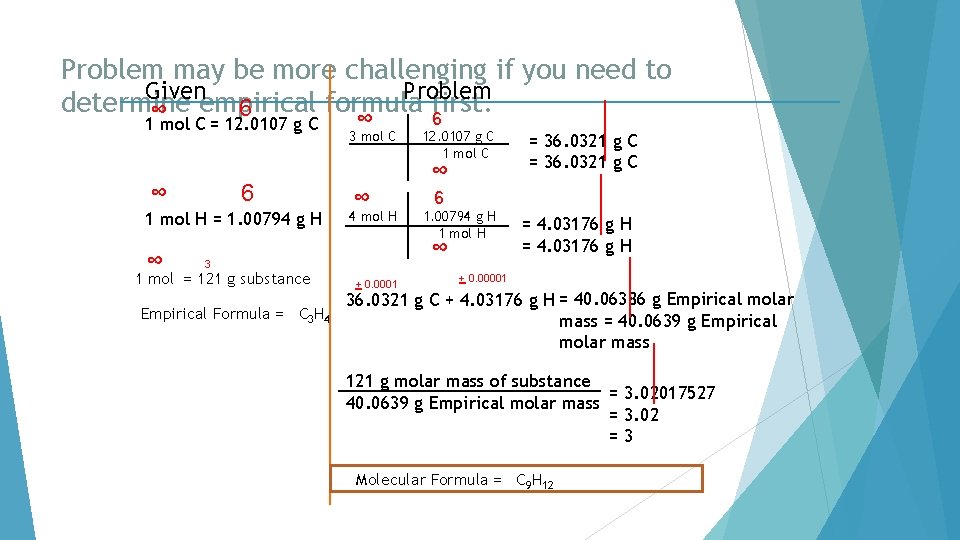
Problem may be more challenging if you need to Given Problem determine formula first. 6 ∞ empirical 6 1 mol C = 12. 0107 g C ∞ 6 1 mol H = 1. 00794 g H ∞ ∞ 3 mol C ∞ ∞ 4 mol H Empirical Formula = C 3 H 4 + 0. 0001 = 36. 0321 g C 6 1. 00794 g H 1 mol H ∞ 3 1 mol = 121 g substance 12. 0107 g C 1 mol C = 4. 03176 g H + 0. 00001 36. 0321 g C + 4. 03176 g H = 40. 06386 g Empirical molar mass = 40. 0639 g Empirical molar mass 121 g molar mass of substance = 3. 02017527 40. 0639 g Empirical molar mass = 3. 02 =3 Molecular Formula = C 9 H 12