Chemical Kinetics The area of chemistry that concerns



- Slides: 13

Chemical Kinetics The area of chemistry that concerns reaction rates and reaction mechanisms.

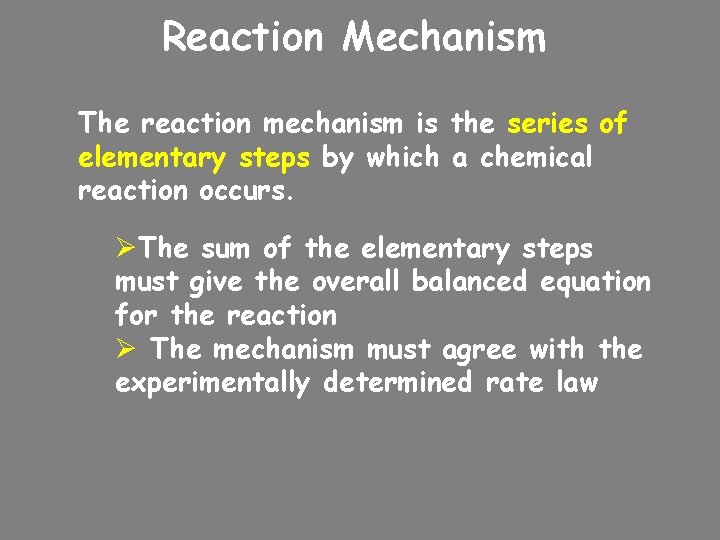
Reaction Mechanism The reaction mechanism is the series of elementary steps by which a chemical reaction occurs. ØThe sum of the elementary steps must give the overall balanced equation for the reaction Ø The mechanism must agree with the experimentally determined rate law

Rate-Determining Step In a multi-step reaction, the slowest step is the rate-determining step. It therefore determines the rate of the reaction. The experimental rate law must agree with the rate-determining step

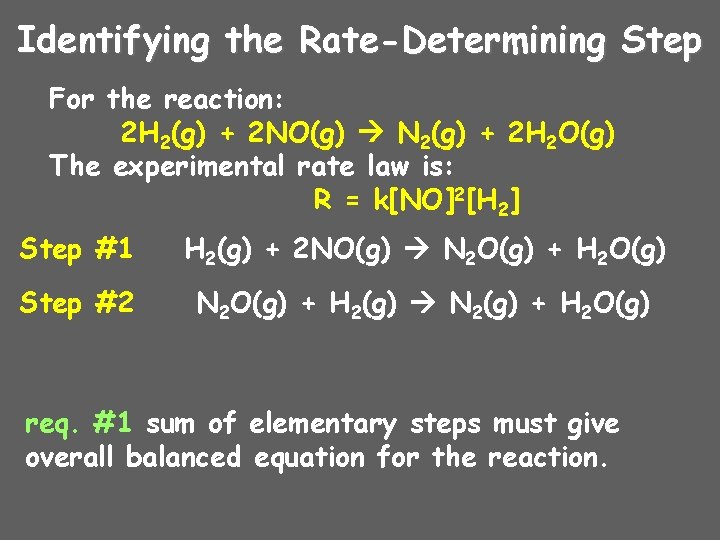
Identifying the Rate-Determining Step For the reaction: 2 H 2(g) + 2 NO(g) N 2(g) + 2 H 2 O(g) The experimental rate law is: R = k[NO]2[H 2] Step #1 H 2(g) + 2 NO(g) N 2 O(g) + H 2 O(g) Step #2 N 2 O(g) + H 2(g) N 2(g) + H 2 O(g) req. #1 sum of elementary steps must give overall balanced equation for the reaction.

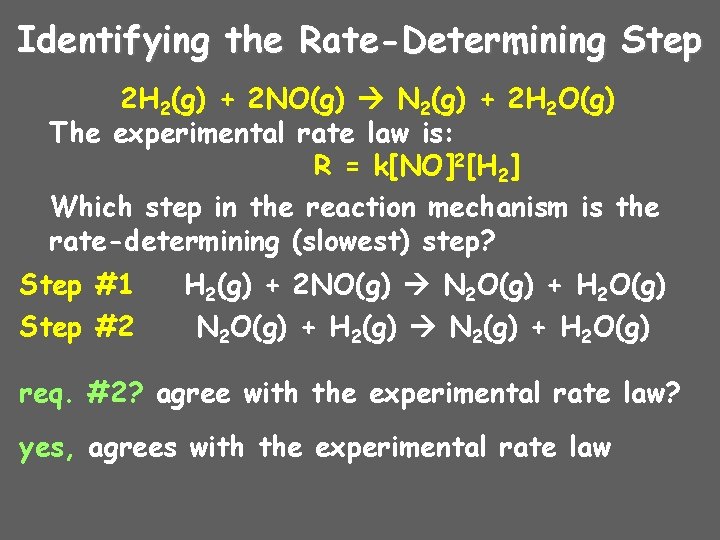
Identifying the Rate-Determining Step 2 H 2(g) + 2 NO(g) N 2(g) + 2 H 2 O(g) The experimental rate law is: R = k[NO]2[H 2] Which step in the reaction mechanism is the rate-determining (slowest) step? Step #1 Step #2 H 2(g) + 2 NO(g) N 2 O(g) + H 2 O(g) N 2 O(g) + H 2(g) N 2(g) + H 2 O(g) req. #2? agree with the experimental rate law? yes, agrees with the experimental rate law

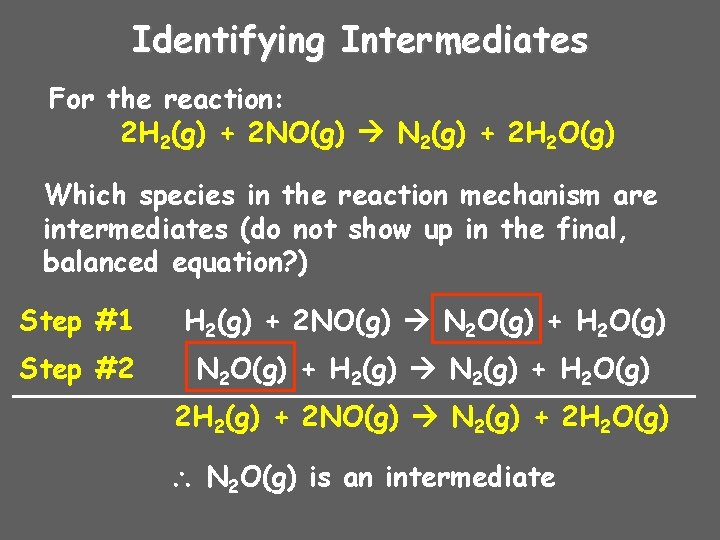
Identifying Intermediates For the reaction: 2 H 2(g) + 2 NO(g) N 2(g) + 2 H 2 O(g) Which species in the reaction mechanism are intermediates (do not show up in the final, balanced equation? ) Step #1 H 2(g) + 2 NO(g) N 2 O(g) + H 2 O(g) Step #2 N 2 O(g) + H 2(g) N 2(g) + H 2 O(g) 2 H 2(g) + 2 NO(g) N 2(g) + 2 H 2 O(g) N 2 O(g) is an intermediate

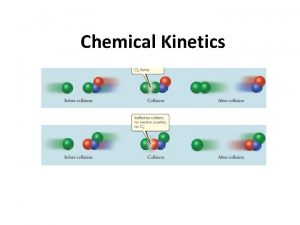
Collision Model Key Idea: Molecules must collide to react. However, only a small fraction of collisions produces a reaction. Why?

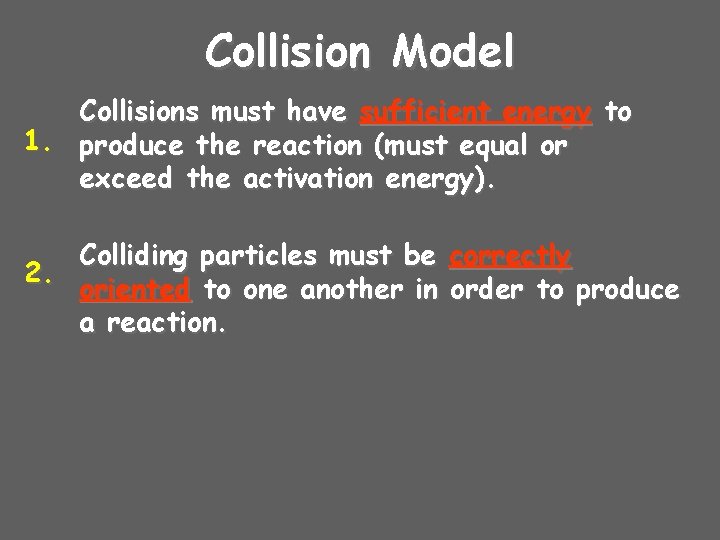
Collision Model Collisions must have sufficient energy to 1. produce the reaction (must equal or exceed the activation energy). Colliding particles must be correctly 2. oriented to one another in order to produce a reaction.

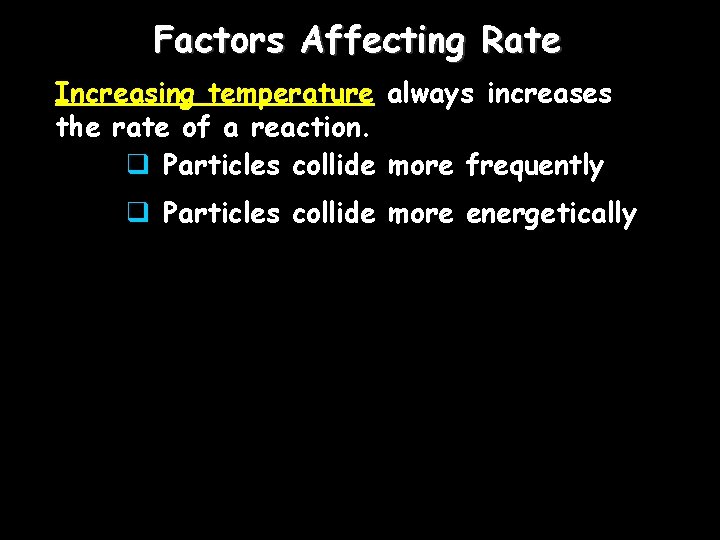
Factors Affecting Rate Increasing temperature always increases the rate of a reaction. q Particles collide more frequently q Particles collide more energetically

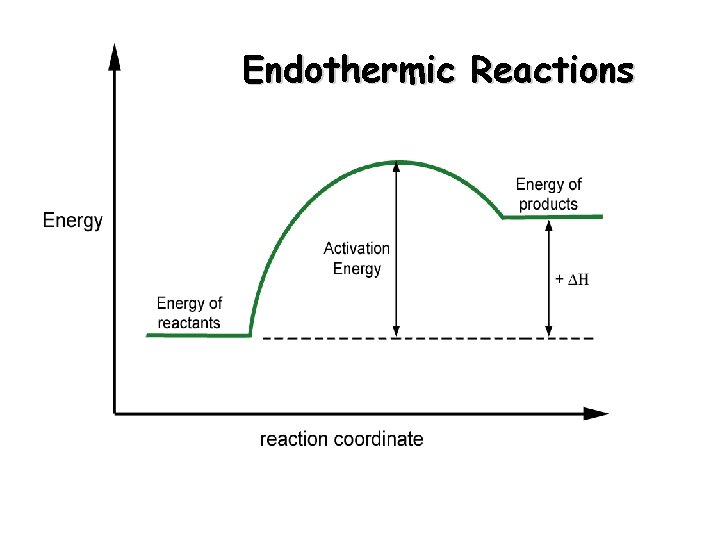
Endothermic Reactions

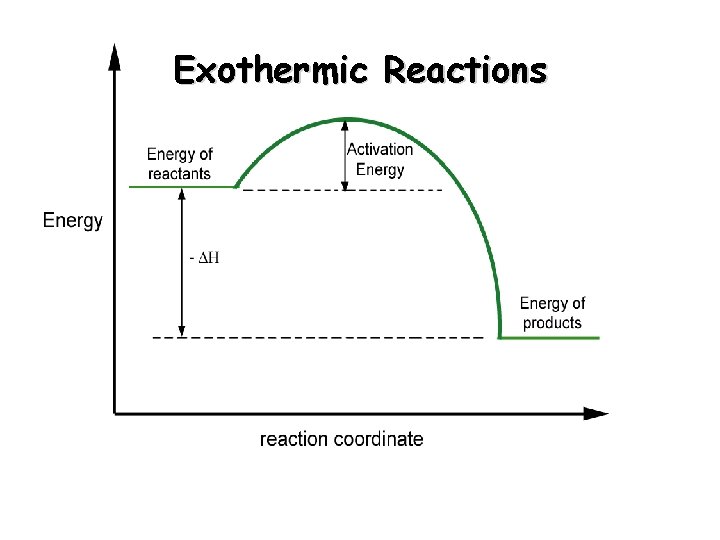
Exothermic Reactions

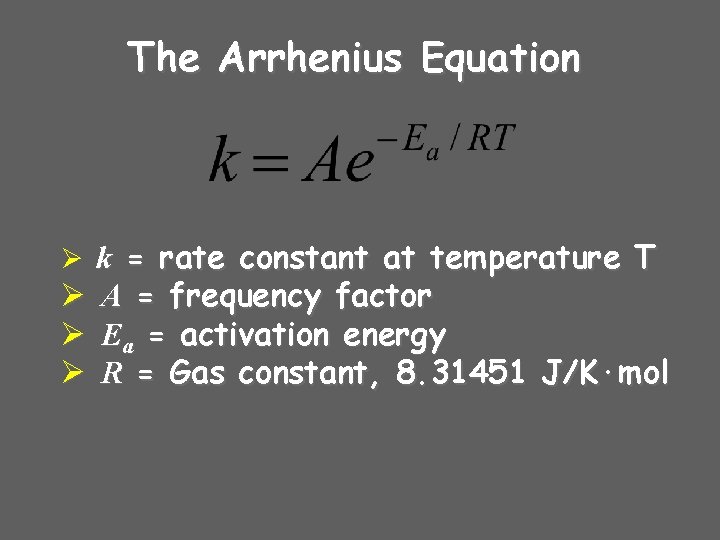
The Arrhenius Equation Ø k = rate constant at temperature T Ø Ø Ø A = frequency factor Ea = activation energy R = Gas constant, 8. 31451 J/K·mol

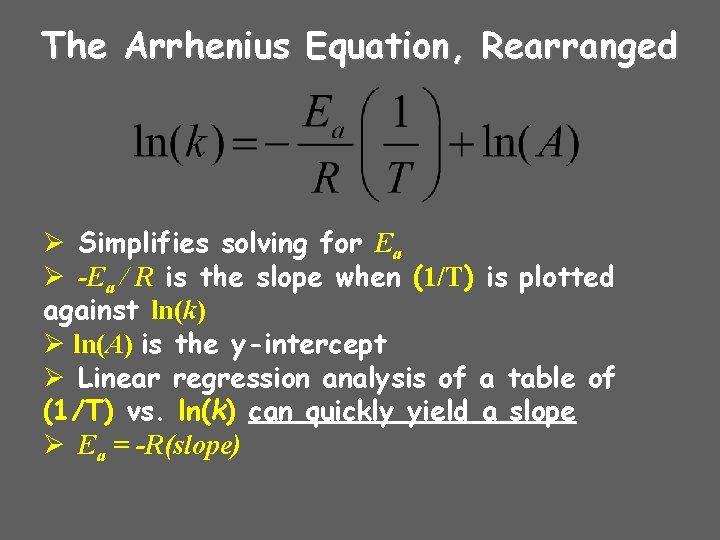
The Arrhenius Equation, Rearranged Ø Simplifies solving for Ea Ø -Ea / R is the slope when (1/T) is plotted against ln(k) Ø ln(A) is the y-intercept Ø Linear regression analysis of a table of (1/T) vs. ln(k) can quickly yield a slope Ø Ea = -R(slope)
 Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 Half life kinetics
Half life kinetics Ap chem kinetics
Ap chem kinetics Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics What is quasi steady state
What is quasi steady state Phân độ lown
Phân độ lown Block xoang nhĩ
Block xoang nhĩ Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng