Cytoskeleton Locomotion Kohidai Laszlo MD Ph D Med





- Slides: 67

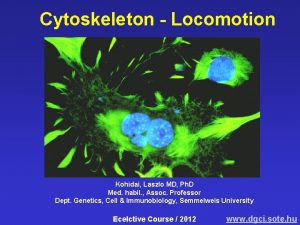
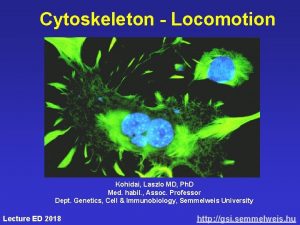
Cytoskeleton - Locomotion Kohidai, Laszlo MD, Ph. D Med. habil. , Assoc. Professor Dept. Genetics, Cell & Immunobiology, Semmelweis University Lecture ED 2017 http: //gsi. semmelweis. hu

Main functions of cytoskeleton • • Determines the shape of the cell Anchores organelles Movement of organelles Tensile strength Movement of chromosomes Polarity Motility !

Cytoskeleton Microfilaments (actin) l Microtubuli (tubulin) l Intermedier filaments l Motor proteins l Actin and microtubule associated proteins l !

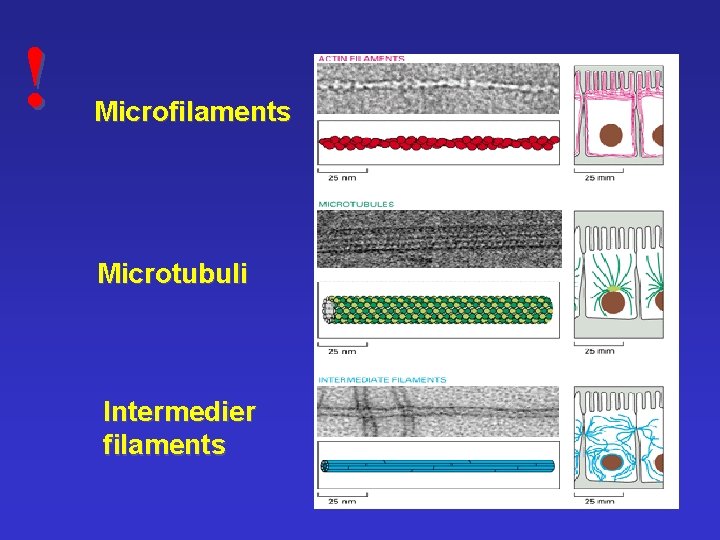
! Microfilaments Microtubuli Intermedier filaments

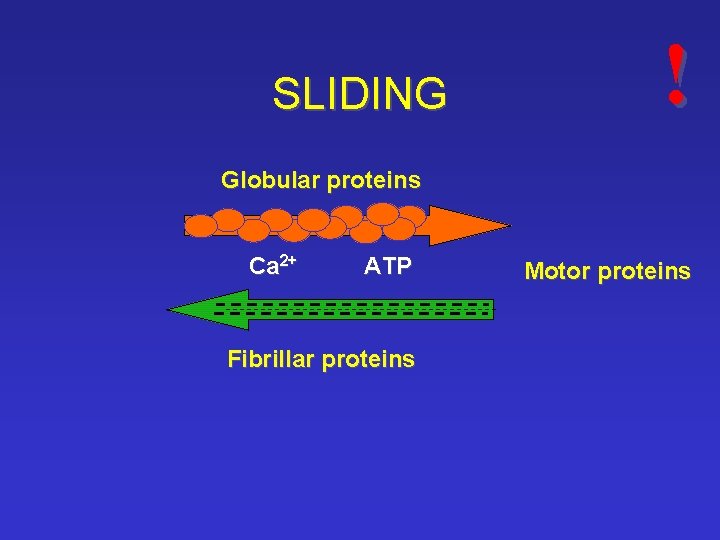
SLIDING ! Globular proteins Ca 2+ ATP Fibrillar proteins Motor proteins

Microfilaments

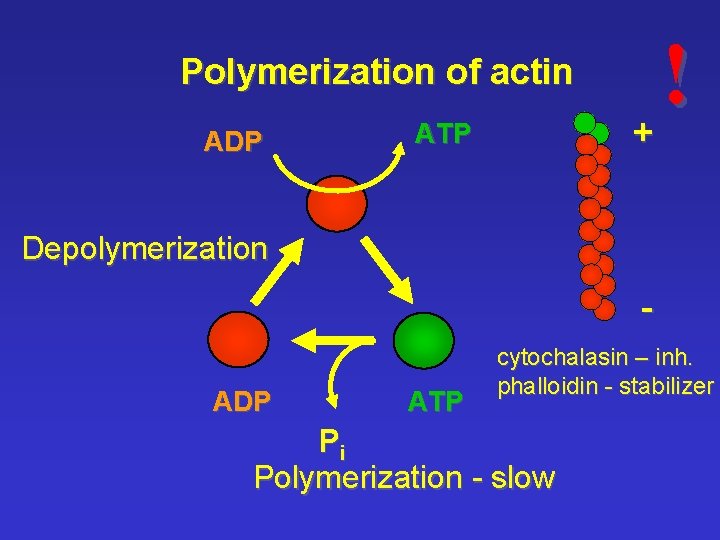
Polymerization of actin ADP ATP ! + Depolymerization ADP ATP cytochalasin – inh. phalloidin - stabilizer Pi Polymerization - slow

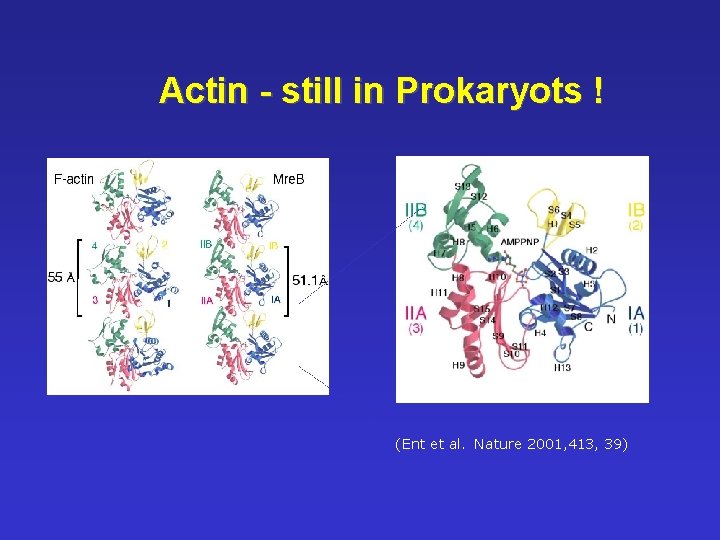
Actin - still in Prokaryots ! ((Ent et al. Nature 2001, 413, 39)

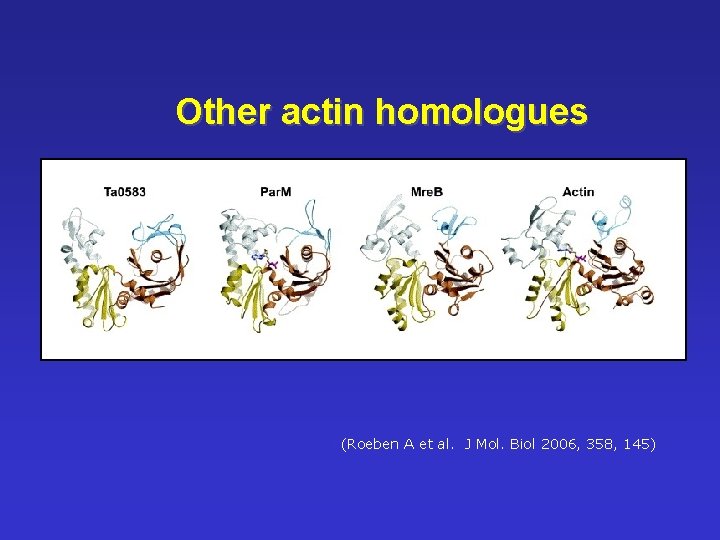
Other actin homologues ((Roeben A et al. J Mol. Biol 2006, 358, 145)

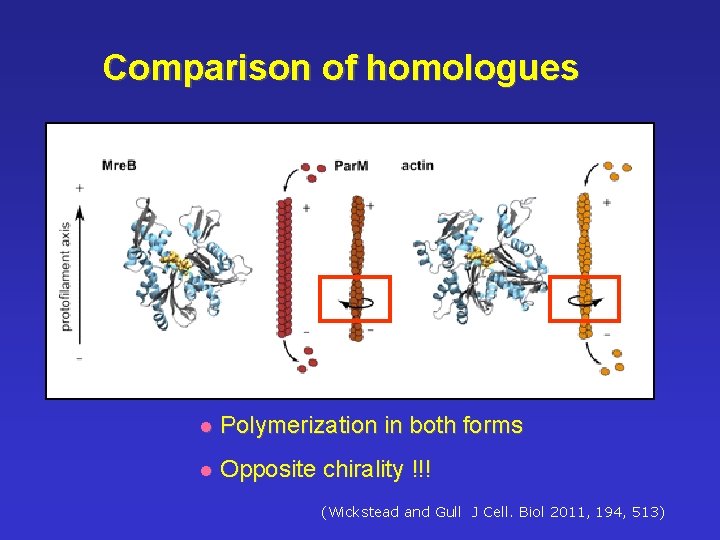
Comparison of homologues l Polymerization in both forms l Opposite chirality !!! ((Wickstead and Gull J Cell. Biol 2011, 194, 513)

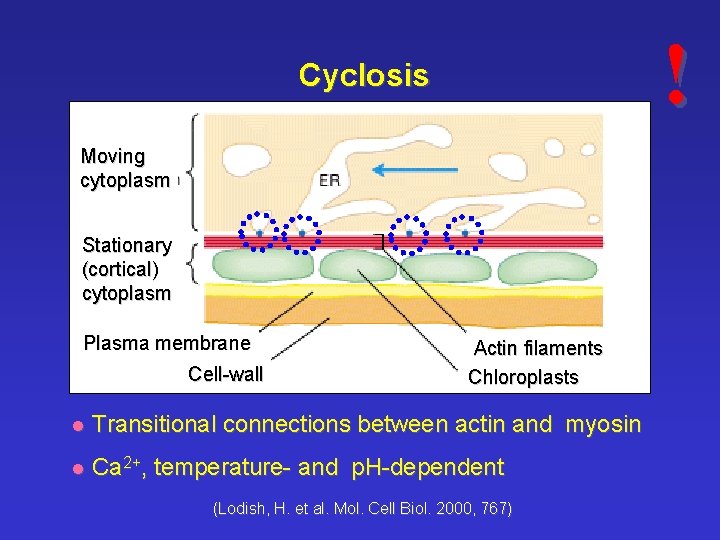
! Cyclosis Moving cytoplasm Stationary (cortical) cytoplasm Plasma membrane Cell-wall Actin filaments Chloroplasts l Transitional connections between actin and myosin l Ca 2+, temperature- and p. H-dependent (Lodish, H. et al. Mol. Cell Biol. 2000, 767)

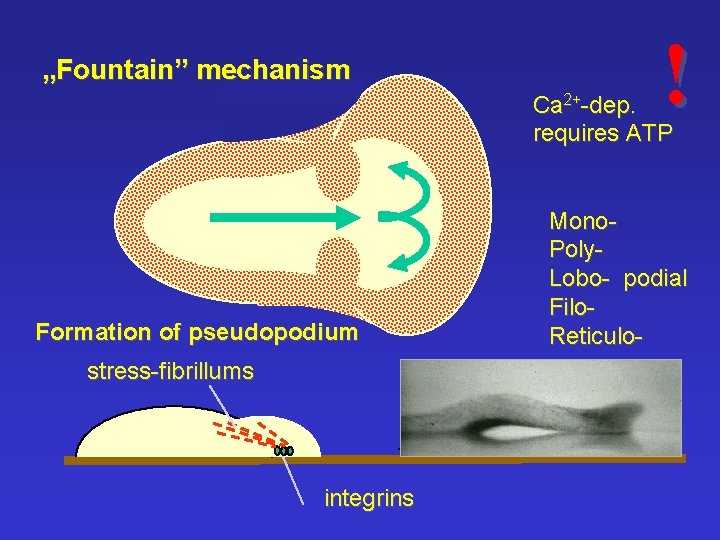
„Fountain” mechanism ! Ca 2+-dep. requires ATP Formation of pseudopodium stress-fibrillums integrins Mono. Poly. Lobo- podial Filo. Reticulo-

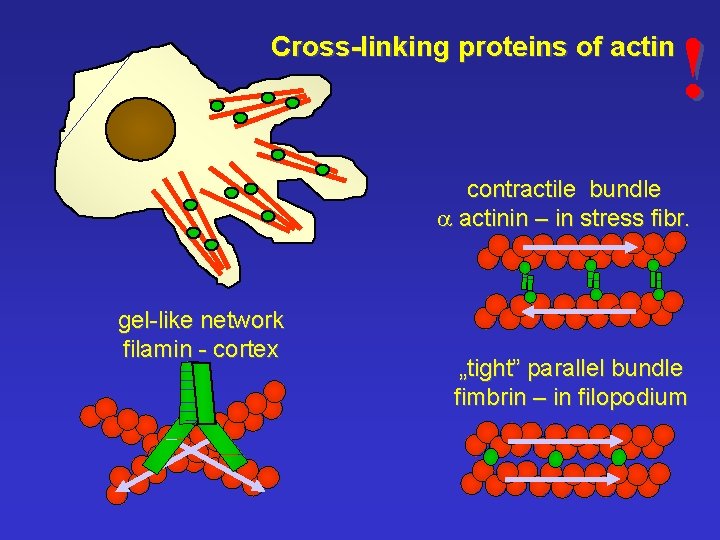
Cross-linking proteins of actin ! contractile bundle a actinin – in stress fibr. gel-like network filamin - cortex „tight” parallel bundle fimbrin – in filopodium

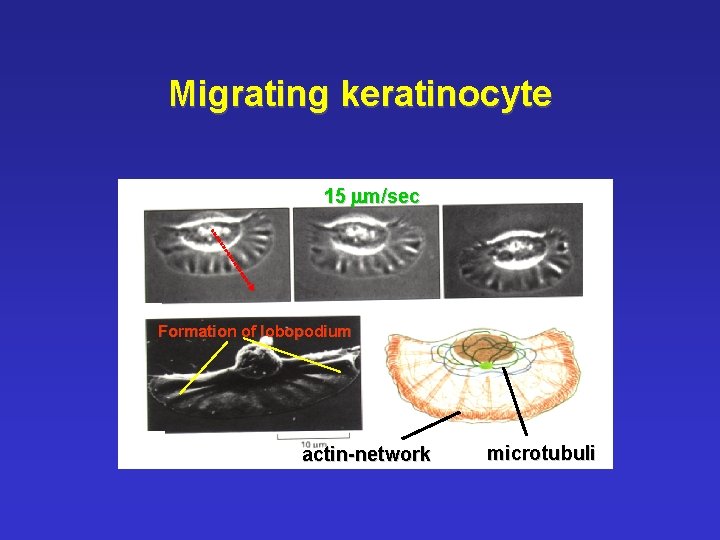
Migrating keratinocyte 15 mm/sec Formation of lobopodium actin-network microtubuli

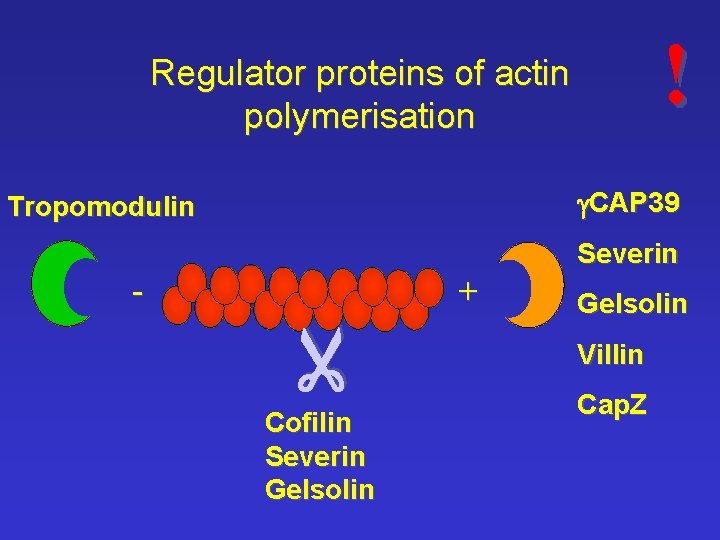
! Regulator proteins of actin polymerisation g. CAP 39 Tropomodulin + - Cofilin Severin Gelsolin Villin Cap. Z

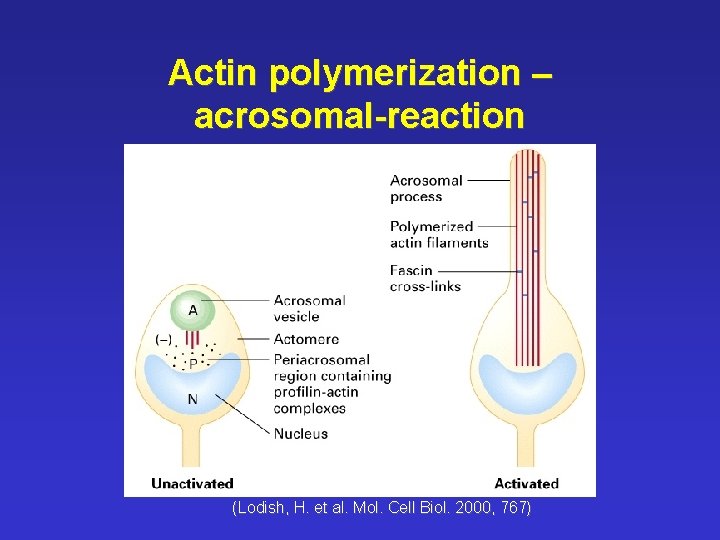
Actin polymerization – acrosomal-reaction (Lodish, H. et al. Mol. Cell Biol. 2000, 767)

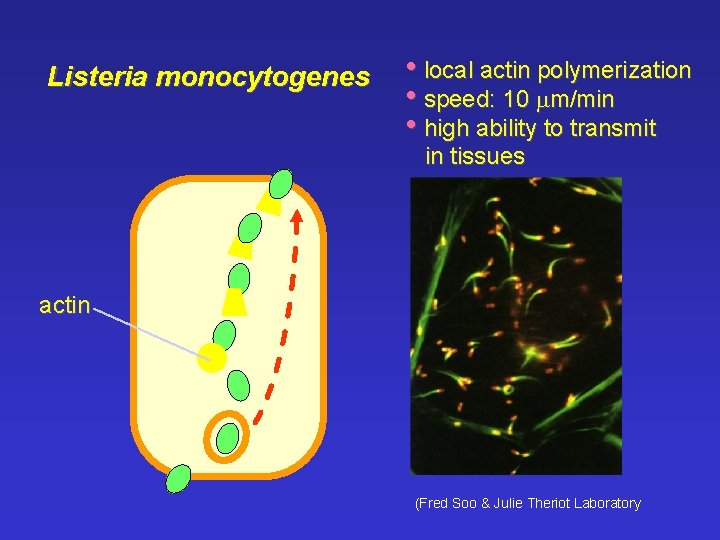
Listeria monocytogenes • local actin polymerization • speed: 10 mm/min • high ability to transmit in tissues actin (Fred Soo & Julie Theriot Laboratory

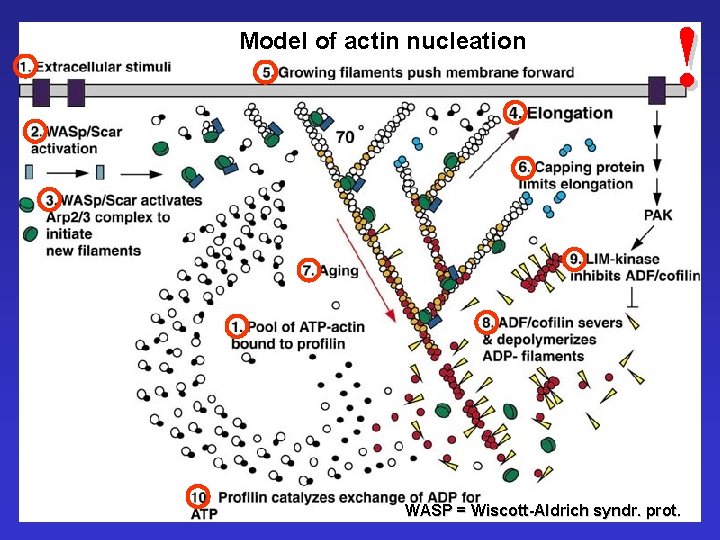
Model of actin nucleation ! WASP = Wiscott-Aldrich syndr. prot.

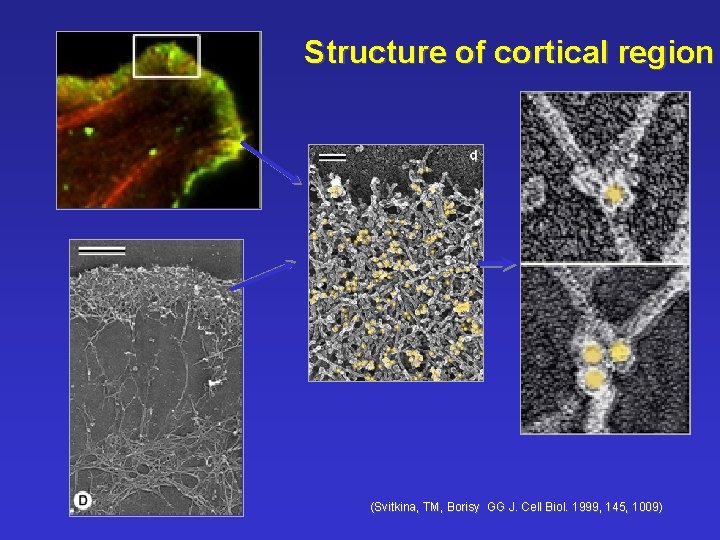
Structure of cortical region (Svitkina, TM, Borisy GG J. Cell Biol. 1999, 145, 1009)

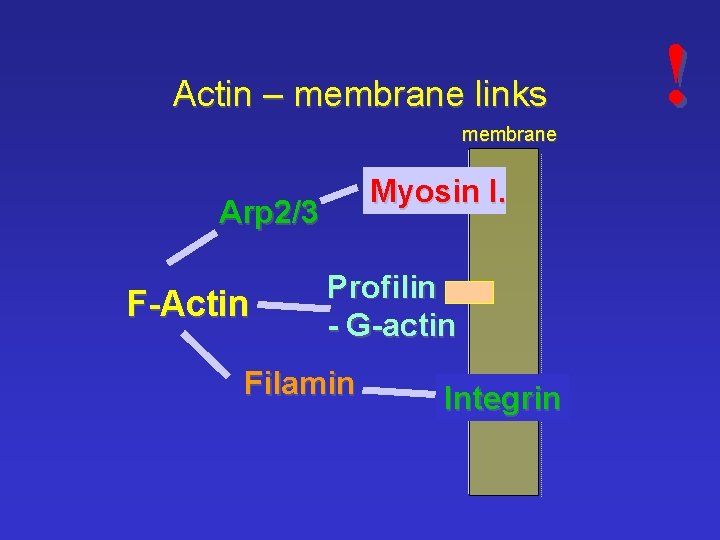
Actin – membrane links membrane Myosin I. Arp 2/3 F-Actin Profilin - G-actin Filamin Integrin !

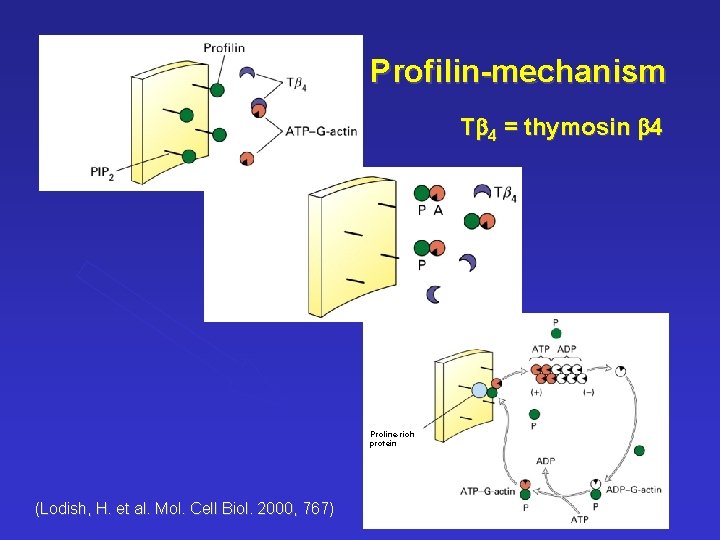
Profilin-mechanism Tb 4 = thymosin b 4 Proline-rich protein (Lodish, H. et al. Mol. Cell Biol. 2000, 767)

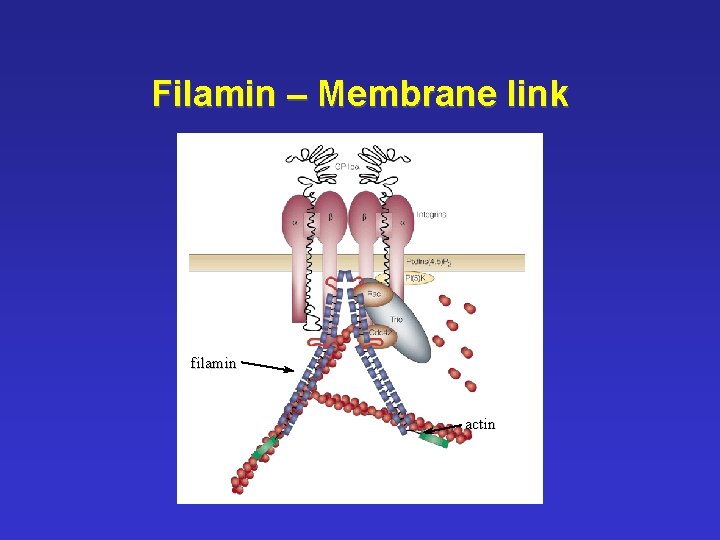
Filamin – Membrane link filamin actin

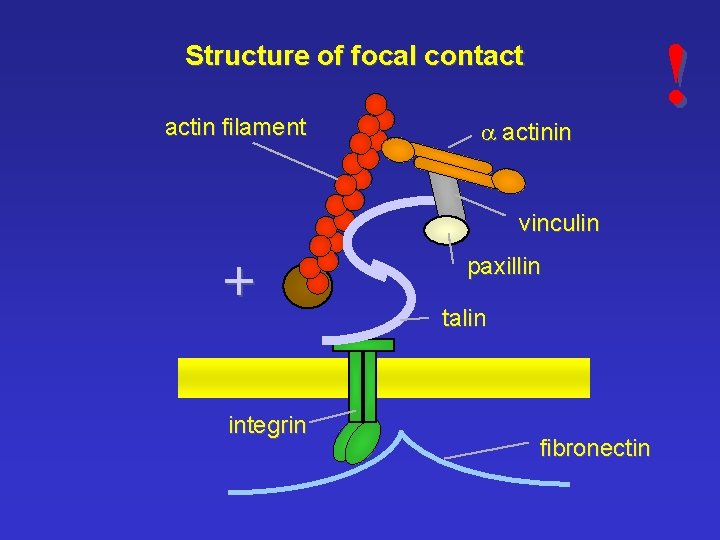
Structure of focal contact actin filament a actinin vinculin + integrin paxillin talin fibronectin !

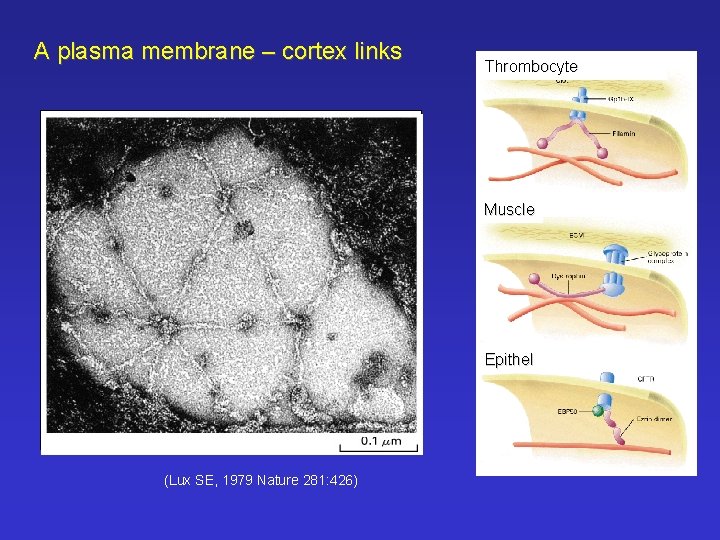
A plasma membrane – cortex links Thrombocyte Glycophorin Ankyrin Spectrin tetramer Muscle Epithel ((Lux SE, 1979 Nature 281: 426)

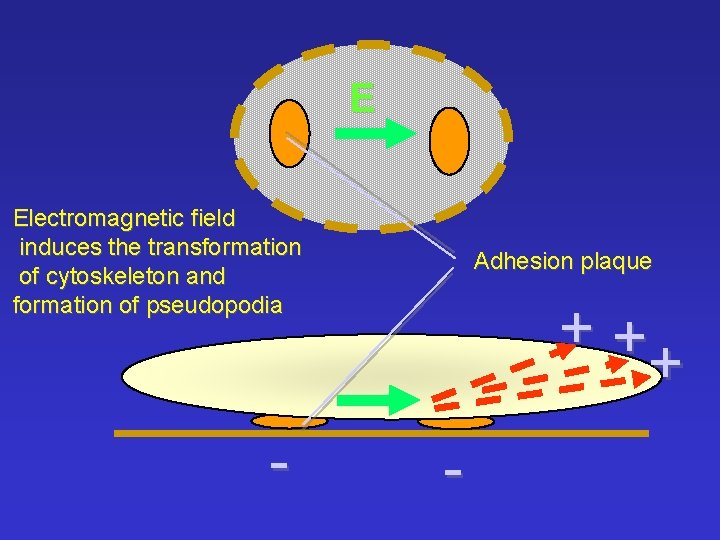
E Electromagnetic field induces the transformation of cytoskeleton and formation of pseudopodia - Adhesion plaque ++ + -

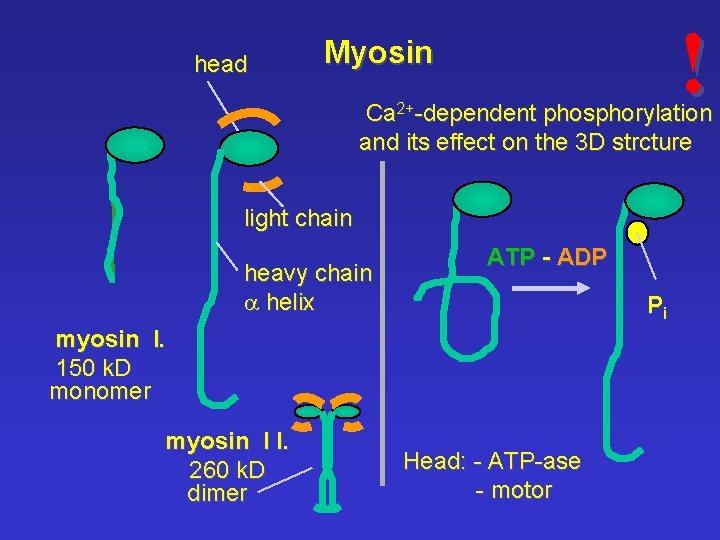
head ! Myosin Ca 2+-dependent phosphorylation and its effect on the 3 D strcture light chain heavy chain a helix ATP - ADP Pi myosin I. 150 k. D monomer myosin I I. 260 k. D dimer Head: - ATP-ase - motor

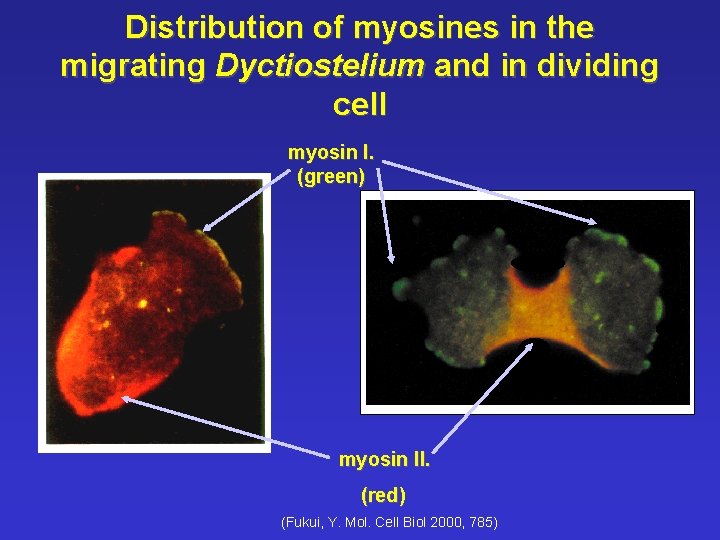
Distribution of myosines in the migrating Dyctiostelium and in dividing cell myosin I. (green) myosin II. (red) (Fukui, Y. Mol. Cell Biol 2000, 785))

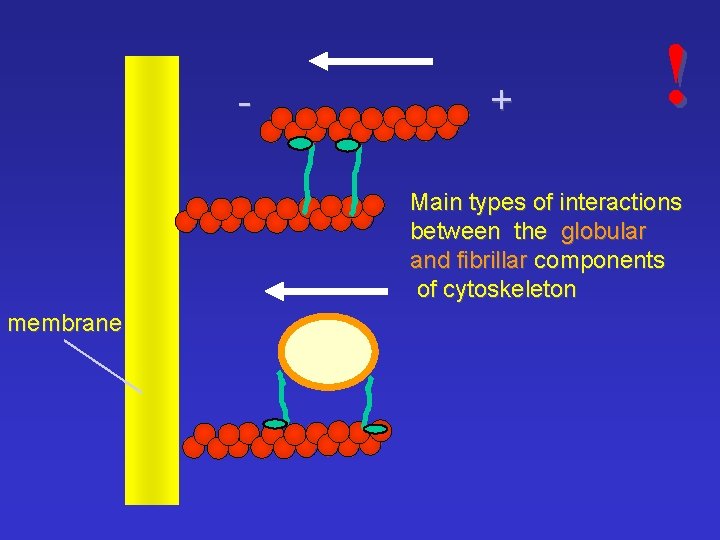
- + ! Main types of interactions between the globular and fibrillar components of cytoskeleton membrane

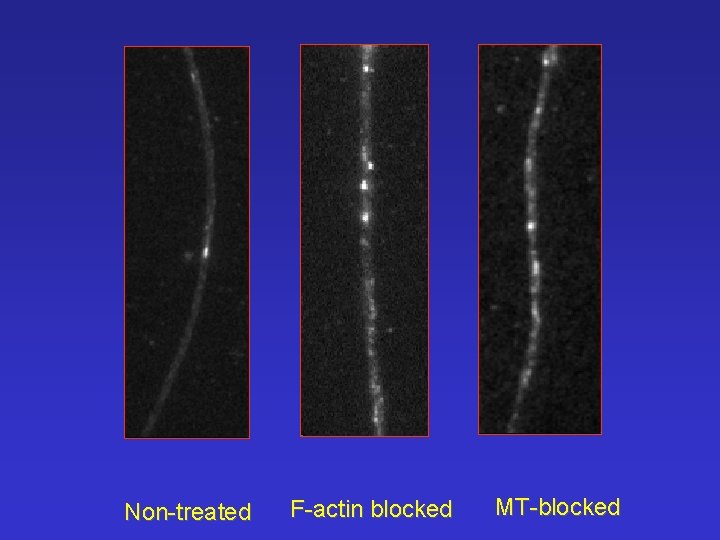
Non-treated F-actin blocked MT-blocked

Microtubules

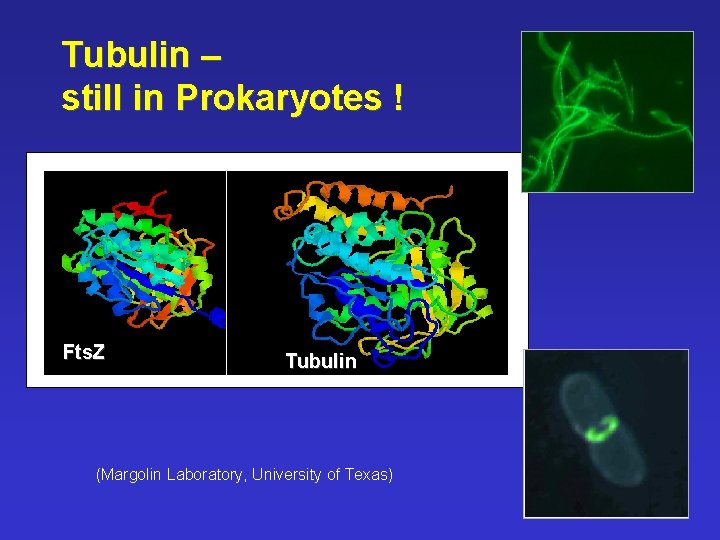
Tubulin – still in Prokaryotes ! Fts. Z Tubulin (Margolin Laboratory, University of Texas)

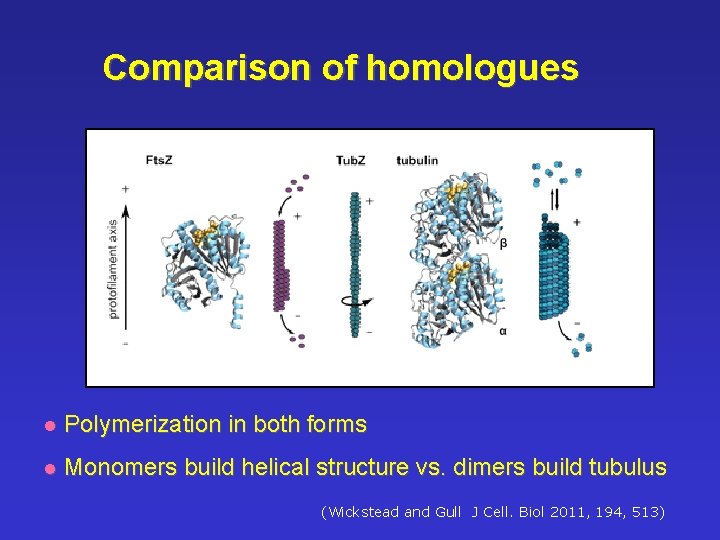
Comparison of homologues l Polymerization in both forms l Monomers build helical structure vs. dimers build tubulus ((Wickstead and Gull J Cell. Biol 2011, 194, 513)

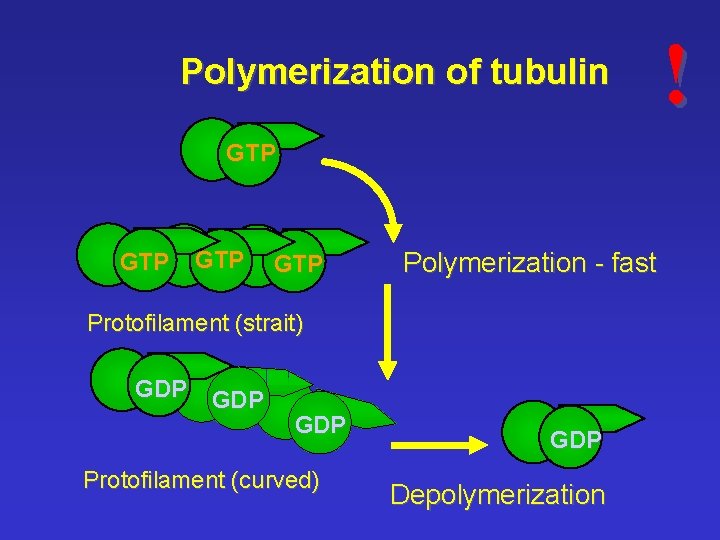
Polymerization of tubulin GTP GTP Polymerization - fast Protofilament (strait) GDP GDP Protofilament (curved) GDP Depolymerization !

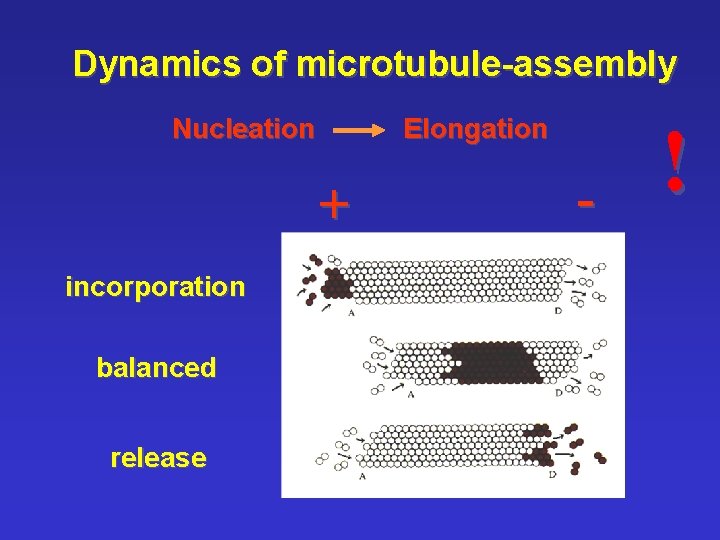
Dynamics of microtubule-assembly Nucleation Elongation + incorporation balanced release - !

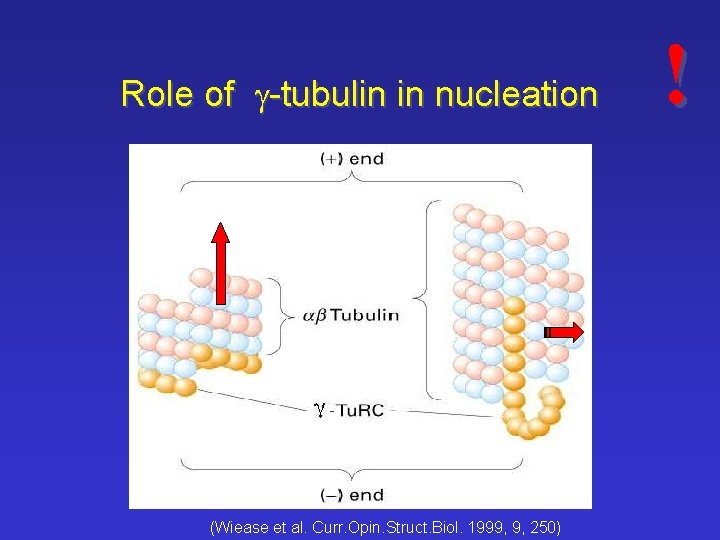
Role of g-tubulin in nucleation (Wiease et al. Curr. Opin. Struct. Biol. 1999, 9, 250) !

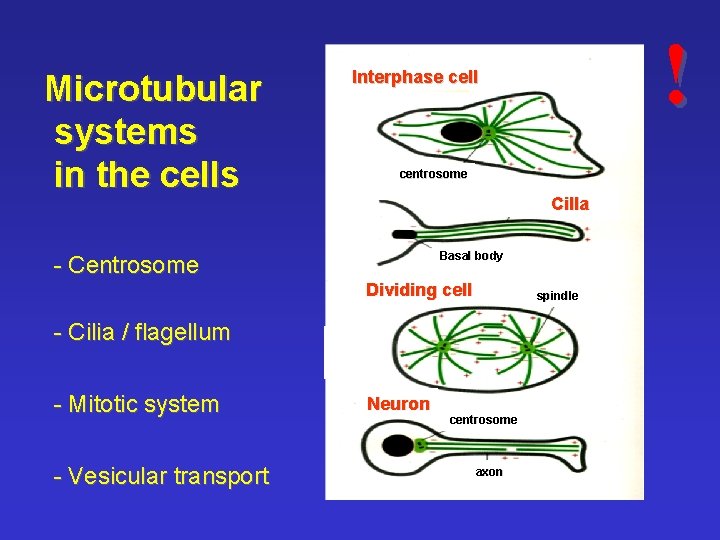
Microtubular systems in the cells centrosome Cilla Basal body - Centrosome Dividing cell spindle - Cilia / flagellum - Mitotic system - Vesicular transport ! Interphase cell Neuron centrosome axon

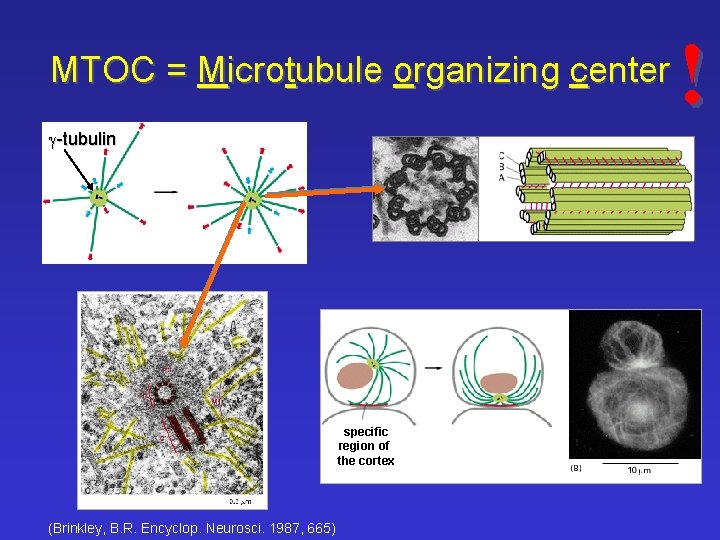
MTOC = Microtubule organizing center g-tubulin specific region of the cortex ((Brinkley, B. R. Encyclop. Neurosci. 1987, 665) !

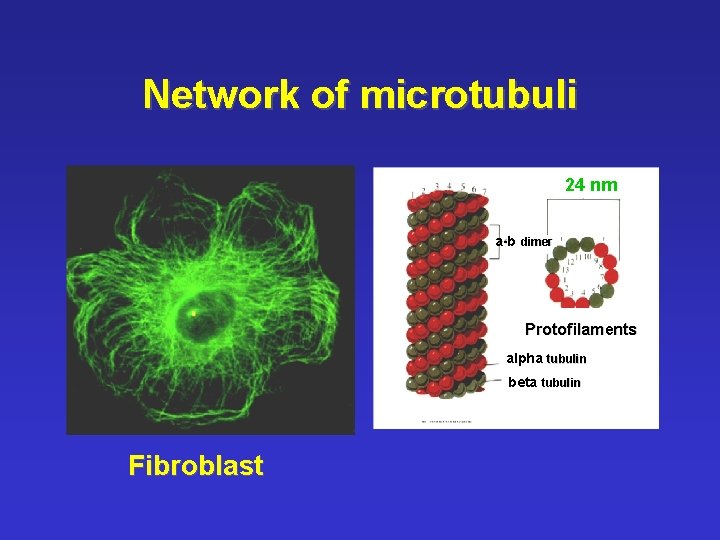
Network of microtubuli 24 nm a-b dimer Protofilaments alpha tubulin beta tubulin Fibroblast

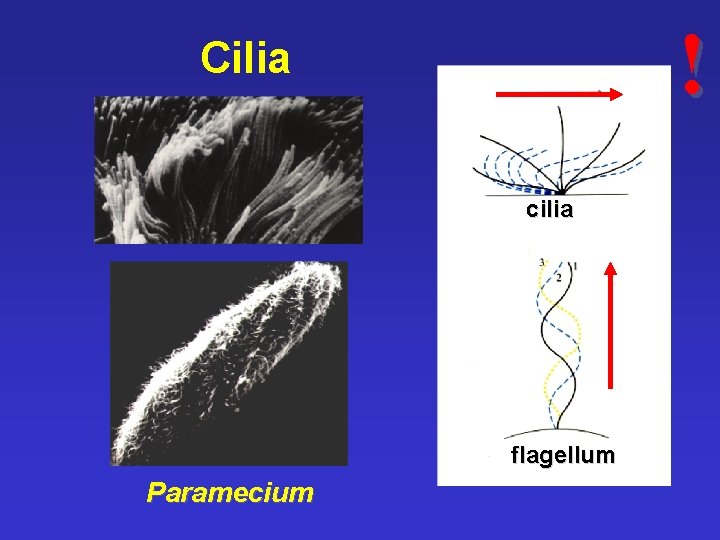
! Cilia cilia flagellum Paramecium

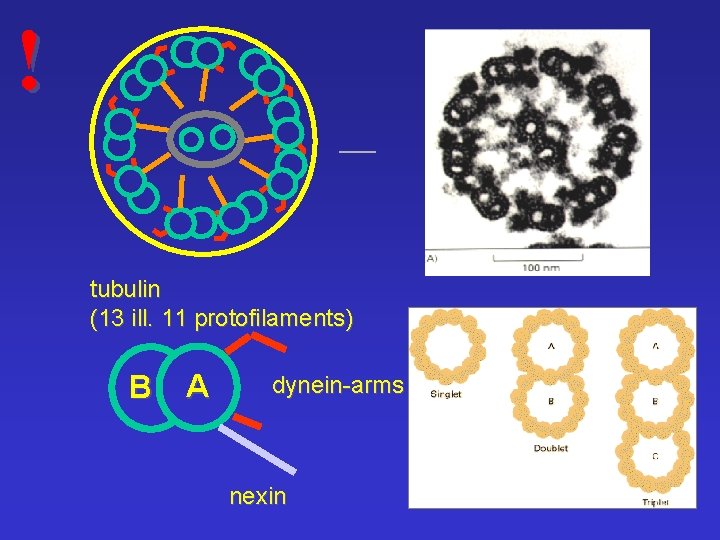
! tubulin (13 ill. 11 protofilaments) B A dynein-arms nexin

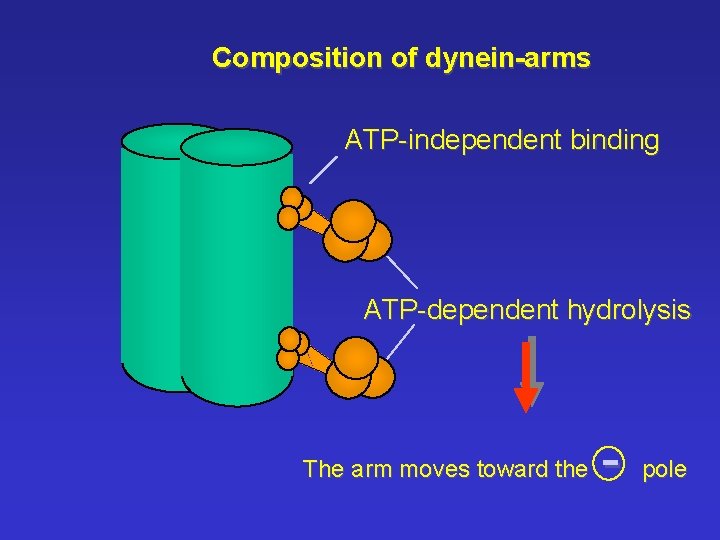
Composition of dynein-arms ATP-independent binding ATP-dependent hydrolysis The arm moves toward the - pole

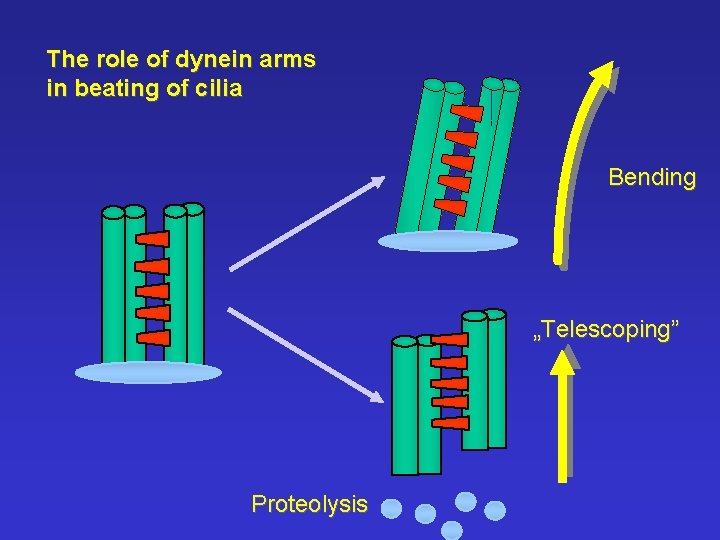
The role of dynein arms in beating of cilia Bending „Telescoping” Proteolysis

Molecules composing the cilia more than 250 types of molecules 70% a and b tubulin l dynein arms outer - 9 polypeptides - ATP-ase inner – composition varies l radial spokes - 17 polypeptides l

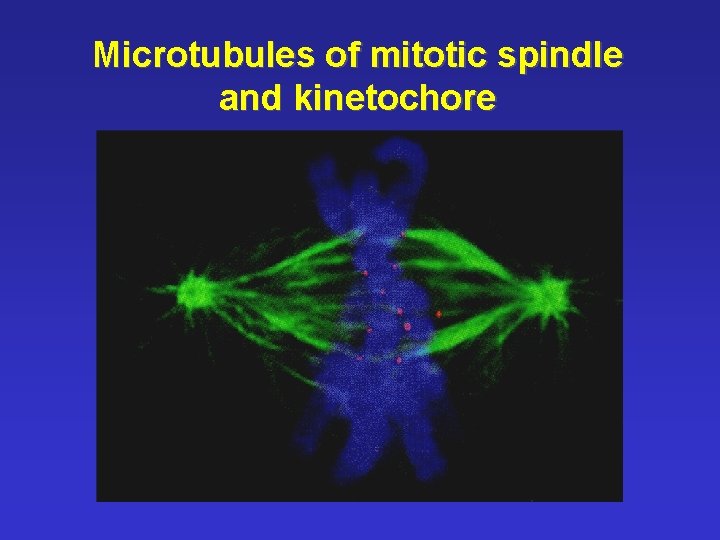
Microtubules of mitotic spindle and kinetochore

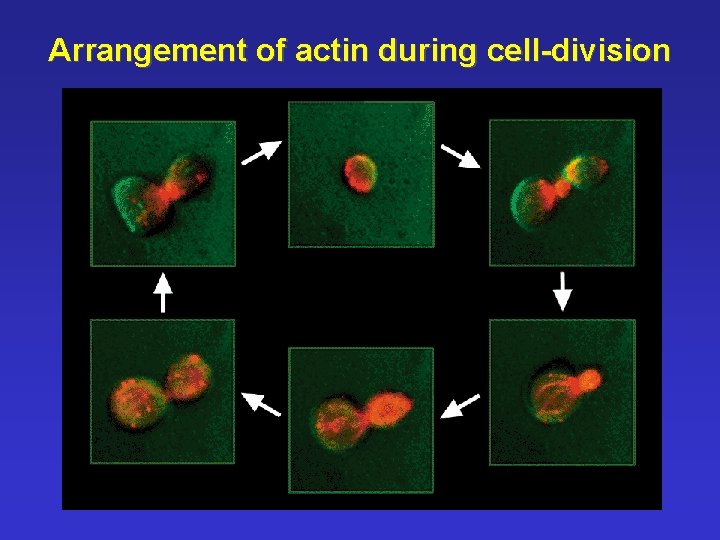
Arrangement of actin during cell-division

Intermedier filaments

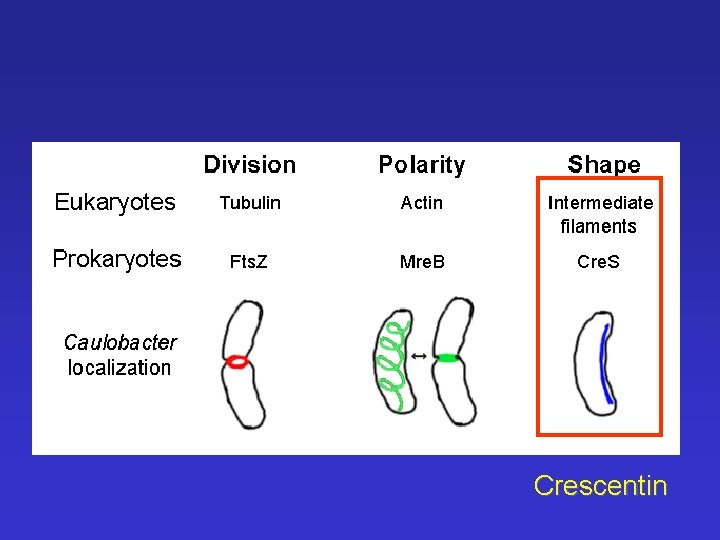
Crescentin

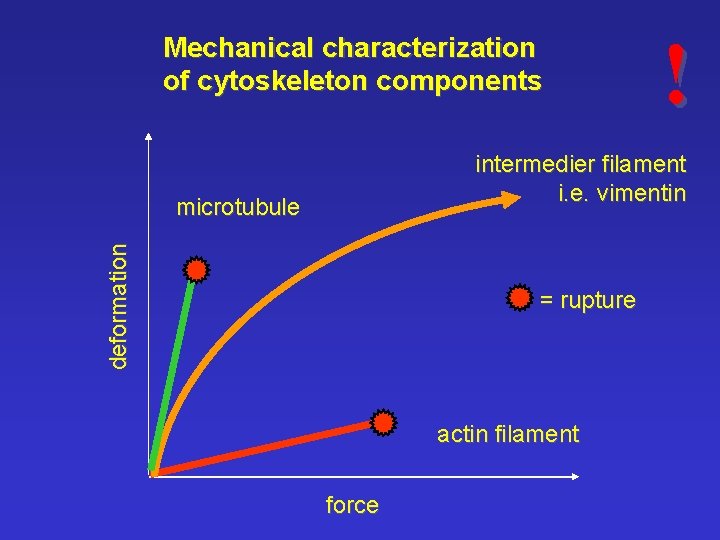
Mechanical characterization of cytoskeleton components ! intermedier filament i. e. vimentin deformation microtubule = rupture actin filament force

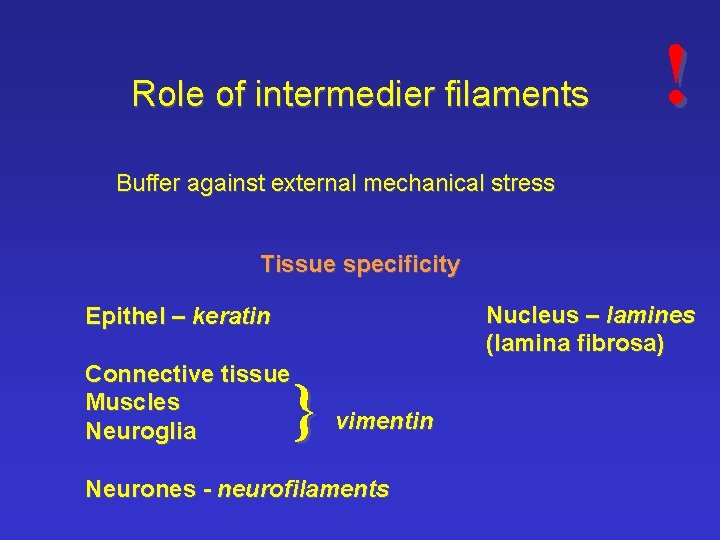
Role of intermedier filaments ! Buffer against external mechanical stress Tissue specificity Nucleus – lamines (lamina fibrosa) Epithel – keratin Connective tissue Muscles Neuroglia } vimentin Neurones - neurofilaments

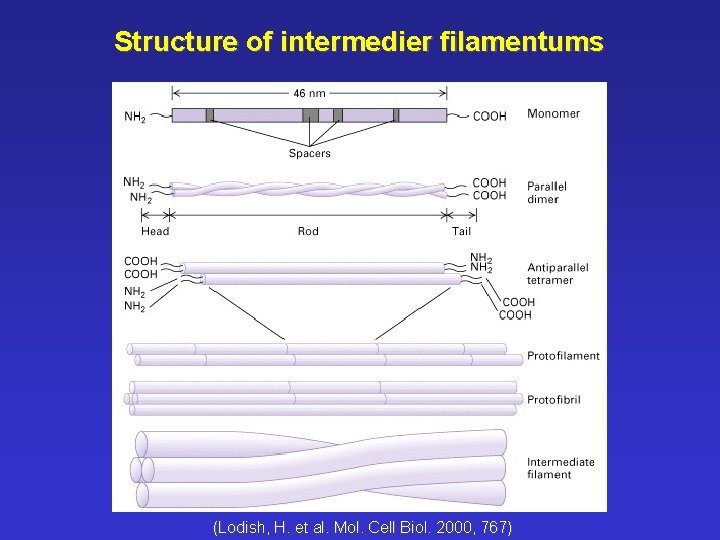
Structure of intermedier filamentums (Lodish, H. et al. Mol. Cell Biol. 2000, 767)

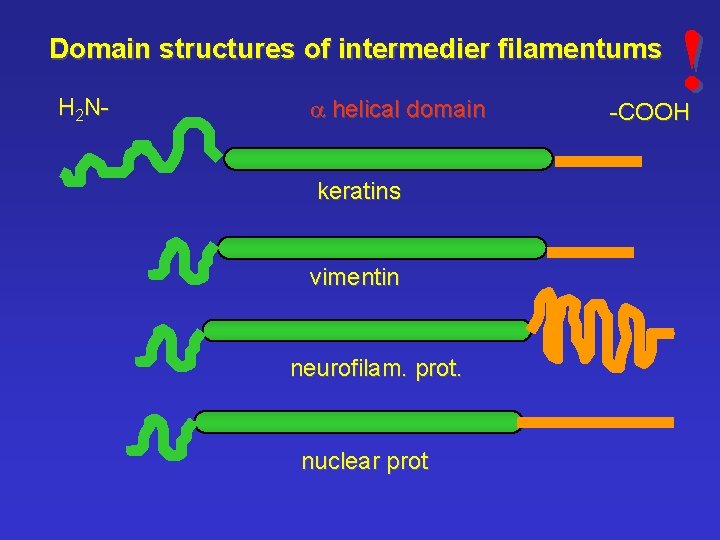
Domain structures of intermedier filamentums H 2 N- a helical domain keratins vimentin neurofilam. prot. nuclear prot ! -COOH

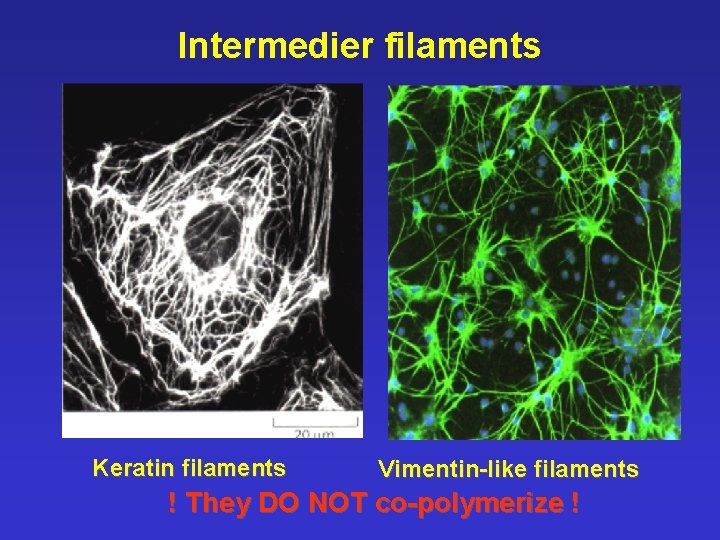
Intermedier filaments Keratin filaments Vimentin-like filaments ! They DO NOT co-polymerize !

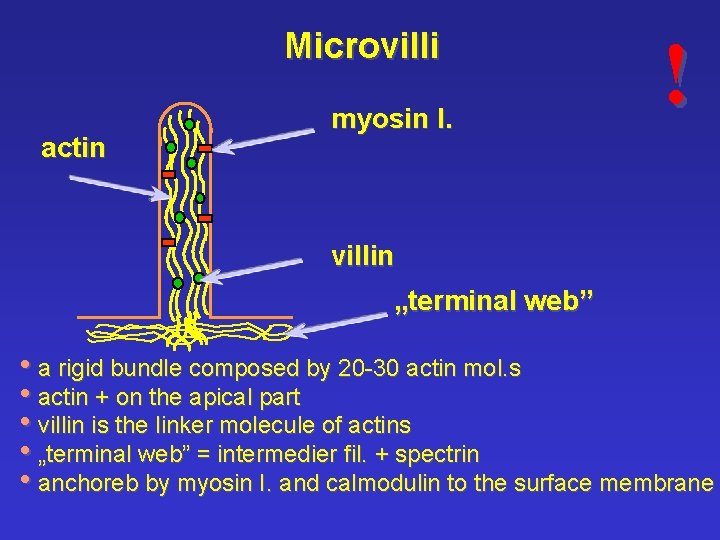
Microvilli actin myosin I. ! villin „terminal web” • a rigid bundle composed by 20 -30 actin mol. s • actin + on the apical part • villin is the linker molecule of actins • „terminal web” = intermedier fil. + spectrin • anchoreb by myosin I. and calmodulin to the surface membrane

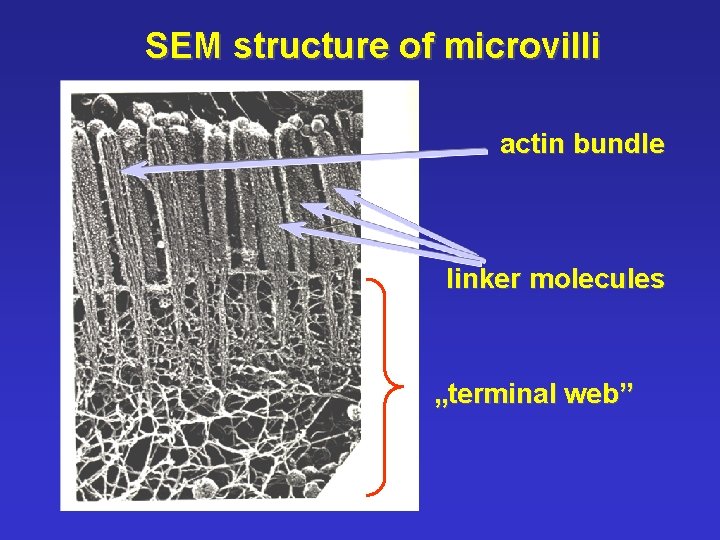
SEM structure of microvilli actin bundle linker molecules „terminal web”

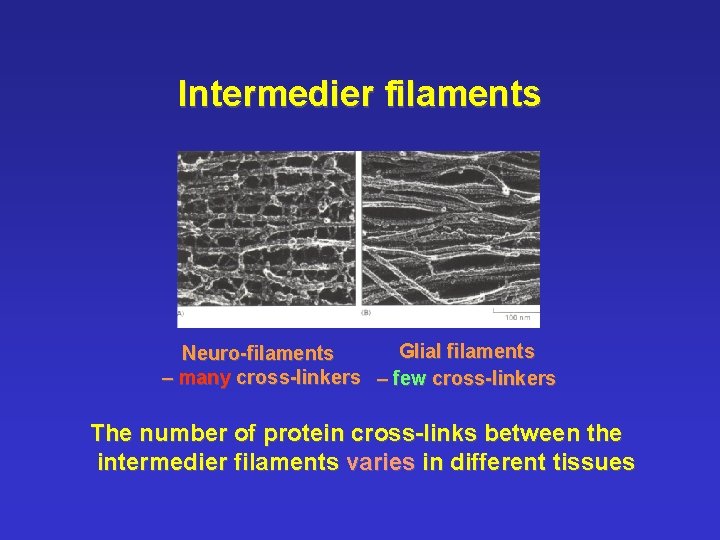
Intermedier filaments Glial filaments Neuro-filaments – many cross-linkers – few cross-linkers The number of protein cross-links between the intermedier filaments varies in different tissues

Microtubuli associated proteins (MAP-s)

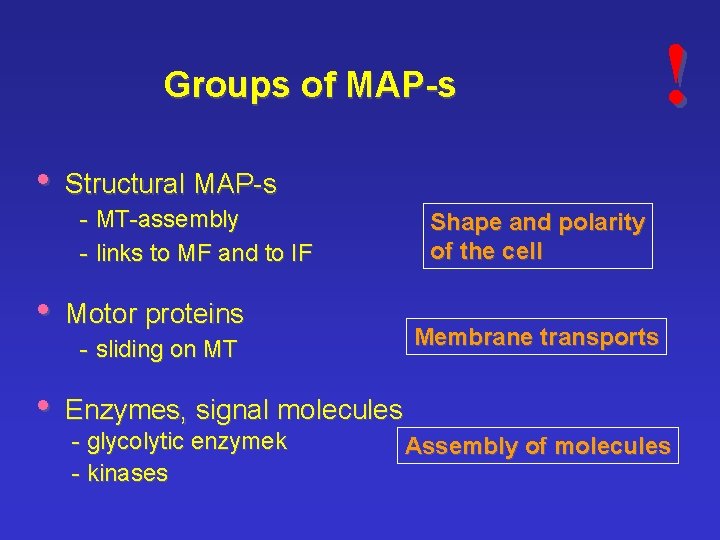
Groups of MAP-s • Structural MAP-s - MT-assembly - links to MF and to IF • Motor proteins - sliding on MT • ! Shape and polarity of the cell Membrane transports Enzymes, signal molecules - glycolytic enzymek - kinases Assembly of molecules

Motor-proteins

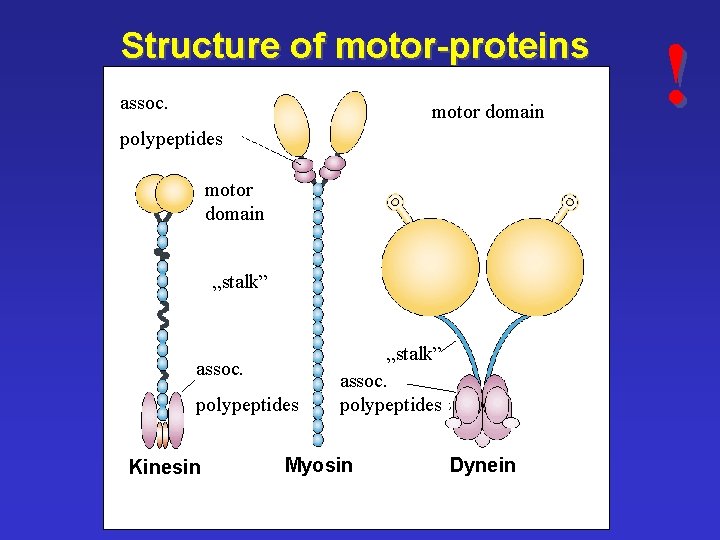
Structure of motor-proteins assoc. motor domain polypeptides motor domain „stalk” assoc. polypeptides Kinesin „stalk” assoc. polypeptides Myosin Dynein !

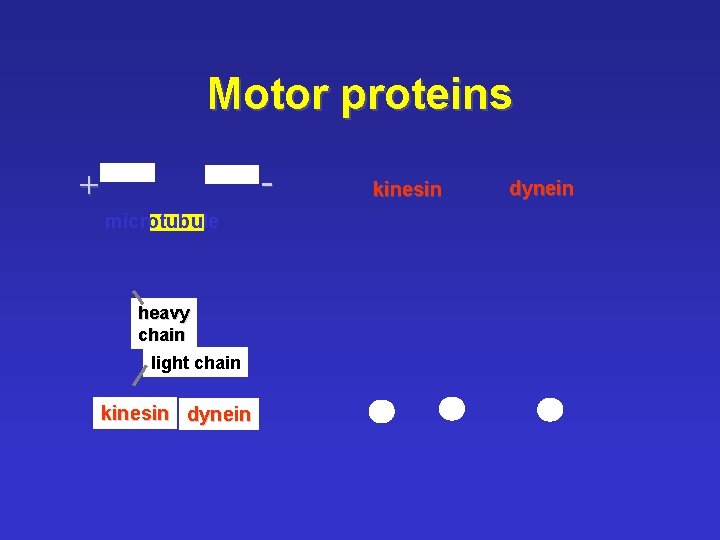
Motor proteins - + microtubule heavy chain light chain kinesin dynein

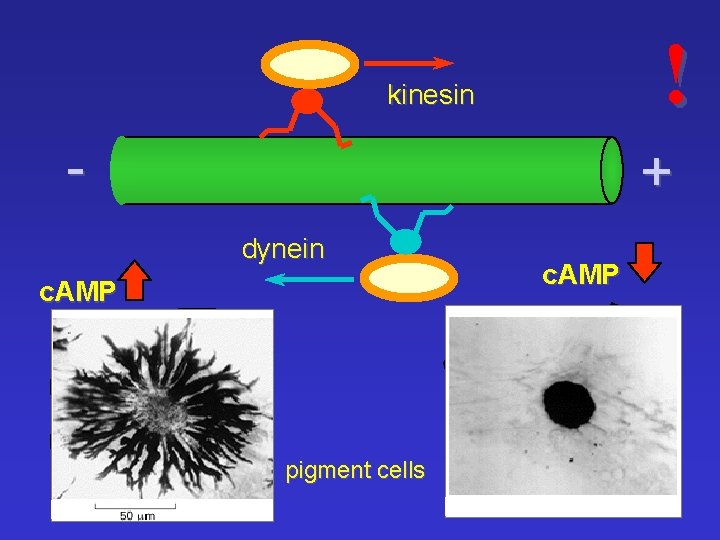
! kinesin - + dynein c. AMP pigment cells c. AMP

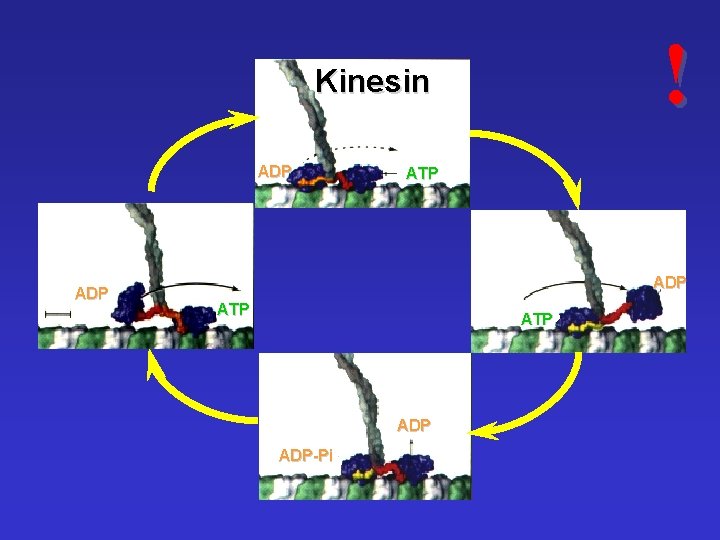
! Kinesin ADP ATP ADP-Pi

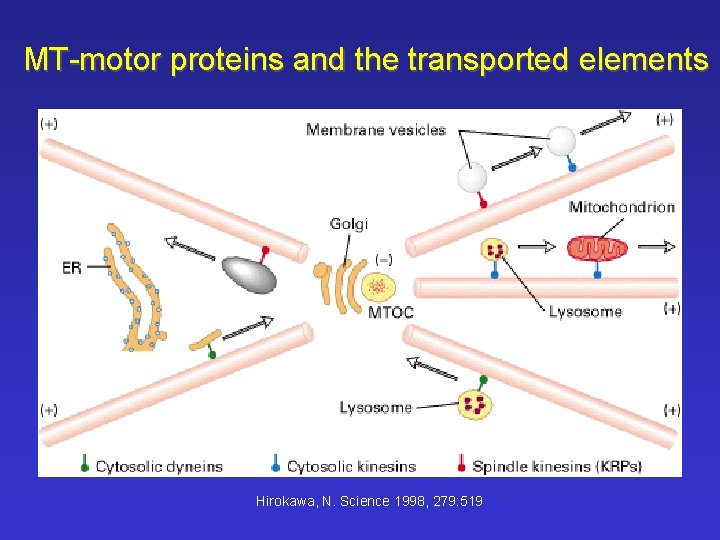
MT-motor proteins and the transported elements (Hirokawa, N. Science 1998, 279: 519

There are other mechanisms over sliding …

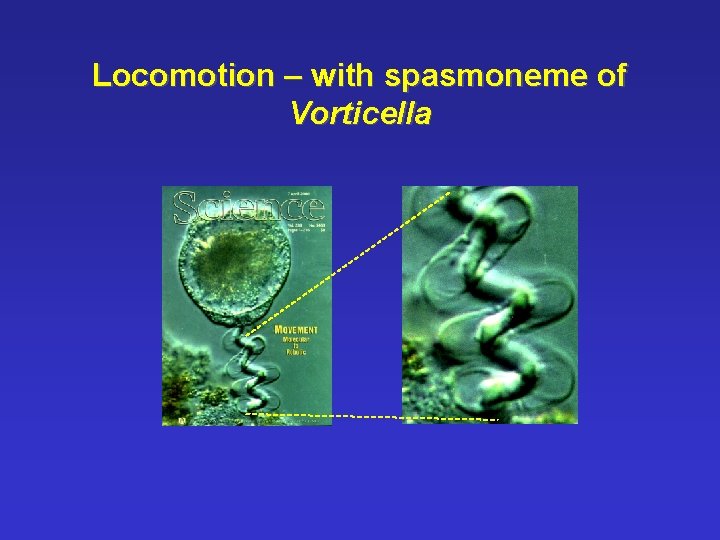
Locomotion – with spasmoneme of Vorticella

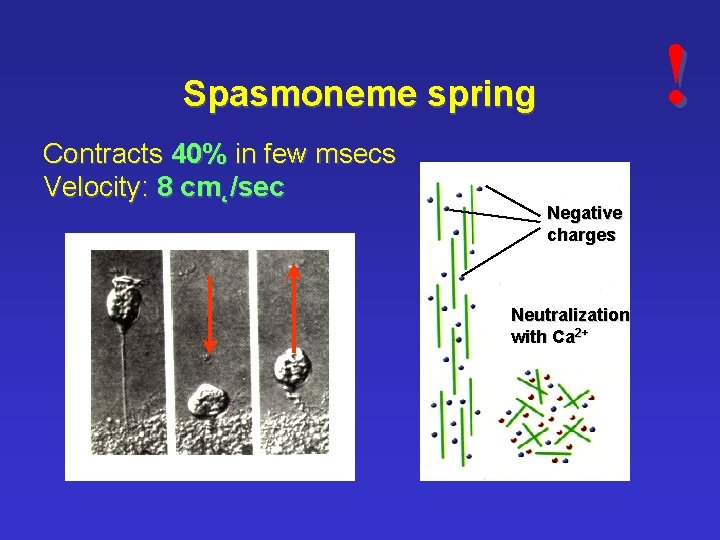
! Spasmoneme spring Contracts 40% in few msecs Velocity: 8 cm˛/sec Negative charges Neutralization with Ca 2+

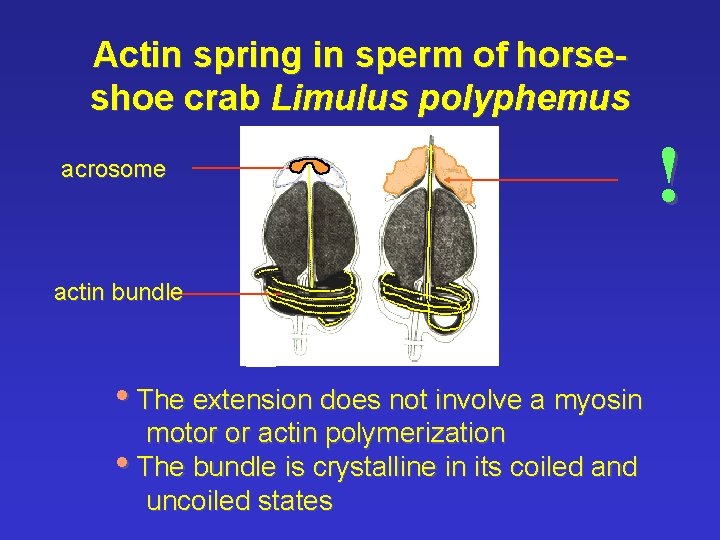
Actin spring in sperm of horseshoe crab Limulus polyphemus acrosome actin bundle • The extension does not involve a myosin motor or actin polymerization • The bundle is crystalline in its coiled and uncoiled states !
 Sekte olan sureler
Sekte olan sureler Prokaryotes and eukaryotes
Prokaryotes and eukaryotes Cytoskeleton organelles
Cytoskeleton organelles Cell analogy to a school
Cell analogy to a school Cytoskeleton components
Cytoskeleton components Chloroplast function
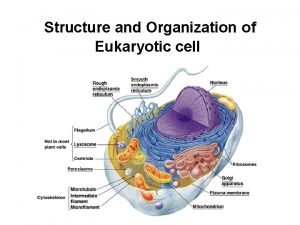
Chloroplast function Eukaryotic cell
Eukaryotic cell Cytoskeleton look like
Cytoskeleton look like Microtubules microfilaments and intermediate filaments
Microtubules microfilaments and intermediate filaments Cilia analogy
Cilia analogy Cytoskeleton
Cytoskeleton Endomembrane system
Endomembrane system Cytoskeleton
Cytoskeleton Cytoskeleton prokaryotic or eukaryotic
Cytoskeleton prokaryotic or eukaryotic 3 cytoskeletal elements
3 cytoskeletal elements Treadmilling microtubules
Treadmilling microtubules Plastoquinine
Plastoquinine Mikrotubule
Mikrotubule Differential drive
Differential drive Reptiles organs for locomotion
Reptiles organs for locomotion Characteristics of phylum apicomplexa
Characteristics of phylum apicomplexa Vorticella locomotion
Vorticella locomotion Locomotion and support
Locomotion and support Flexible muscle-based locomotion for bipedal creatures
Flexible muscle-based locomotion for bipedal creatures Phylum porifera locomotion
Phylum porifera locomotion Physics of swimming
Physics of swimming What is an axial movement
What is an axial movement Locomotion book summary
Locomotion book summary Zygnema locomotion
Zygnema locomotion Locomotion
Locomotion Reptiles organs for locomotion
Reptiles organs for locomotion Parapodia and setae
Parapodia and setae Scombridae
Scombridae Tarsier locomotion
Tarsier locomotion Amiiform
Amiiform Eukaryotic cells
Eukaryotic cells Reptiles organs for locomotion
Reptiles organs for locomotion Protection support and locomotion answer key
Protection support and locomotion answer key Volvox locomotion
Volvox locomotion Eubacteria pictures
Eubacteria pictures Locomotion and support
Locomotion and support Reptiles organs for locomotion
Reptiles organs for locomotion Dr poór lászló
Dr poór lászló Terméskén
Terméskén Mrts
Mrts Laszlo systems
Laszlo systems Jeno laszlo vargha
Jeno laszlo vargha Dr. friedrich lászló
Dr. friedrich lászló Nav felsővezető
Nav felsővezető Dr balla lászló
Dr balla lászló Dr halvax lászló
Dr halvax lászló Huzsvai lászló statisztika
Huzsvai lászló statisztika Art humeroradialis
Art humeroradialis Dr kohut lászló
Dr kohut lászló Laszlo zsolnai
Laszlo zsolnai Kopcsay lászló
Kopcsay lászló Huzsvai lászló statisztika
Huzsvai lászló statisztika Skin tunneled catheter
Skin tunneled catheter Méhes lászló festő
Méhes lászló festő Papanek lászló
Papanek lászló Graphs and geometry lászló lovász
Graphs and geometry lászló lovász Fehér pedagógia
Fehér pedagógia Bruckner lászló
Bruckner lászló Berek lászló
Berek lászló Kohut lászló
Kohut lászló Huzsvai lászló
Huzsvai lászló Kajdocsi lászló
Kajdocsi lászló Pitlik
Pitlik