Chemistry Jeopardy Molar Relationships Phases of Matter Atomic




















- Slides: 50

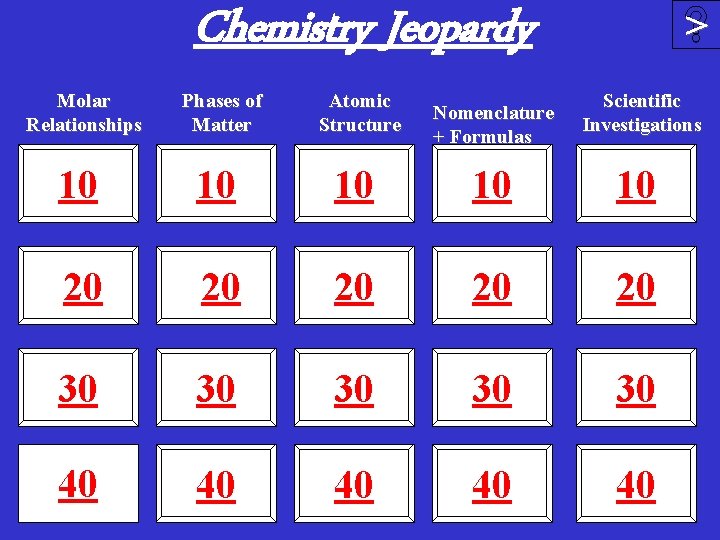
Chemistry Jeopardy Molar Relationships Phases of Matter Atomic Structure Nomenclature + Formulas > Scientific Investigations 10 10 10 20 20 20 30 30 30 40 40 40

How many grams of sodium chloride are required to prepare 500. 0 m. L of a 0. 100 M solution? What is 2. 93 g?

How many grams of product Z will be formed if 12. 0 g of W react with 10. 0 g of X to form 8. 0 g of product Y in the reaction shown? What is 14. 0 g?

Daily Double

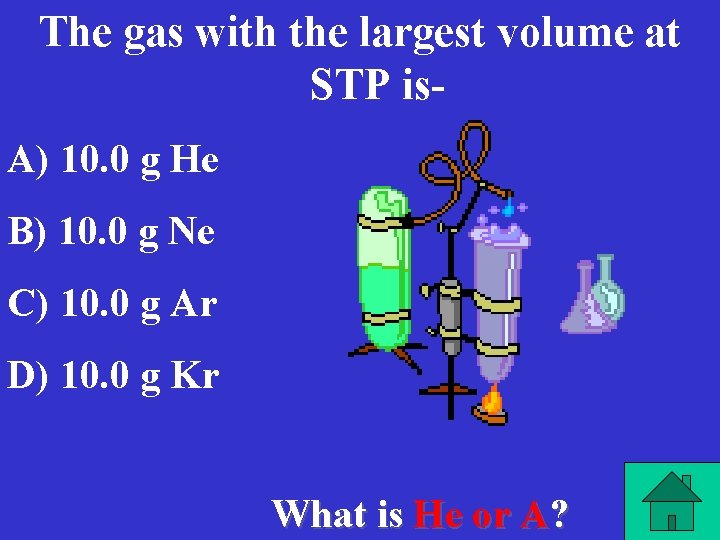
The gas with the largest volume at STP is. A) 10. 0 g He B) 10. 0 g Ne C) 10. 0 g Ar D) 10. 0 g Kr What is He or A?

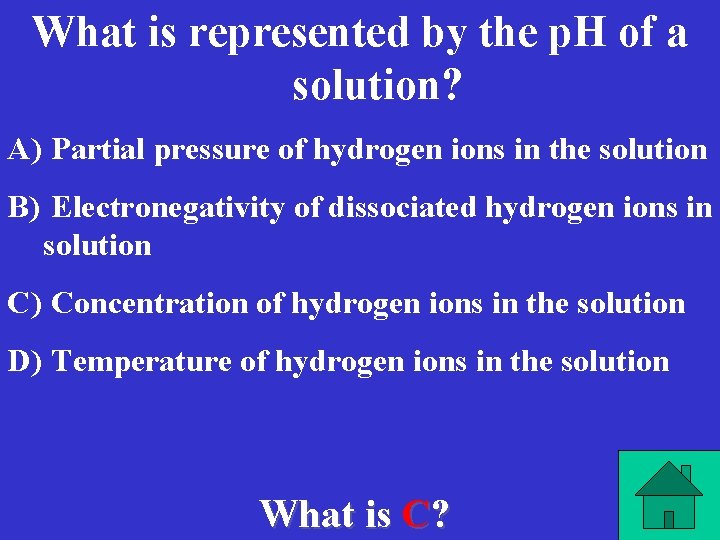
What is represented by the p. H of a solution? A) Partial pressure of hydrogen ions in the solution B) Electronegativity of dissociated hydrogen ions in solution C) Concentration of hydrogen ions in the solution D) Temperature of hydrogen ions in the solution What is C?

Which of the following could cause a gaseous substances to liquify? A) An increase in pressure B) An increase in volume C) An increase in temperature D) A decrease in number of moles What is A?

According to Charles’ law, the volume of a fixed amount of gas is directly proportional to. A) isoelectric mixture B) vapor concentration C) barometric pressure D) kelvin temperature What is D?

The energy required to melt a solid into a liquid is called. A) heat of vaporization B) heat of fusion C) cooling curve D) triple point What is B?

If a deep-sea diver’s tank is filled with a helium-oxygen mixture to a pressure of 170 atmospheres and the partial pressure of helium is 110 atmospheres, the partial pressure of oxygen is- A) 60 atm B) 110 atm C) 140 atm D) 280 atm What is 60 atm or A?

Daily Double

What is the main similarity among elements in group 2? A) Atomic radius B) Chemical properties C) Mass number D) Boiling point What is B?

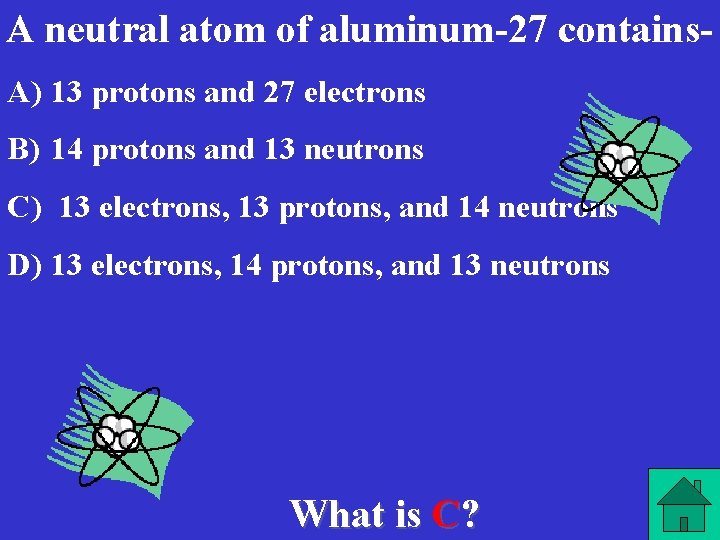
A neutral atom of aluminum-27 contains. A) 13 protons and 27 electrons B) 14 protons and 13 neutrons C) 13 electrons, 13 protons, and 14 neutrons D) 13 electrons, 14 protons, and 13 neutrons What is C?

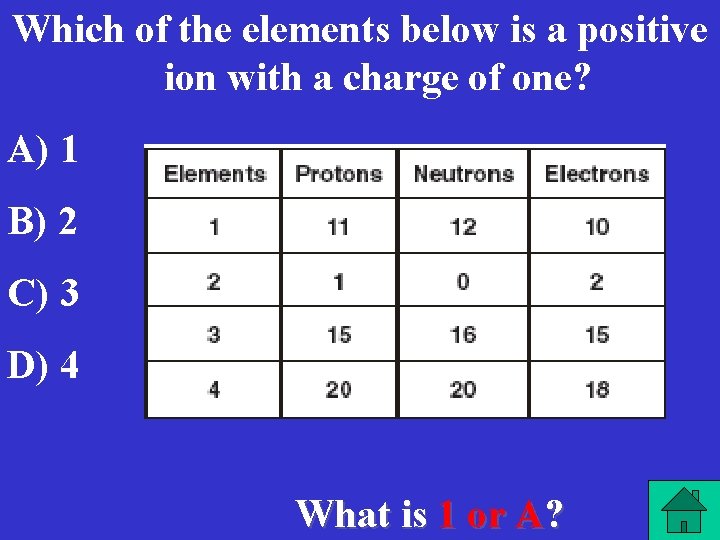
Which of the elements below is a positive ion with a charge of one? A) 1 B) 2 C) 3 D) 4 What is 1 or A?

Cations are formed when neutral atoms lose- A)electrons B) protons C) neutrons D) positrons What is A?

Which of these is the general formula for a double-replacement reaction? A) A+B→AB B) AB+XY→BA+YX C) AB+XY→AY+XB D) A+B+XY→AX+BY What is C?

The correct formula of an ionic compound containing Al 3+ and CO 32 - is- A) Al. CO 3 B) Al(CO 3)3 C) Al 2(CO 3)3 D) Al 3(CO 3)2 What is C?

Which of these is the balanced chemical equation for the reaction shown above? A) Al+H 2 SO 4→Al 2(SO 4)3+H 2 B) 2 Al+3 H 2 SO 4→Al 2(SO 4)3+3 H 2 C) 2 Al+3 H 2 SO 4→Al 2(SO 4)3+H 2 D) 2 Al+H 2 SO 4→Al 2(SO 4)3+H 2 What is B?

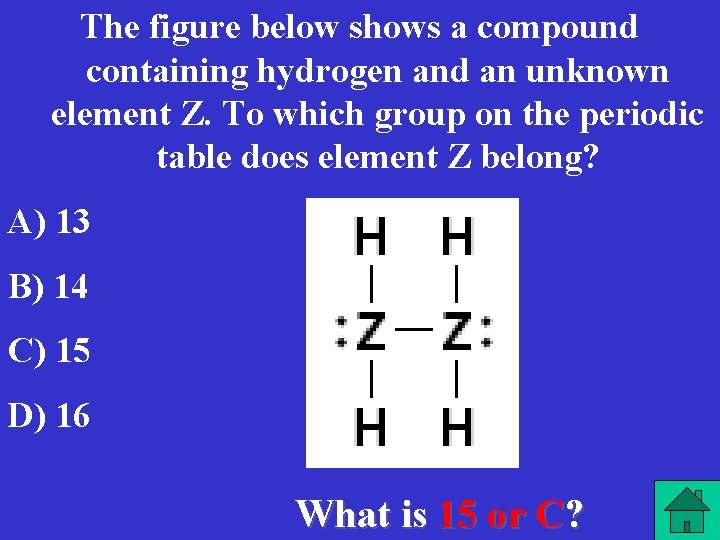
The figure below shows a compound containing hydrogen and an unknown element Z. To which group on the periodic table does element Z belong? A) 13 B) 14 C) 15 D) 16 What is 15 or C?

If a student needed to obtain 8. 0 m. L of a liquid for an experiment, the appropriate piece of laboratory equipment to use would be a- A) 50 m. L beaker B) 1. 0 m. L pipet C) 50 m. L flask D) 10. 0 m. L graduated cylinder What is D?

Which of the following best describes why an experiment should be repeated? A) To organize the data B) To produce a variety of results C) To include another variable D) To verify the observed results What is D?

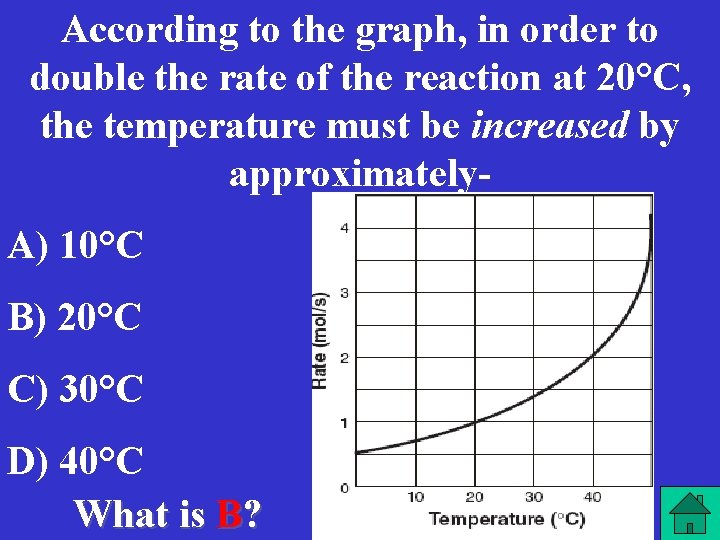
According to the graph, in order to double the rate of the reaction at 20°C, the temperature must be increased by approximately. A) 10°C B) 20°C C) 30°C D) 40°C What is B?

Which basic lab technique involves the separation of a mixture’s components through differences in particle size? A) Filtration B) Extraction C) Distillation D) Crystallization What is A?

Double Chem. Jeopardy More Molar Fun! Changes Matter! The correct Formula Who Knows? Beats Me 20 20 20 40 40 40 60 60 60 80 80 80

How many grams of nitrogen are present in 2 moles of HNO 3? A) 1 B) 2 C) 14 D) 28 What is 28 or D?

How many moles of copper are equivalent to 3. 44 X 1023 atoms of copper? A) 0. 571 moles B) 1. 75 moles C) 5. 41 X 1021 moles D) 5. 71 X 1022 moles What is 0. 571 moles or A?

Daily Double

What is the molar mass of Al(NO 3)3? A) 57 g/mol B) 103 g/mol C) 165 g/mol D) 213 g/mol What is 213 g/mol or D?

What is the mass in grams of one mole of sulfur dioxide (SO 2)? A) 48. 1 g B) 64. 1 g C) 80. 1 g D) 96. 1 g What is 64. 1 g or B?

A sample of nitrogen occupies 10. 0 liters at 25°C and 98. 7 k. Pa. What would be the volume at 20°C and 102. 7 k. Pa? A) 7. 87 L B) 9. 45 L C) 10. 2 L D) 10. 6 L What is 9. 45 L or B?

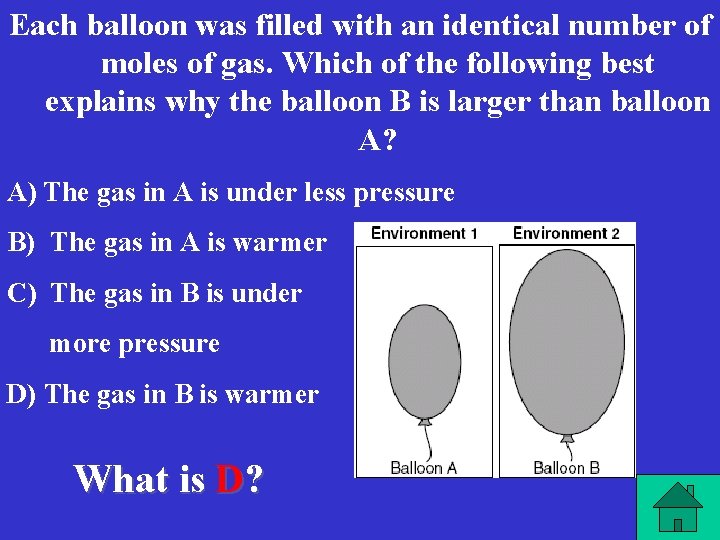
Each balloon was filled with an identical number of moles of gas. Which of the following best explains why the balloon B is larger than balloon A? A) The gas in A is under less pressure B) The gas in A is warmer C) The gas in B is under more pressure D) The gas in B is warmer What is D?

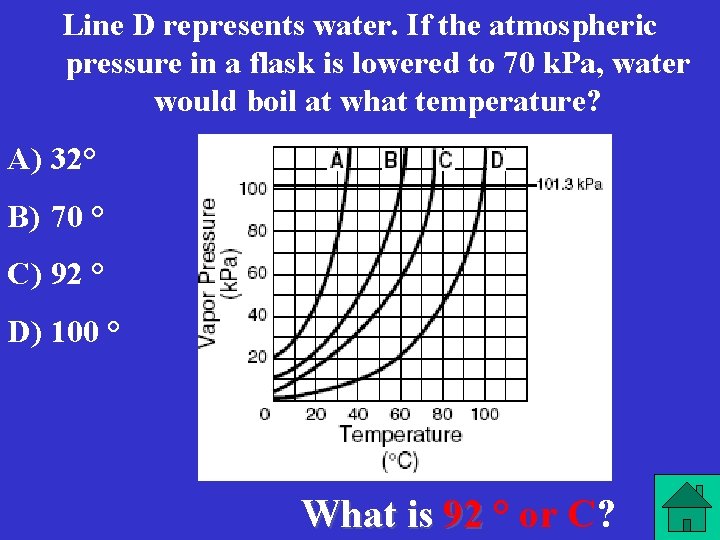
Line D represents water. If the atmospheric pressure in a flask is lowered to 70 k. Pa, water would boil at what temperature? A) 32° B) 70 ° C) 92 ° D) 100 ° What is 92 ° or C?

Solid magnesium has a specific heat of 1. 01 J/g°C. How much heat is given off by a 20. 0 gram sample of magnesium when it cools from 70. 0°C to 50°C? A) 202 J B) 404 J C) 808 J D) 1010 J What is 404 J or B?

Daily Double

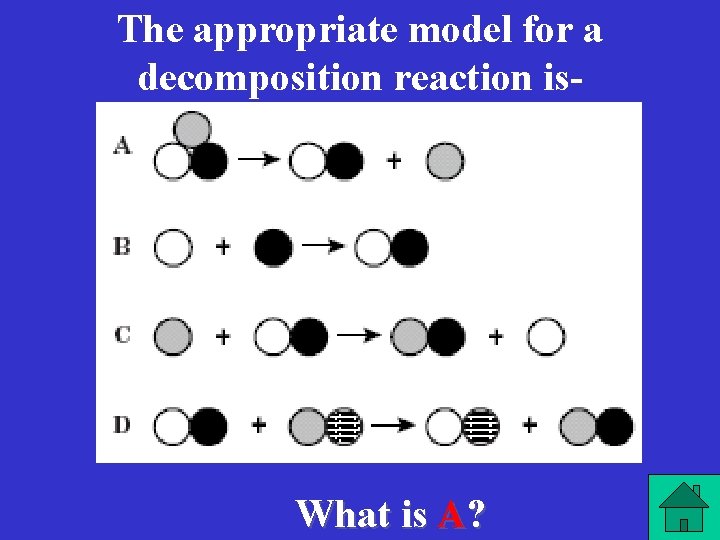
The appropriate model for a decomposition reaction is- What is A?

The formula for dinitrogen tetroxide is- A) N 2 O 4 B) N 3 O 3 C) N 2 O 2 D) NO What is A?

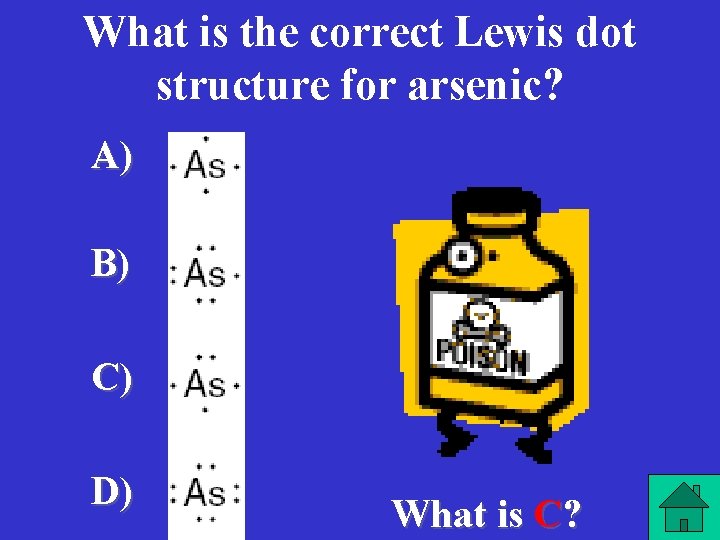
What is the correct Lewis dot structure for arsenic? A) B) C) D) What is C?

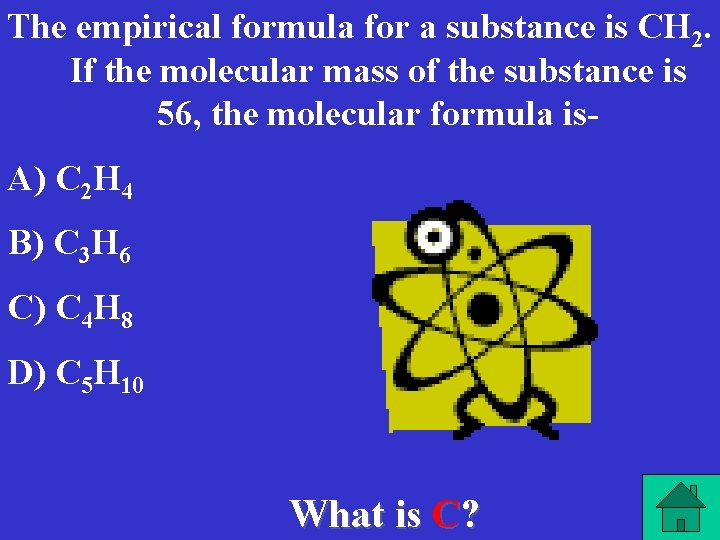
The empirical formula for a substance is CH 2. If the molecular mass of the substance is 56, the molecular formula is- A) C 2 H 4 B) C 3 H 6 C) C 4 H 8 D) C 5 H 10 What is C?

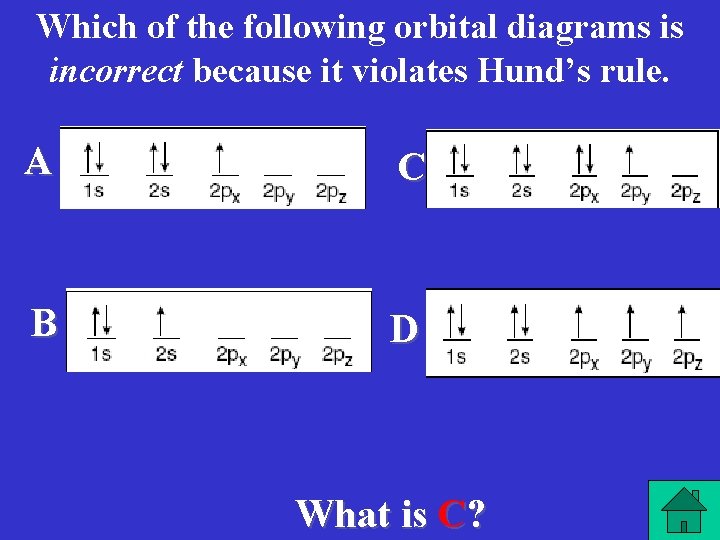
Which of the following orbital diagrams is incorrect because it violates Hund’s rule. A C B D What is C?

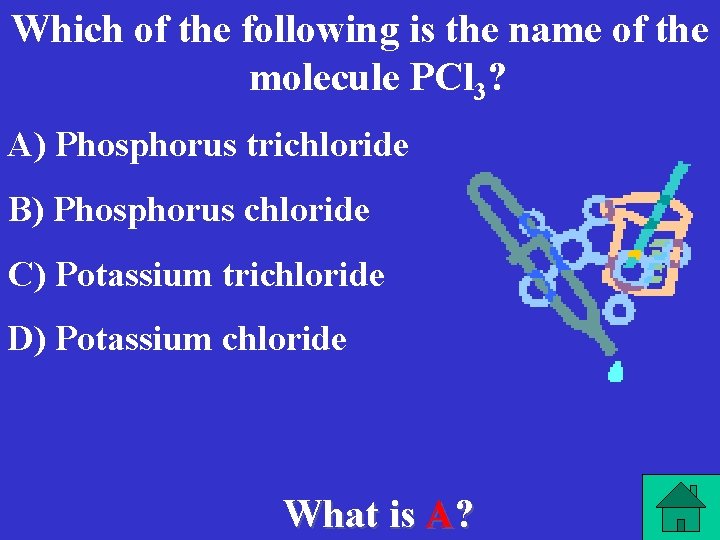
Which of the following is the name of the molecule PCl 3? A) Phosphorus trichloride B) Phosphorus chloride C) Potassium trichloride D) Potassium chloride What is A?

Which of the following properties decreases from left to right across a period? A) Atomic number B) Electronegativity C) Atomic radius D) Ionization energy What is C?

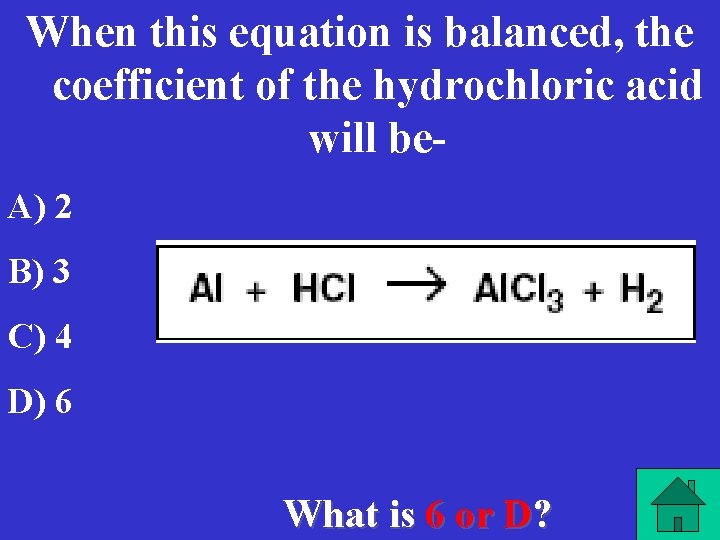
When this equation is balanced, the coefficient of the hydrochloric acid will be. A) 2 B) 3 C) 4 D) 6 What is 6 or D?

The atomic number corresponds to an atom’s number of- A) protons B) neutrons C) electrons D) positrons What is A?

Which element naturally occurs as a diatomic molecule? A) Zn B) C C) K D) H What is D?

What shape does the molecule BF 3 have? A) Bent B) Linear C) Tetrahedral D) Trigonal planar What is D?

How should 0. 000365 be expressed in proper scientific notation? A) 3. 65 X 104 B) 365 C) 3. 65 D) 3. 65 X 10 -4 What is D?


FINAL JEOPARDY

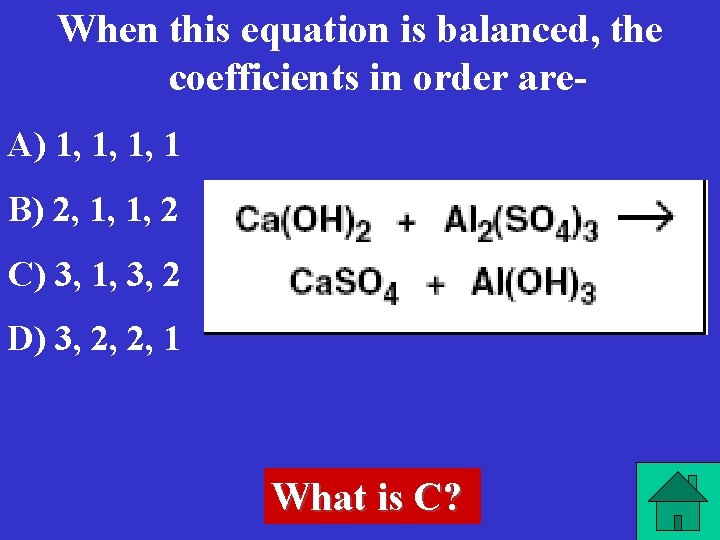
When this equation is balanced, the coefficients in order are. A) 1, 1, 1, 1 B) 2, 1, 1, 2 C) 3, 1, 3, 2 D) 3, 2, 2, 1 What is C?

Thanks for playing JEOPARDY Now, go home and study!
 Molar relationships chemistry
Molar relationships chemistry Converting mass to moles
Converting mass to moles Atomic mass vs molar mass
Atomic mass vs molar mass Four states of matter
Four states of matter Four states of matter
Four states of matter Phases changes of matter
Phases changes of matter 3 phases of matter
3 phases of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases Phases of matter foldable
Phases of matter foldable Phases of matter
Phases of matter Phases of matter concept map
Phases of matter concept map Relative formula mass of hcl
Relative formula mass of hcl Periodic trends in the periodic table
Periodic trends in the periodic table Atomic radius increases from left to right
Atomic radius increases from left to right Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Difference between atomic mass and atomic number
Difference between atomic mass and atomic number Atomic number vs atomic radius
Atomic number vs atomic radius Moon phases jeopardy
Moon phases jeopardy Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Ib chemistry atomic structure
Ib chemistry atomic structure Ap chemistry chapter 7
Ap chemistry chapter 7 Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity First ionization energy of calcium equation
First ionization energy of calcium equation Atomic structure jeopardy
Atomic structure jeopardy Indirect relationship example
Indirect relationship example Section 1 composition of matter
Section 1 composition of matter Gray and white matter
Gray and white matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter Grey matter and white matter in brain
Grey matter and white matter in brain Ecological succession
Ecological succession Healthy boundaries jeopardy
Healthy boundaries jeopardy Healthy relationships jeopardy
Healthy relationships jeopardy Unit rate triangle
Unit rate triangle Angle relationships jeopardy
Angle relationships jeopardy Is a vertical angle 90 degrees
Is a vertical angle 90 degrees Chemistry matter and its changes
Chemistry matter and its changes Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 assessment the mole answer key
Chapter 10 assessment the mole answer key Examples of matter in chemistry
Examples of matter in chemistry Matter flowchart chemistry
Matter flowchart chemistry 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Flowchart undissolved solids
Flowchart undissolved solids Classification graphic organizer
Classification graphic organizer Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter