CHEMICAL FOUNDATIONS ELEMENTS ATOMS and IONS Chapter 4













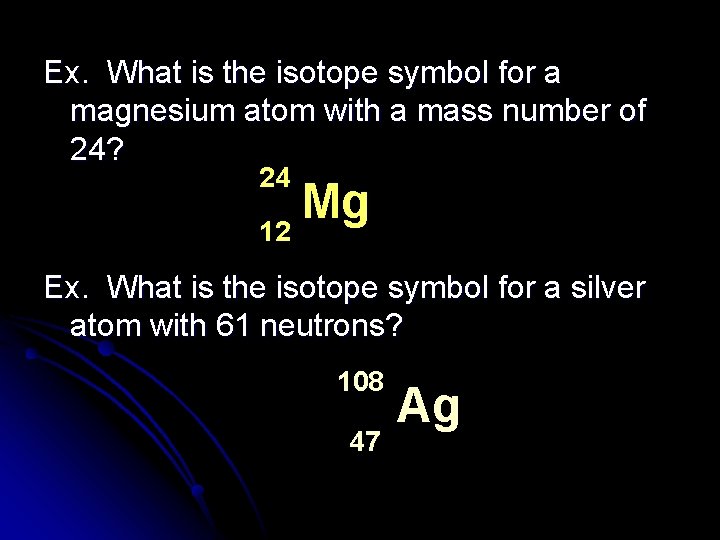














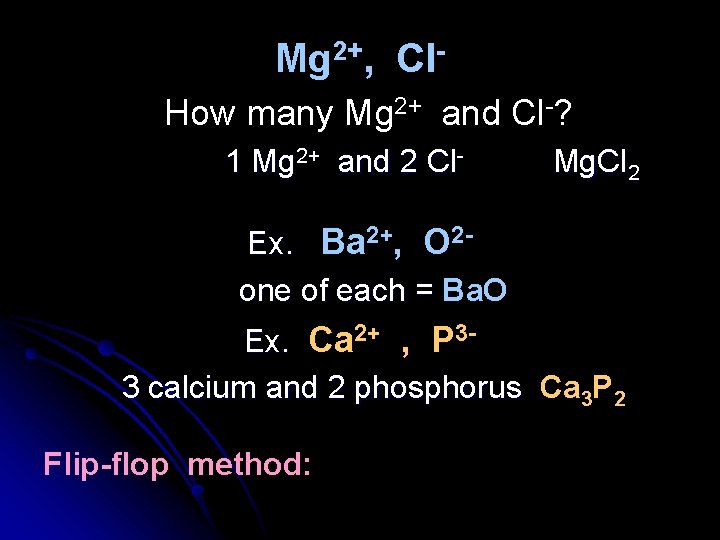
- Slides: 44

CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS and IONS Chapter 4

4. 1 The Elements 4. 2 Symbols for the Elements Read.

4. 3 Dalton’s Atomic Theory Scientists determined: l most natural materials are mixtures l pure substances are elements and compounds l a certain compound always has the same proportion of elements by mass regardless of where it is found. (Law of constant ( composition)

Dalton was a scientist and in 1808 gave an explanation for the above. (Dalton’s Atomic Theory)

Dalton’s Atomic Theory: 1. Elements consist of tiny particles called atoms. 2. All atoms of a certain element are identical. 3. Atoms of one element differ from atoms of another. 4. Atoms of different elements combine to form compounds. 5. Atoms are indivisible in chemical reactions. Not created or destroyed, rearranged. Atom comes from the word atomos = indivisible

4. 4 Formulas of Compounds Chemical formulas express the kind of atom and how many in a compound, using symbols and numbers. CO 2 NO SO 2

Rules for Writing Formulas: 1. 2. 3. Each atom present is represented by its element symbol. The number of each type of atom is indicated by a subscript written to the right of the element symbol. When only one atom of a given type is present, the subscript 1 is not written.

Ex. A molecule from acid rain contains one sulfur atom and three oxygen atoms. SO 3

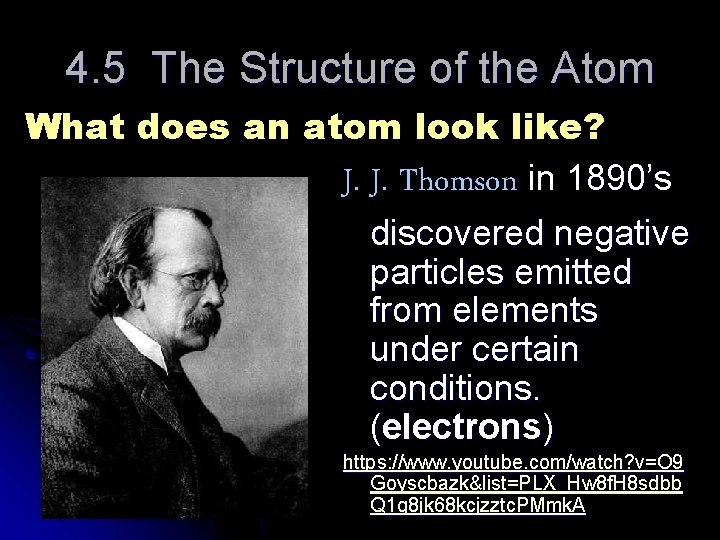
4. 5 The Structure of the Atom What does an atom look like? J. J. Thomson in 1890’s discovered negative particles emitted from elements under certain conditions. (electrons) https: //www. youtube. com/watch? v=O 9 Goyscbazk&list=PLX_Hw 8 f. H 8 sdbb Q 1 g 8 jk 68 kcjzztc. PMmk. A

JJ Thomson thought the atom was a uniform “pudding” of positive charge with electrons scattered throughout. (Plum pudding model)

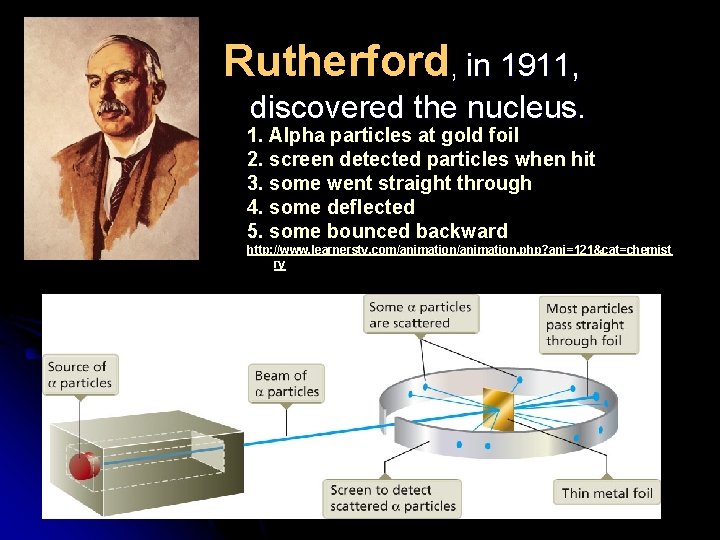
Rutherford, in 1911, discovered the nucleus. 1. Alpha particles at gold foil 2. screen detected particles when hit 3. some went straight through 4. some deflected 5. some bounced backward http: //www. learnerstv. com/animation. php? ani=121&cat=chemist ry

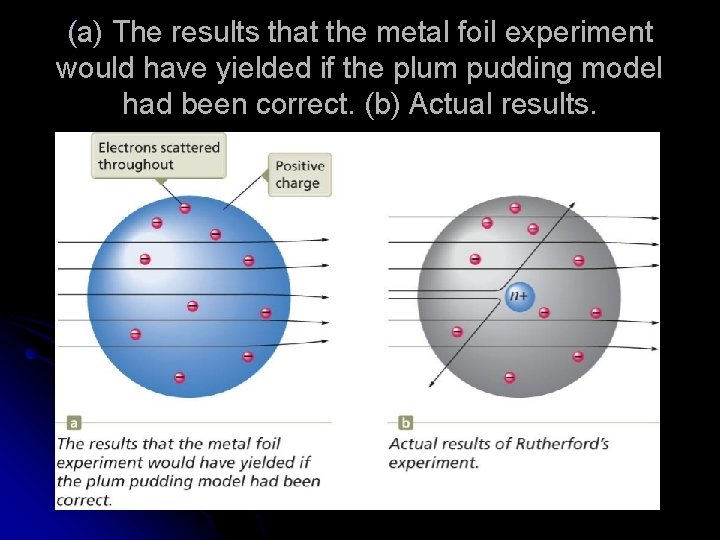
(a) The results that the metal foil experiment would have yielded if the plum pudding model had been correct. (b) Actual results.

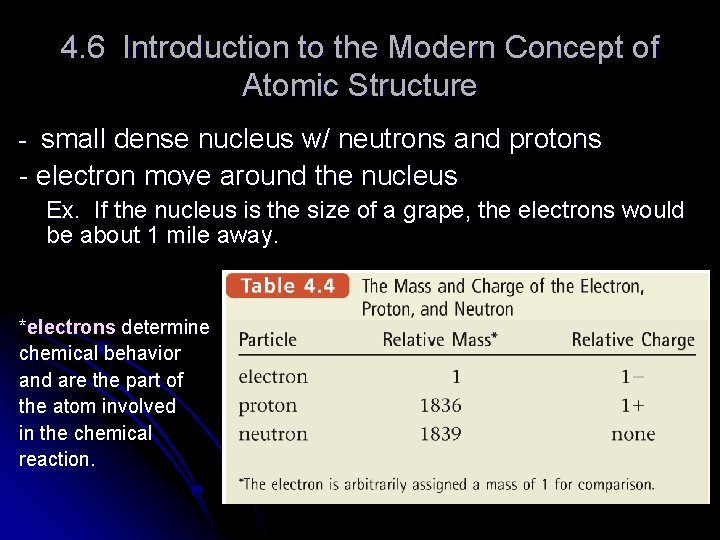
Thus center must be dense and positive. 1919 he concluded protons are in the nucleus. Electron = negative charge (about 1800 times smaller than a proton) Protons = positive charge Chadwick and Rutherford in 1932 showed that neutrons are in the nucleus. (no charge)

4. 6 Introduction to the Modern Concept of Atomic Structure - small dense nucleus w/ neutrons and protons - electron move around the nucleus Ex. If the nucleus is the size of a grape, the electrons would be about 1 mile away. *electrons determine chemical behavior and are the part of the atom involved in the chemical reaction.

4. 7 Isotopes All atoms have protons and electrons. The number of protons equals the number of electrons in an atom. Ex. Sodium, Na, has 11 protons. How many electrons does it have? Neutrons are also found in the nucleus of atoms. All sodium atoms have 11 protons, but sodium atoms can have different numbers of neutrons.

Neutrons are also found in the nucleus of atoms. All sodium atoms have 11 protons, but sodium atoms can have different numbers of neutrons. Dalton’s idea that all atoms of a given element are identical has changed.

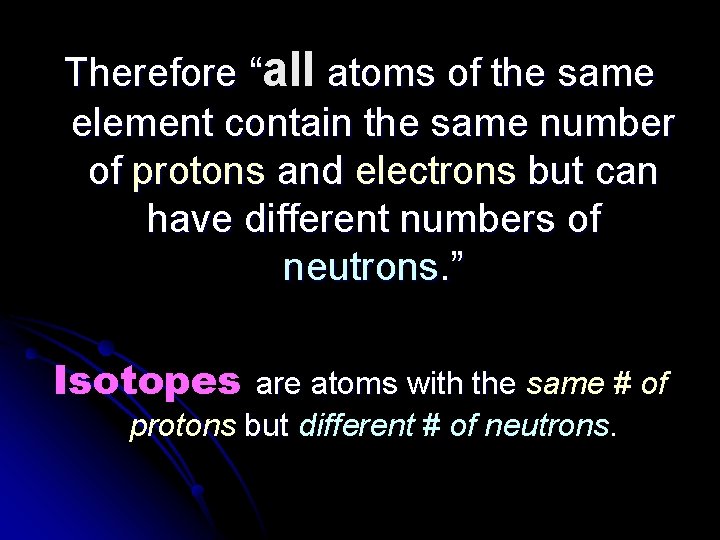
Therefore “all atoms of the same element contain the same number of protons and electrons but can have different numbers of neutrons. ” Isotopes are atoms with the same # of protons but different # of neutrons.

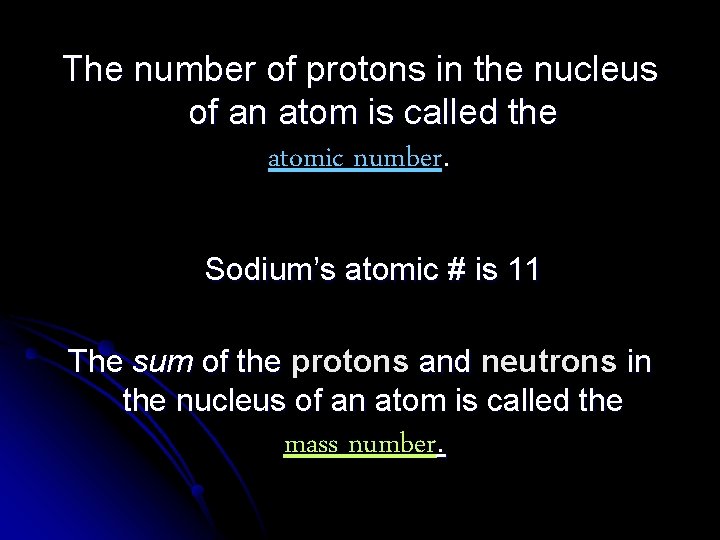
The number of protons in the nucleus of an atom is called the atomic number. Sodium’s atomic # is 11 The sum of the protons and neutrons in the nucleus of an atom is called the mass number.

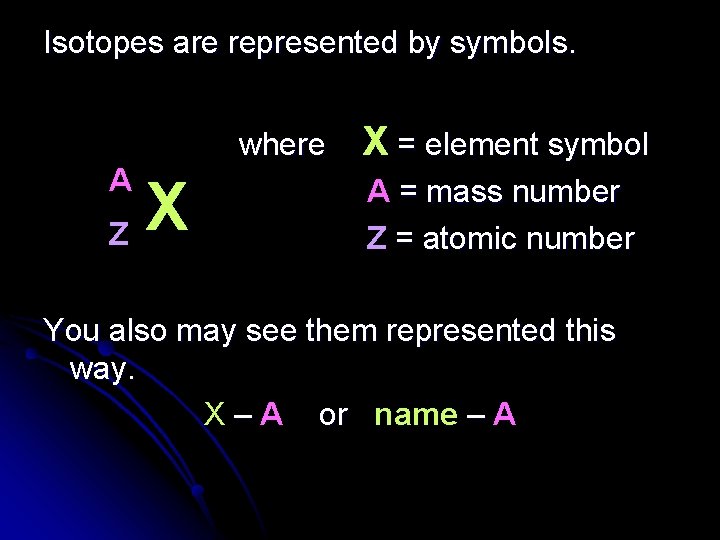
Isotopes are represented by symbols. A X Z where X = element symbol A = mass number Z = atomic number You also may see them represented this way. X – A or name – A

Ex. 23 11 Na has a total of 23 protons and neutrons and only 11 protons. How many neutrons does this sodium atom have? (aka sodium-23) 12 24 Na How many protons does this sodium atom have? How many neutrons? How many electrons? 11, 13, 11 11

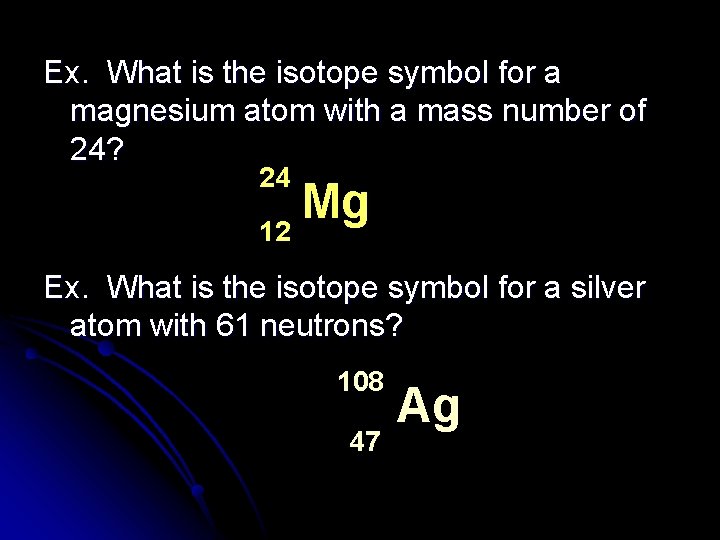
Ex. What is the isotope symbol for a magnesium atom with a mass number of 24? 24 Mg 12 Ex. What is the isotope symbol for a silver atom with 61 neutrons? 108 47 Ag

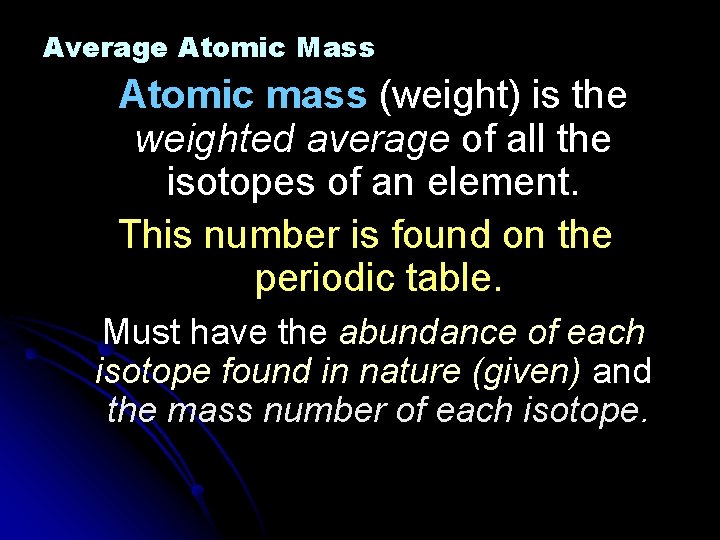
Average Atomic Mass Atomic mass (weight) is the weighted average of all the isotopes of an element. This number is found on the periodic table. Must have the abundance of each isotope found in nature (given) and the mass number of each isotope.

Ex. Determine the average atomic mass of chlorine. Chlorine – 35 is found 75. 77% in nature and chlorine – 37 is found 24. 23% in nature. (mass number x relative abundance, as decimal) 35 x. 7577 = 26. 5195 +37 + x. 2423 = 8. 9651 35. 4846 This is the mass found on the periodic table.

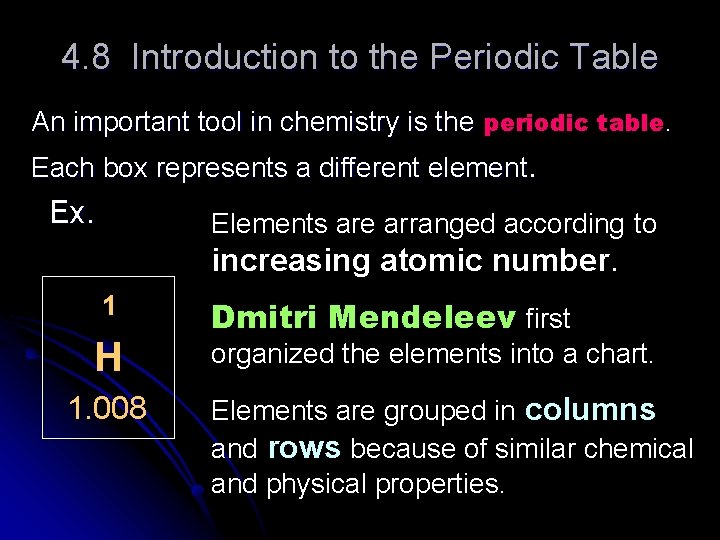
4. 8 Introduction to the Periodic Table An important tool in chemistry is the periodic table. Each box represents a different element. Ex. Elements are arranged according to increasing atomic number. 1 H 1. 008 Dmitri Mendeleev first organized the elements into a chart. Elements are grouped in columns and rows because of similar chemical and physical properties.

Columns are called: groups, or families. Elements found within a column have similar chemical properties. Columns are numbered with a digit and a Letter A. (Representative elements, ( Group A. ) Group 1 = alkali metals Group 2 = alkali earth metals Group 7 = halogens Group 8 = noble gases


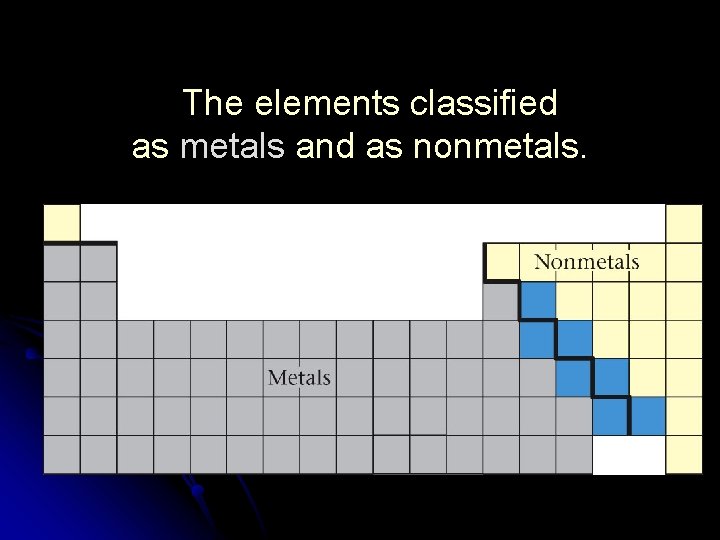
The elements in the short columns are called “transition elements. ” (Group B) Most elements are metals. They have similar physical properties: 1. 2. 3. 4. conduct electricity malleable ductile lustrous Metals are found below and to the left of the zig-zag line. (except Hydrogen: it’s a ( nonmetal)

Nonmetals lack the properties of metals. Many nonmetals are gases. These elements are found in the upper righthand corner of the periodic table. (right of the zig-zag line) Metalloids or semi-metals have mixtures of properties. (found along the zig-zag line, except Al. It is a pure metal)

The elements classified as metals and as nonmetals.

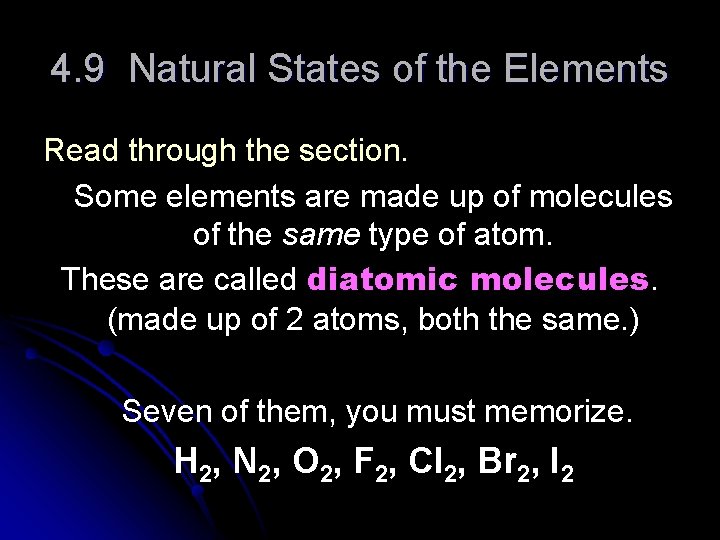
4. 9 Natural States of the Elements Read through the section. Some elements are made up of molecules of the same type of atom. These are called diatomic molecules. (made up of 2 atoms, both the same. ) Seven of them, you must memorize. H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2

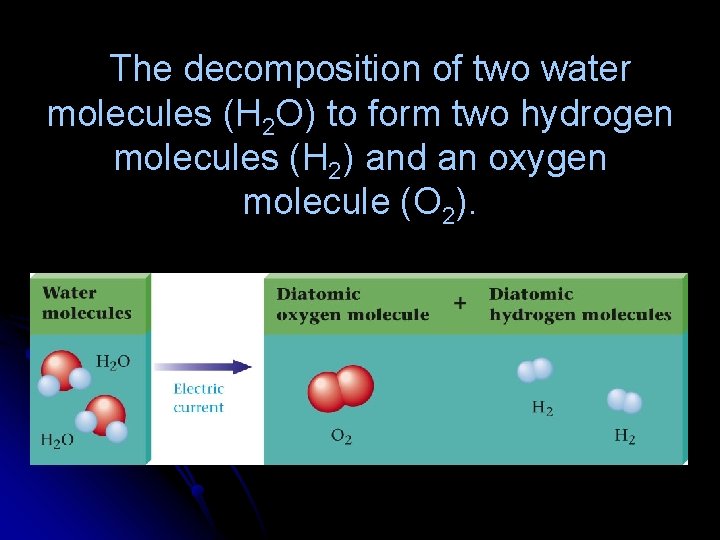
The decomposition of two water molecules (H 2 O) to form two hydrogen molecules (H 2) and an oxygen molecule (O 2).

Some atoms of elements can arrange themselves into different forms or crystal structures. These are called allotropes. (carbon- graphite, diamond, bucky ball)

4. 10 Ions Atoms have a balance of positive and negative charge, they are neutral. (p = e) If electrons are gained or given off by an atom, the atom carries a charge. It either has more protons than electrons, positive charge, or has more electrons than protons, negative charge. These are called ions.

Ex. If sodium loses one electron it now has 10 electrons and 11 protons. (+11) + (-10) = +1 charge Na → Na+ + e. If the atom loses electrons it becomes positive and is called a cation. Al loses 3 electrons, thus: Al → Al 3+ + 3 e(cation) The name of the ion is the element name plus the word ion. (aluminum ion)

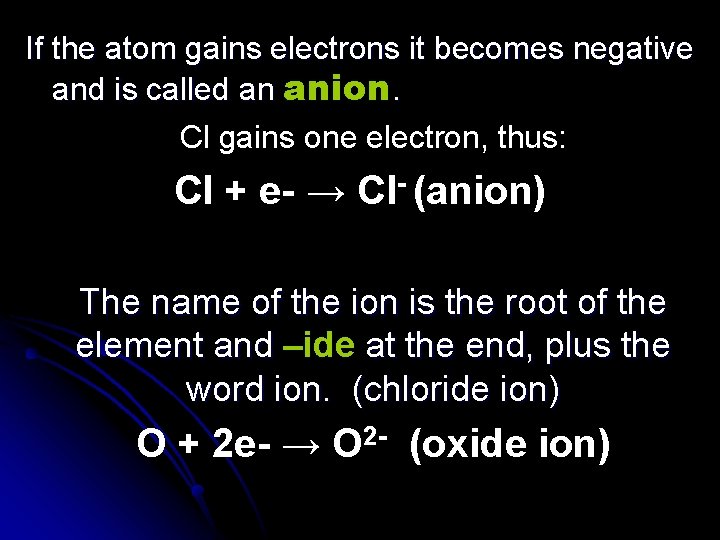
If the atom gains electrons it becomes negative and is called an anion. Cl gains one electron, thus: Cl + e- → Cl- (anion) The name of the ion is the root of the element and –ide at the end, plus the word ion. (chloride ion) O + 2 e- → O 2 - (oxide ion)

*ions are not formed by changing the number of protons, only the number of electrons. * (ions do not form on their own, but in the presence of some other element. Usually metals and nonmetal. ) nitride, oxide, fluoride, chloride, sulfide, phosphide, selenide, bromide, iodide

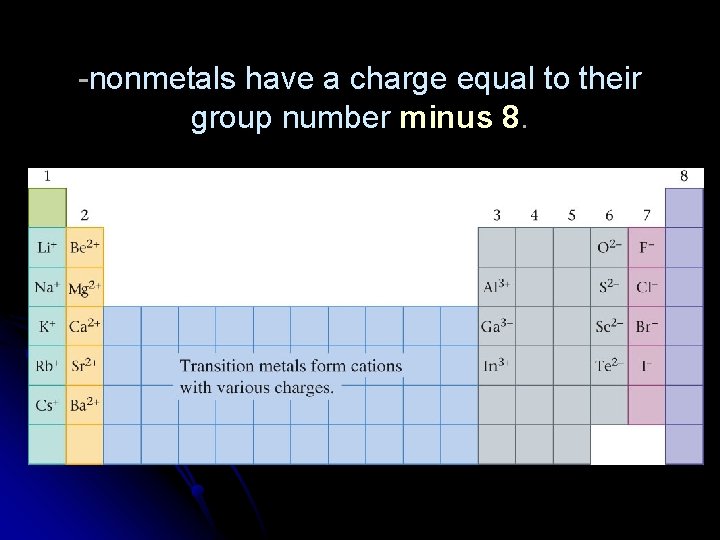
Ionic charges: First: metals form positive ions (cations) which means they lose electrons. Second: nonmetals form negative ions (anions) which means they gain electrons. We can determine the charge for many ions from the periodic table. -metals in groups 1, 2, and 3 all have a charge equal to their group number. Ex. Na, group 1 = Na+ Al, group 3 = Al 3+ Ca, group 2 = Ca 2+ Transition metal charges can not be predicted from the periodic table.

-nonmetals have a charge equal to their group number minus 8.

Ex. selenium, group 6; 6 -8 = -2 Se 2 argon, group 8; 8 -8 = 0 Ar chlorine, group 7; 7 -8 = -1 Cl- Groups 4 and 5 can form multiple charges, or not at all. Nitrogen and phosphorus from group 5 form 3 - charges.


4. 11 Compounds that Contain Ions Ionic compounds (salts) are composed of a metal and a nonmetal, which have become ions in the presence of each other. Properties of ionic compounds are: -high melting and boiling points -crystalline solids at room temperature -conduct electricity when dissolved in water and when melted *All chemical compounds have a net charge of zero, even if it is composed of ions*

So, in an ionic compound the number of cations and anions must be such that the net charge is zero. Ex. sodium chloride Na+ Cl- How many Na+ and Cl- to be neutral? One of each. Na. Cl (metals are written first, nonmetals second) for ionic compunds this called a “formula unit”
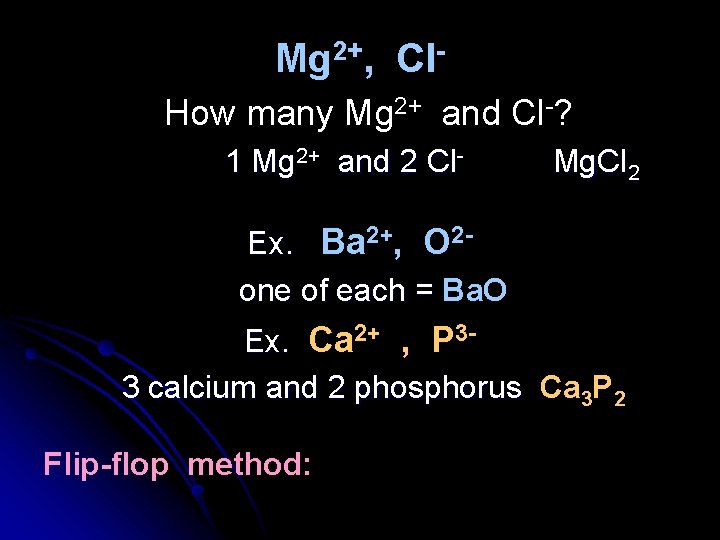
Mg 2+, Cl. How many Mg 2+ and Cl-? 1 Mg 2+ and 2 Cl- Mg. Cl 2 Ex. Ba 2+, O 2 one of each = Ba. O Ex. Ca 2+ , P 33 calcium and 2 phosphorus Ca 3 P 2 Flip-flop method: