Chapter 12 Solids and Modern Materials Outline I






- Slides: 51

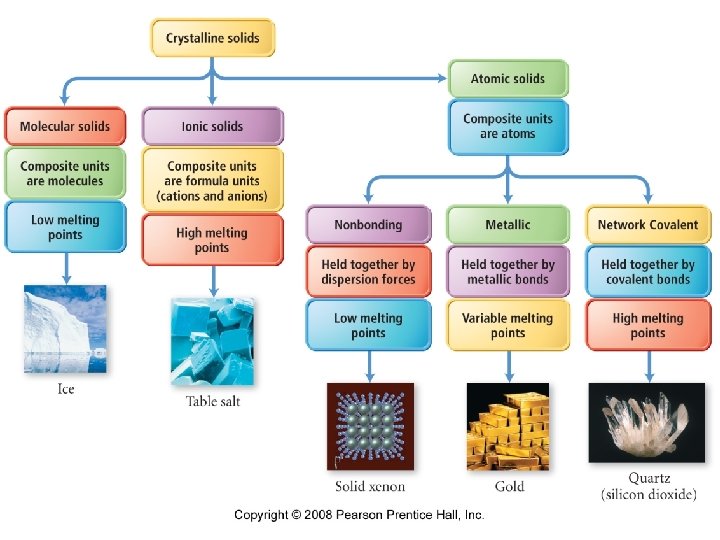
Chapter 12 Solids and Modern Materials Outline I. III. IV. V. Properties and Types of Solids X-ray Diffraction Units Cells and Structures Band Theory Polymers


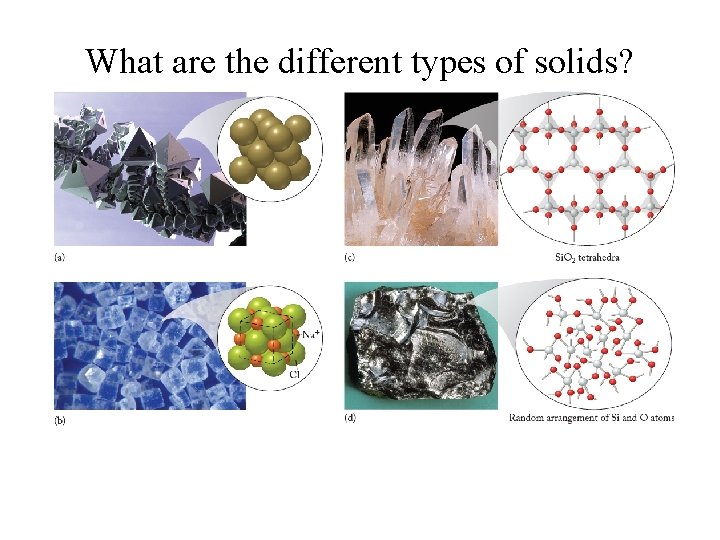
What are the different types of solids?

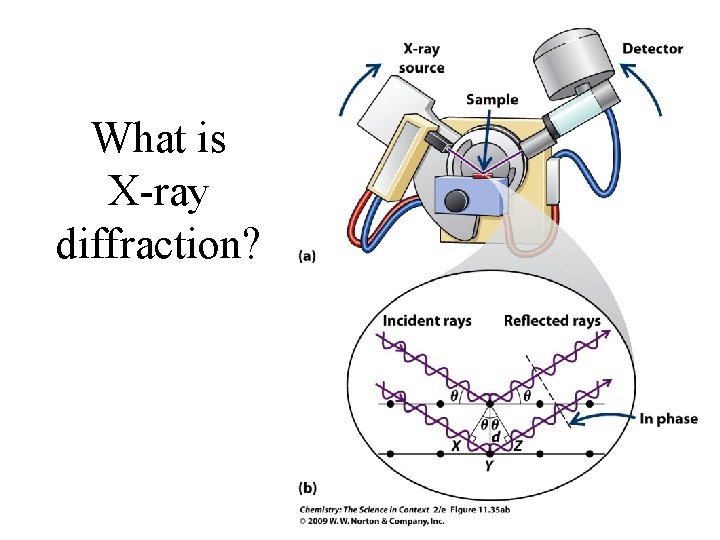
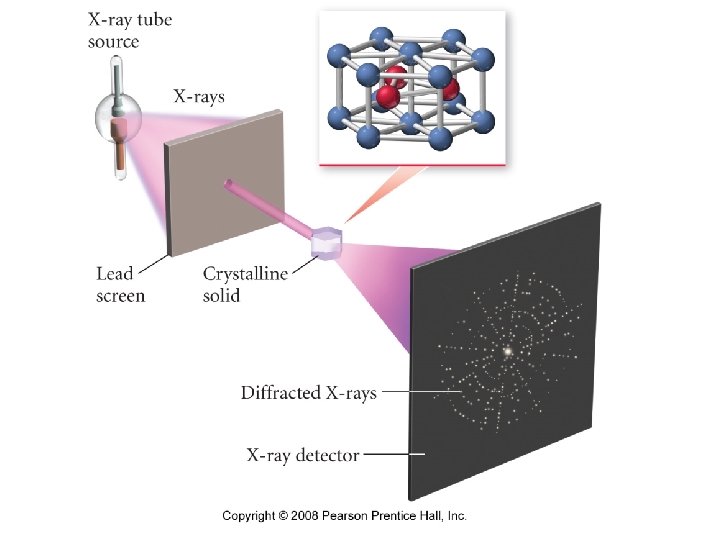
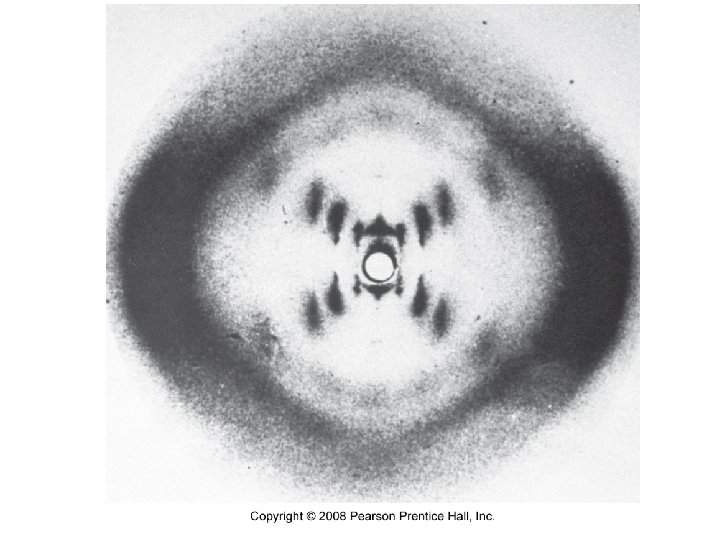
What is X-ray diffraction?

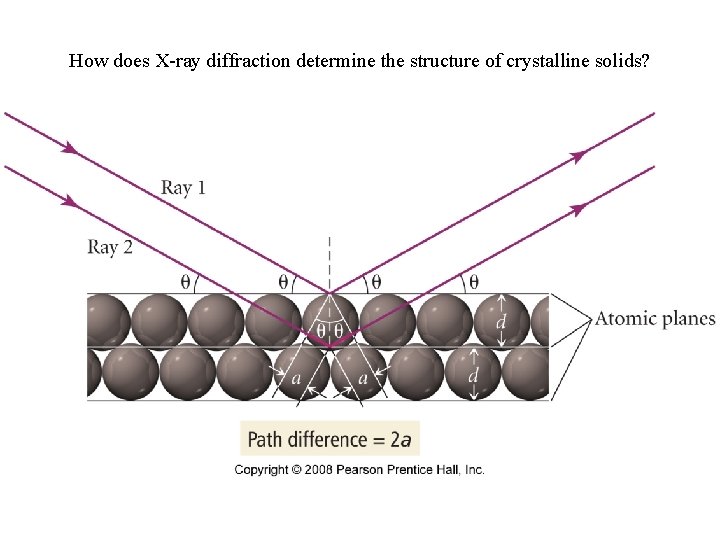
How does X-ray diffraction determine the structure of crystalline solids?



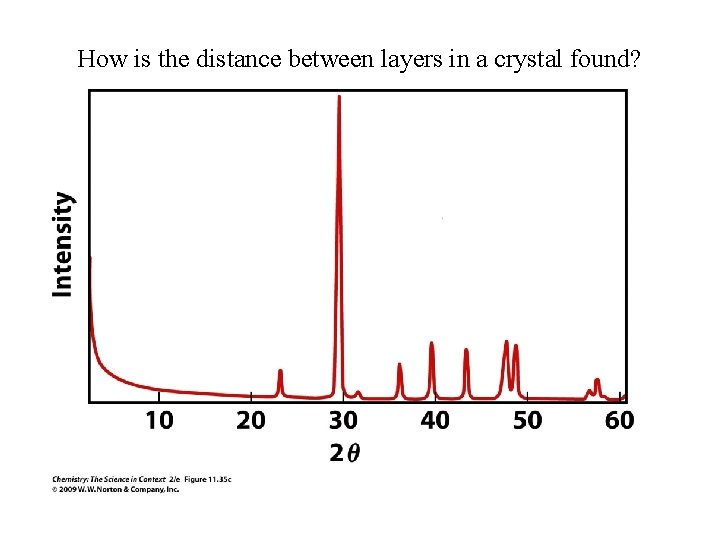
How is the distance between layers in a crystal found?

Example – Bragg Equation Using an x-ray of wavelength 154 pm, a spot is observed at an angle of 17. 5˚. What is the distance between the layer of atoms?

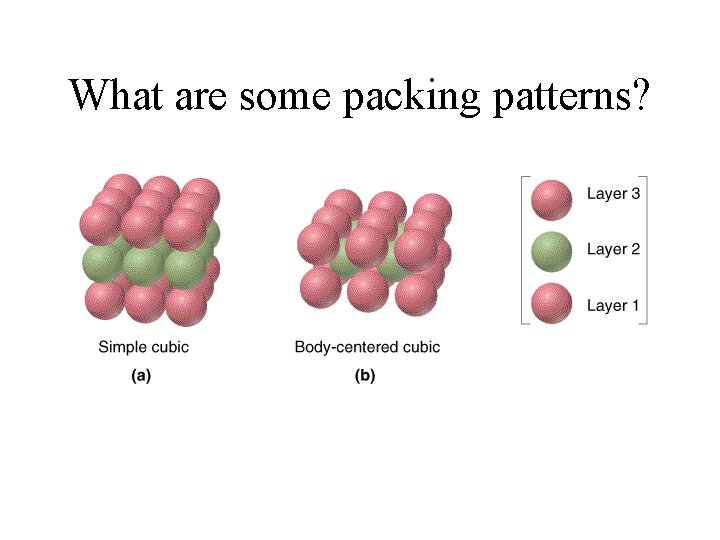
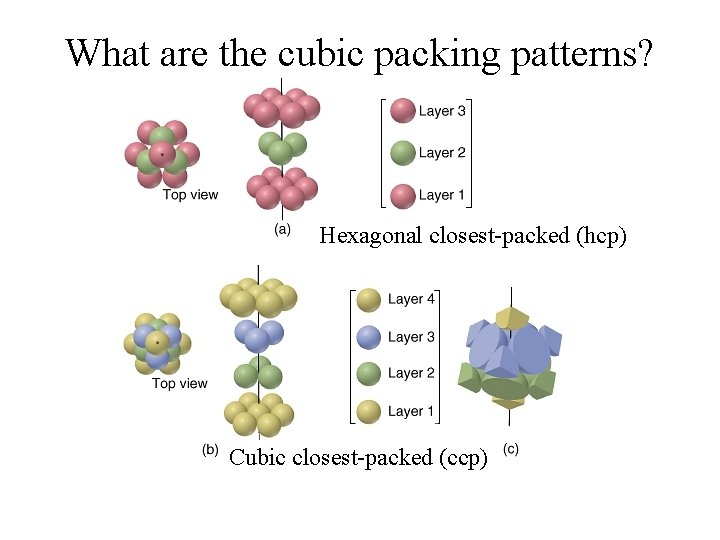
What are some packing patterns?

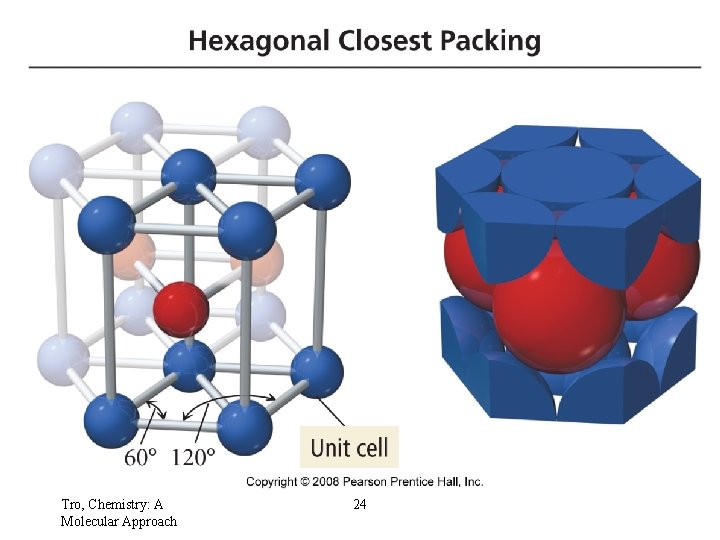
What are the cubic packing patterns? Hexagonal closest-packed (hcp) Cubic closest-packed (ccp)

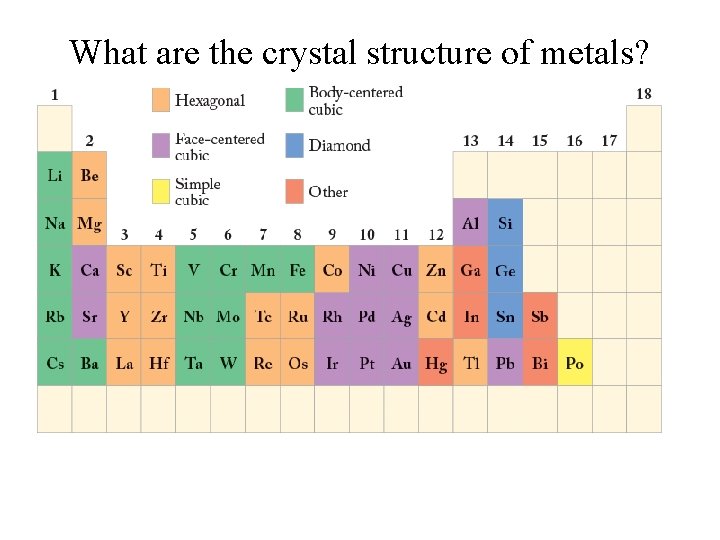
What are the crystal structure of metals?

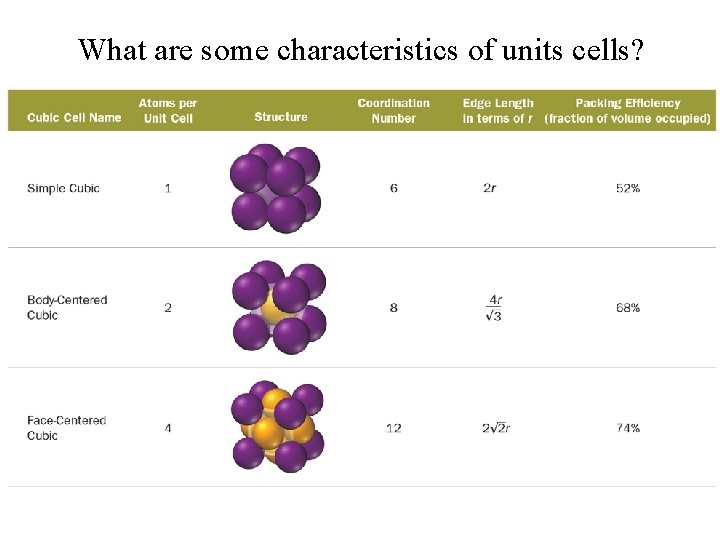
What are some characteristics of units cells?

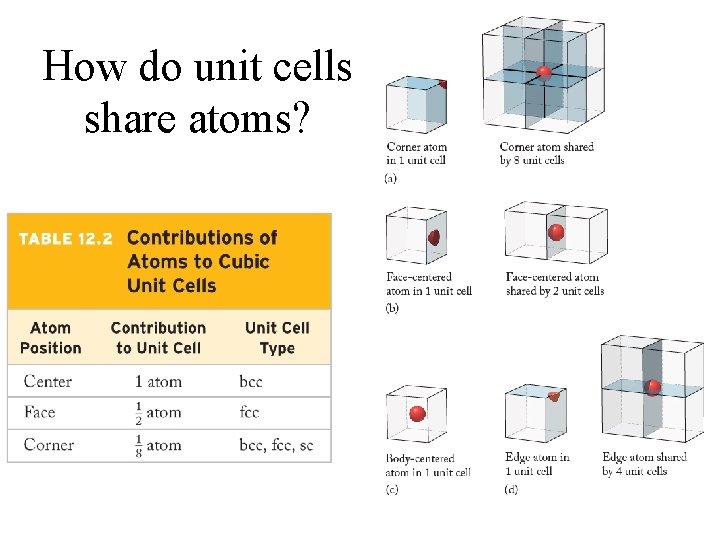
How do unit cells share atoms?

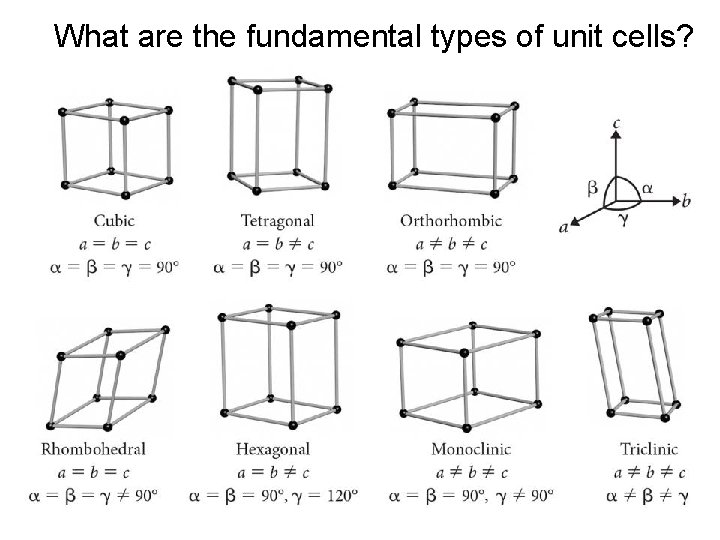
What are the fundamental types of unit cells?

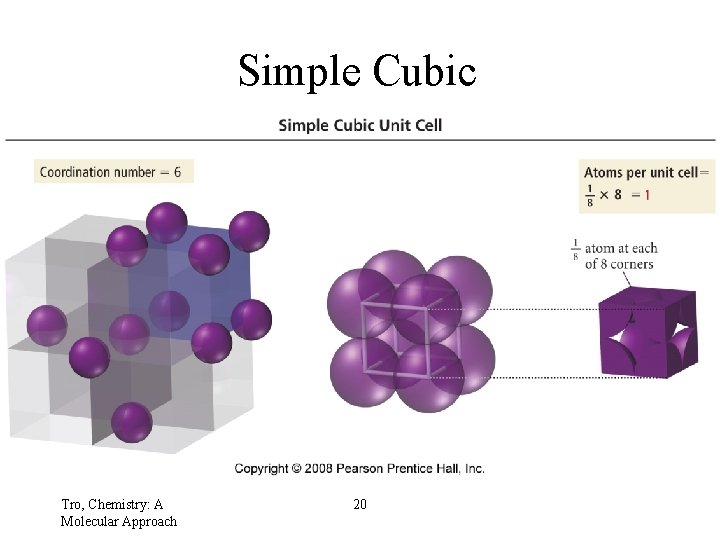
Simple Cubic Tro, Chemistry: A Molecular Approach 20

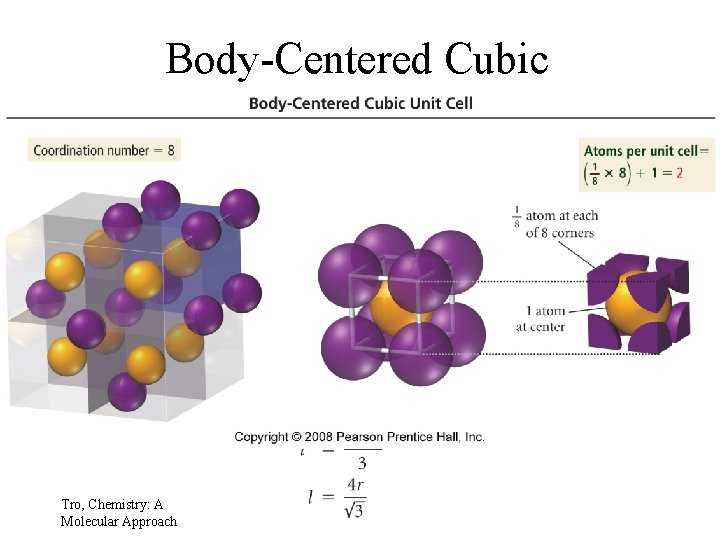
Body-Centered Cubic Tro, Chemistry: A Molecular Approach 21

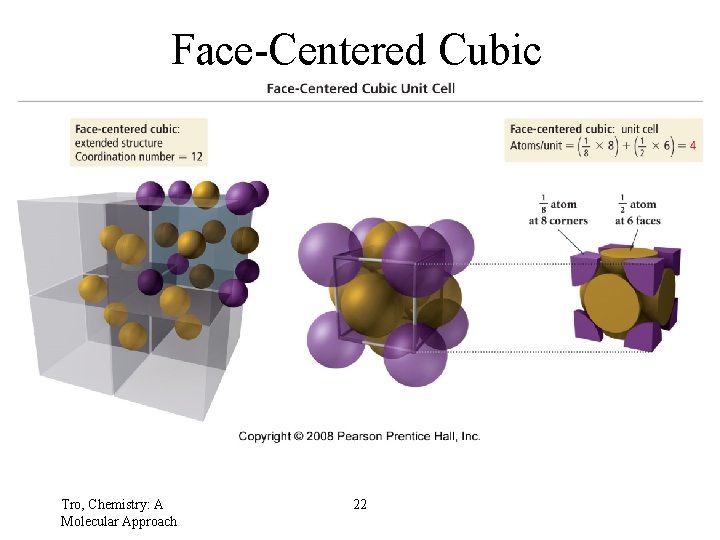
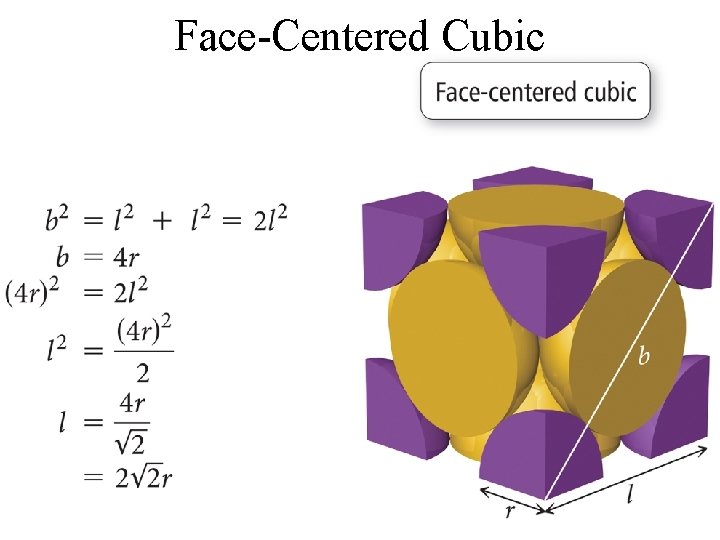
Face-Centered Cubic Tro, Chemistry: A Molecular Approach 22

Face-Centered Cubic

Hexagonal Closest-Packed Structures Tro, Chemistry: A Molecular Approach 24

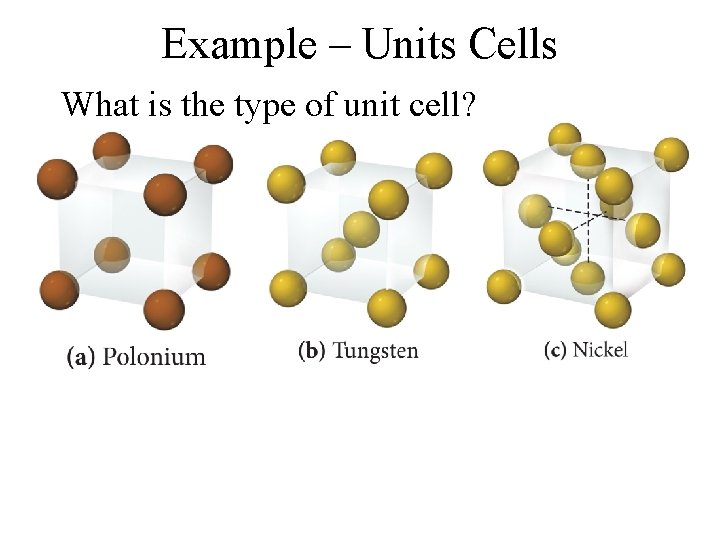
Example – Units Cells What is the type of unit cell?

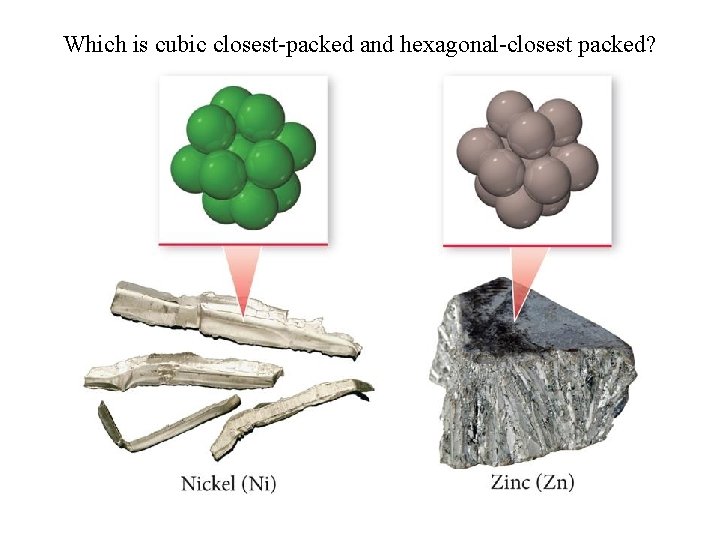
Which is cubic closest-packed and hexagonal-closest packed?

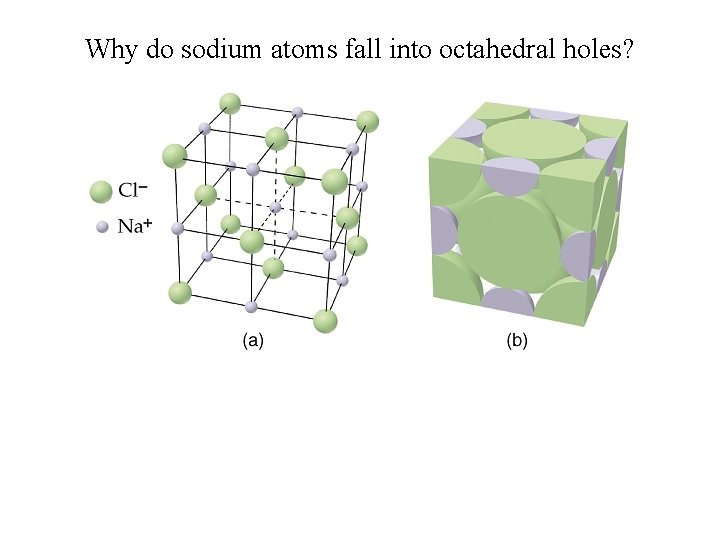
Why do sodium atoms fall into octahedral holes?

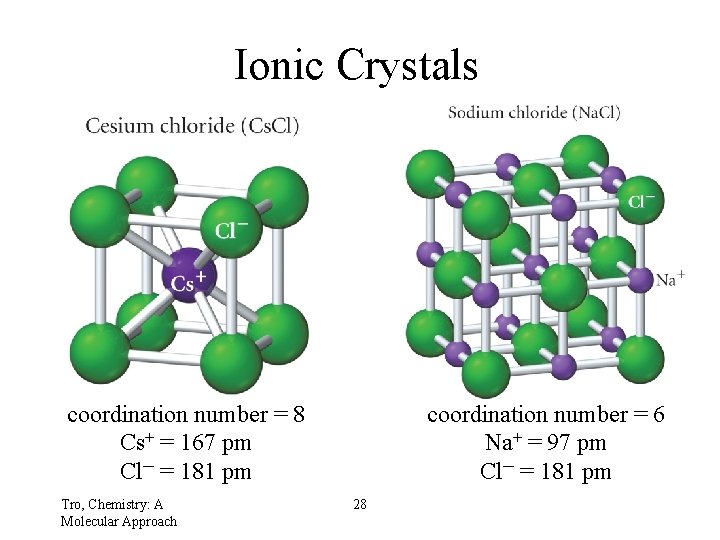
Ionic Crystals Cs. Cl coordination number = 8 Cs+ = 167 pm Cl─ = 181 pm Tro, Chemistry: A Molecular Approach Na. Cl coordination number = 6 Na+ = 97 pm Cl─ = 181 pm 28

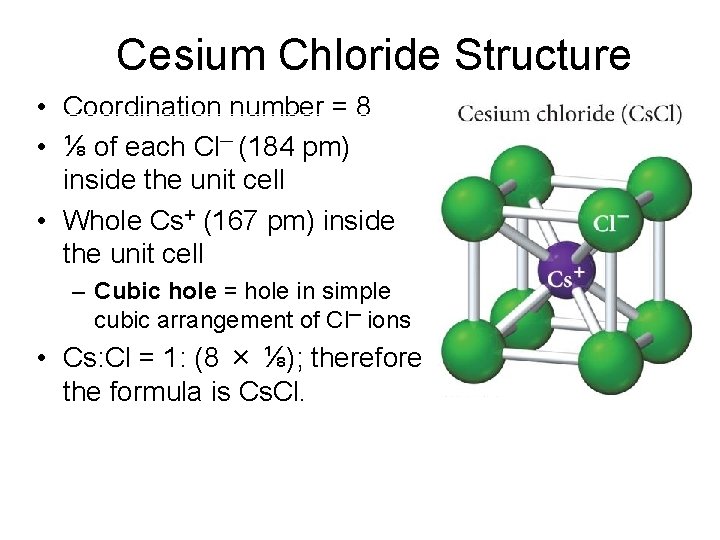
Cesium Chloride Structure • Coordination number = 8 • ⅛ of each Cl─ (184 pm) inside the unit cell • Whole Cs+ (167 pm) inside the unit cell – Cubic hole = hole in simple cubic arrangement of Cl─ ions • Cs: Cl = 1: (8 × ⅛); therefore the formula is Cs. Cl.

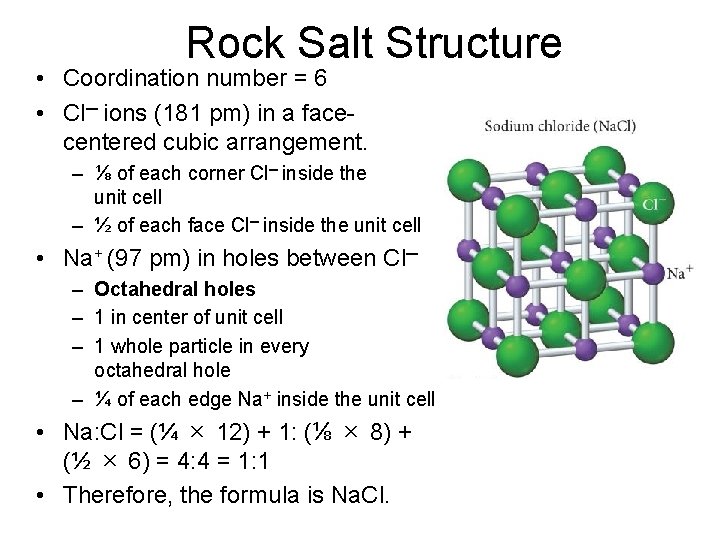
Rock Salt Structure • Coordination number = 6 • Cl─ ions (181 pm) in a facecentered cubic arrangement. – ⅛ of each corner Cl─ inside the unit cell – ½ of each face Cl─ inside the unit cell • Na+ (97 pm) in holes between Cl─ – Octahedral holes – 1 in center of unit cell – 1 whole particle in every octahedral hole – ¼ of each edge Na+ inside the unit cell • Na: Cl = (¼ × 12) + 1: (⅛ × 8) + (½ × 6) = 4: 4 = 1: 1 • Therefore, the formula is Na. Cl.

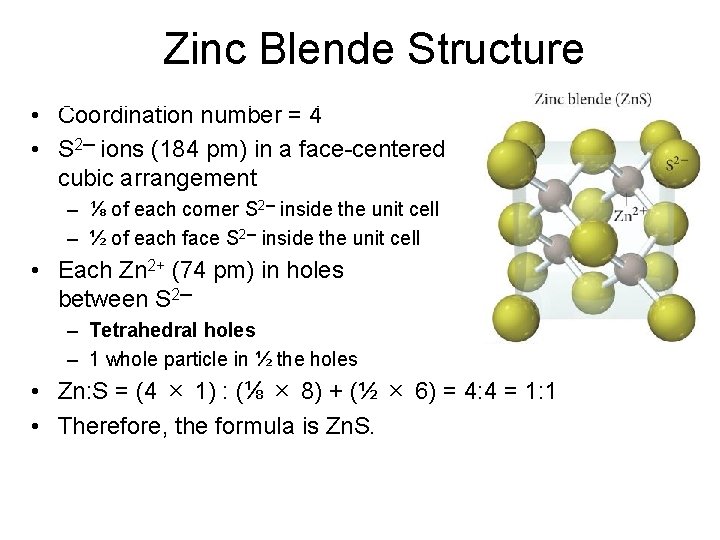
Zinc Blende Structure • Coordination number = 4 • S 2─ ions (184 pm) in a face-centered cubic arrangement – ⅛ of each corner S 2─ inside the unit cell – ½ of each face S 2─ inside the unit cell • Each Zn 2+ (74 pm) in holes between S 2─ – Tetrahedral holes – 1 whole particle in ½ the holes • Zn: S = (4 × 1) : (⅛ × 8) + (½ × 6) = 4: 4 = 1: 1 • Therefore, the formula is Zn. S.

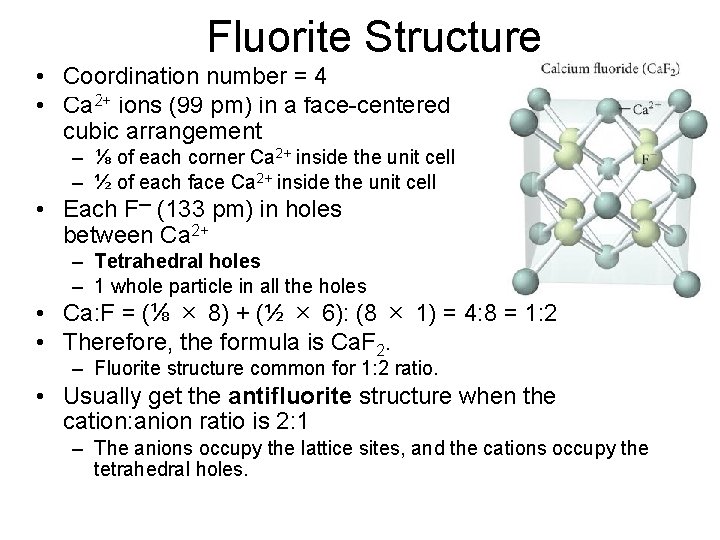
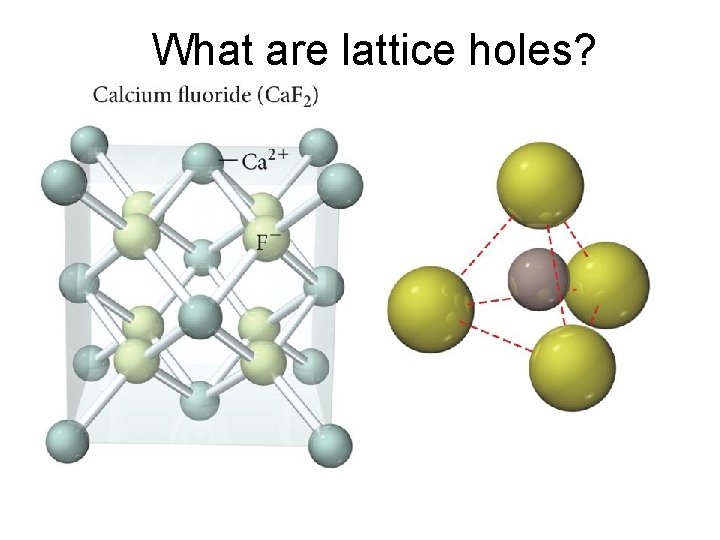
Fluorite Structure • Coordination number = 4 • Ca 2+ ions (99 pm) in a face-centered cubic arrangement – ⅛ of each corner Ca 2+ inside the unit cell – ½ of each face Ca 2+ inside the unit cell • Each F─ (133 pm) in holes between Ca 2+ – Tetrahedral holes – 1 whole particle in all the holes • Ca: F = (⅛ × 8) + (½ × 6): (8 × 1) = 4: 8 = 1: 2 • Therefore, the formula is Ca. F 2. – Fluorite structure common for 1: 2 ratio. • Usually get the antifluorite structure when the cation: anion ratio is 2: 1 – The anions occupy the lattice sites, and the cations occupy the tetrahedral holes.

What are lattice holes?


Example – Unit Cells The atomic radius of copper is 128 pm, and its density is 8. 92 g/cm 3. Is it close packed?

Example – Unit Cells Sodium metal has a density of 0. 970 g/cm 3 and forms a body centered lattice. a) Calculate the length in pm of the edge of the unit cell. b) What is the atomic radius of sodium?

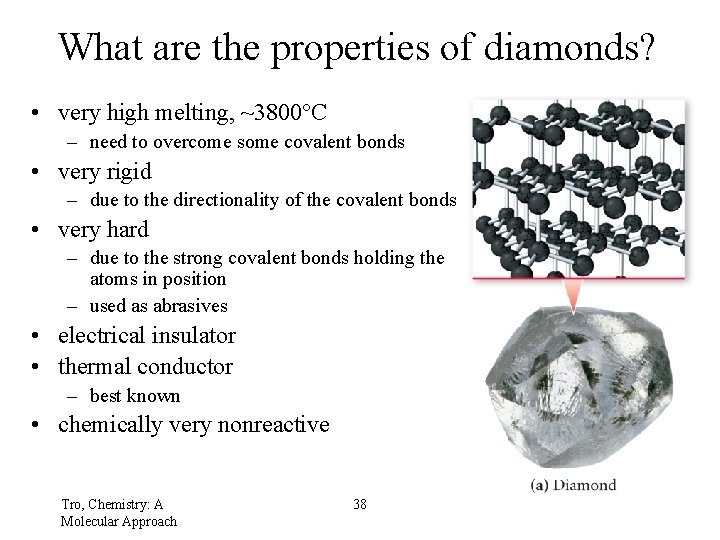
What are the properties of diamonds? • very high melting, ~3800°C – need to overcome some covalent bonds • very rigid – due to the directionality of the covalent bonds • very hard – due to the strong covalent bonds holding the atoms in position – used as abrasives • electrical insulator • thermal conductor – best known • chemically very nonreactive Tro, Chemistry: A Molecular Approach 38

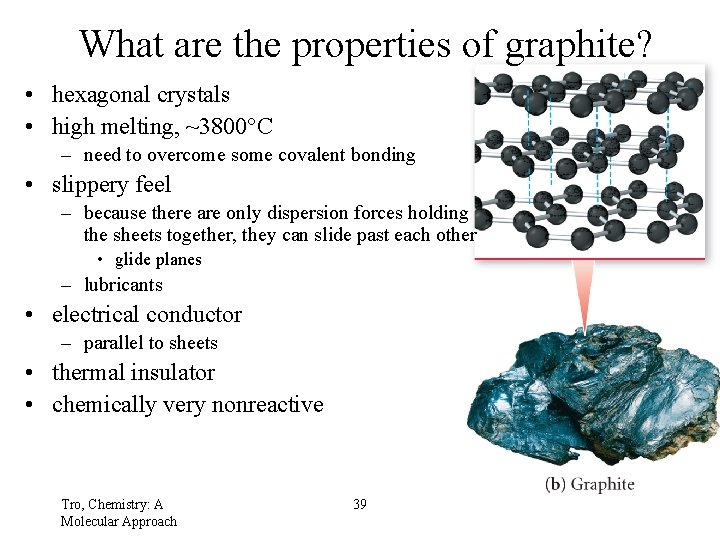
What are the properties of graphite? • hexagonal crystals • high melting, ~3800°C – need to overcome some covalent bonding • slippery feel – because there are only dispersion forces holding the sheets together, they can slide past each other • glide planes – lubricants • electrical conductor – parallel to sheets • thermal insulator • chemically very nonreactive Tro, Chemistry: A Molecular Approach 39

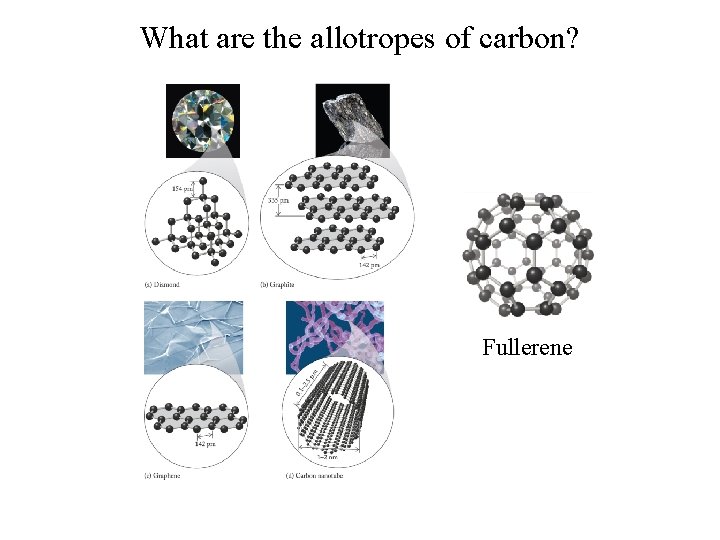
What are the allotropes of carbon? Fullerene

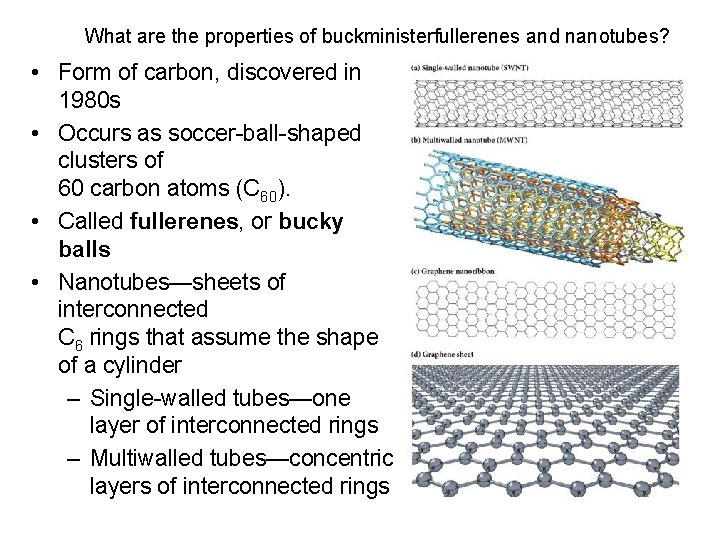
What are the properties of buckministerfullerenes and nanotubes? • Form of carbon, discovered in 1980 s • Occurs as soccer-ball-shaped clusters of 60 carbon atoms (C 60). • Called fullerenes, or bucky balls • Nanotubes—sheets of interconnected C 6 rings that assume the shape of a cylinder – Single-walled tubes—one layer of interconnected rings – Multiwalled tubes—concentric layers of interconnected rings

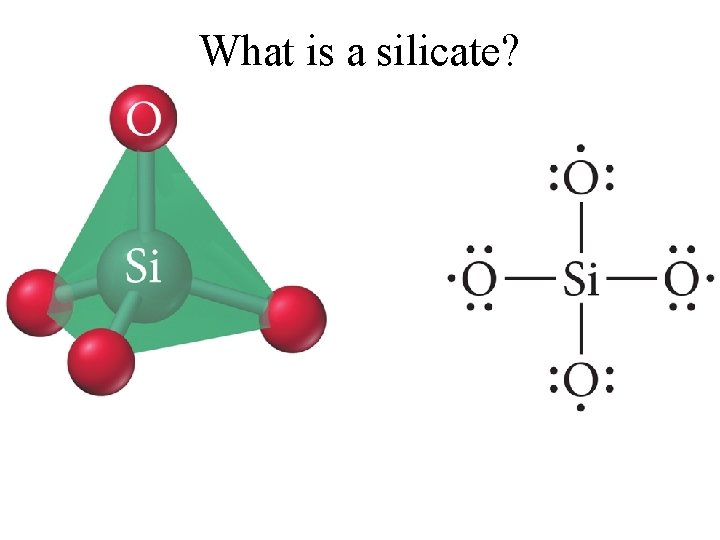
What is a silicate?

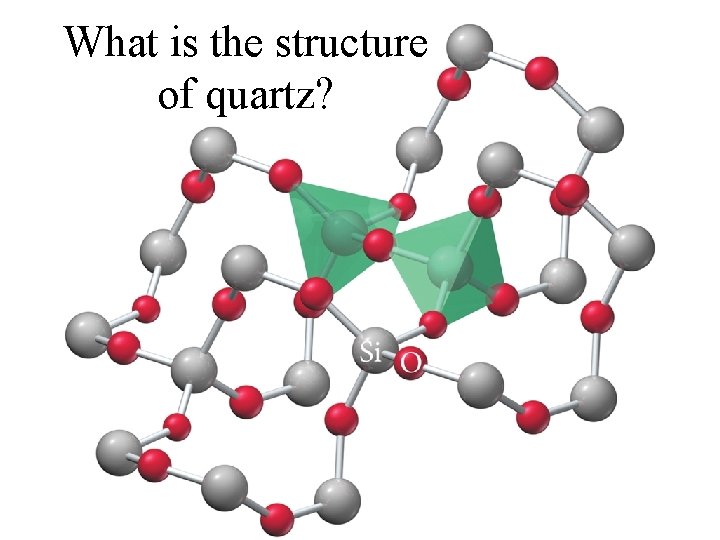
What is the structure of quartz?

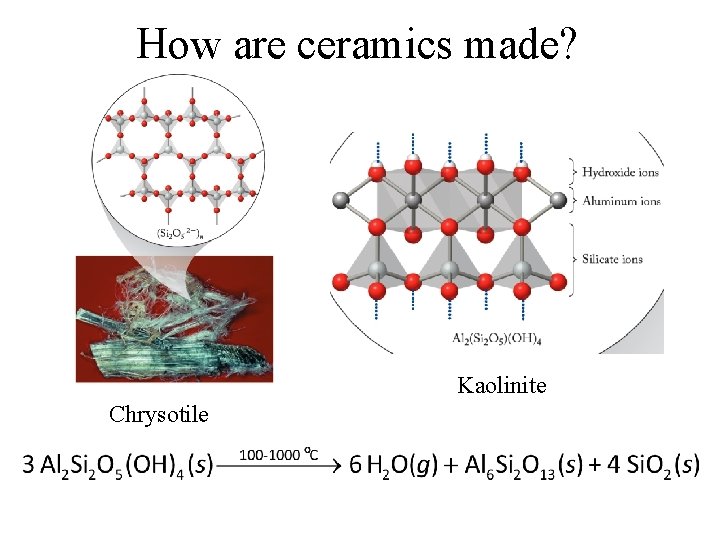
How are ceramics made? Kaolinite Chrysotile

What are some applications of ceramics?

What are some applications of glass?

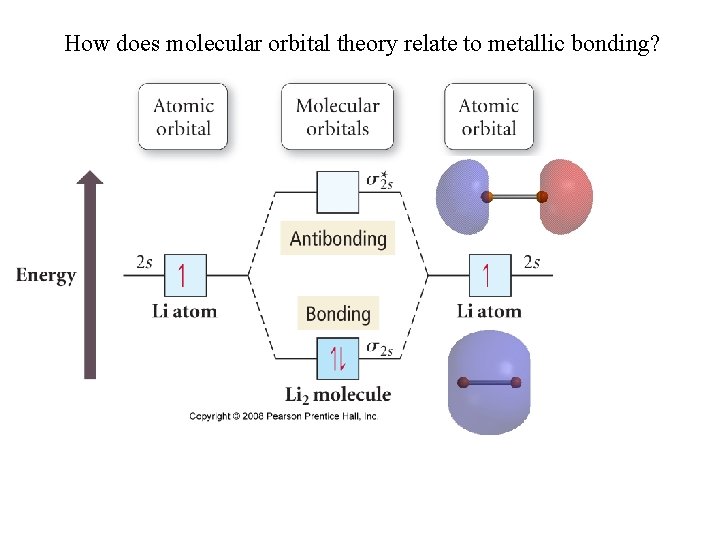
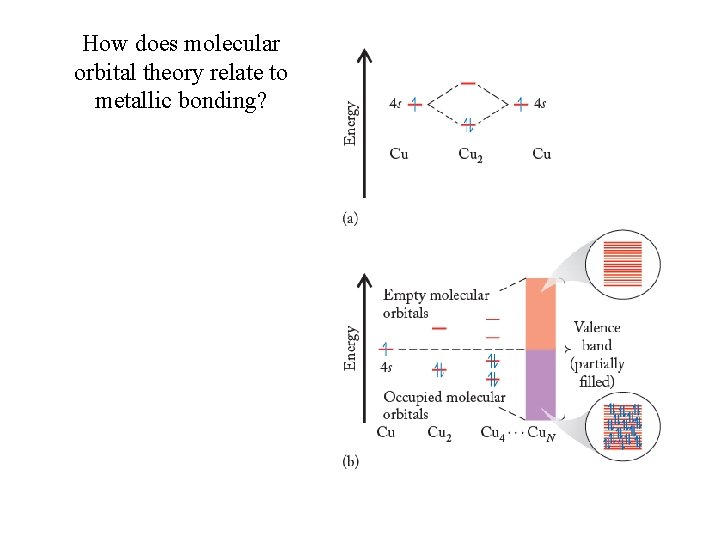
How does molecular orbital theory relate to metallic bonding?

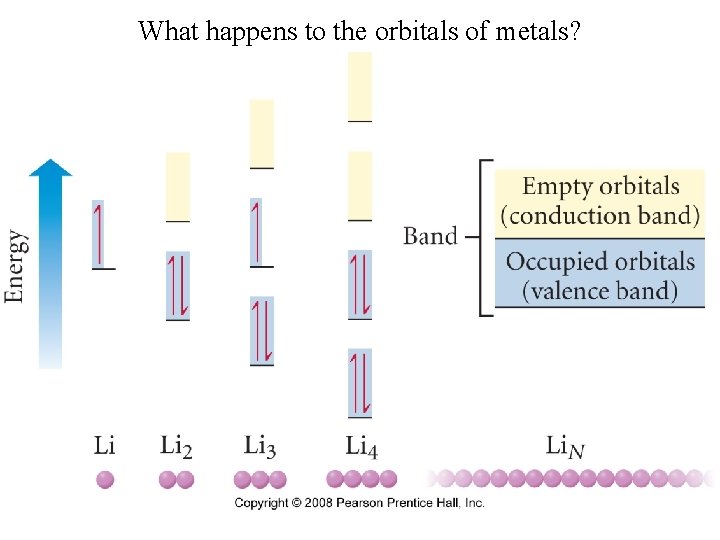
What happens to the orbitals of metals?

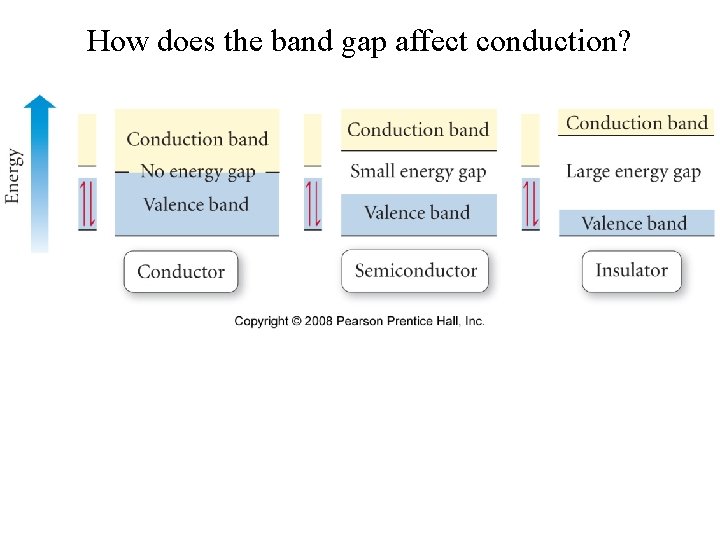
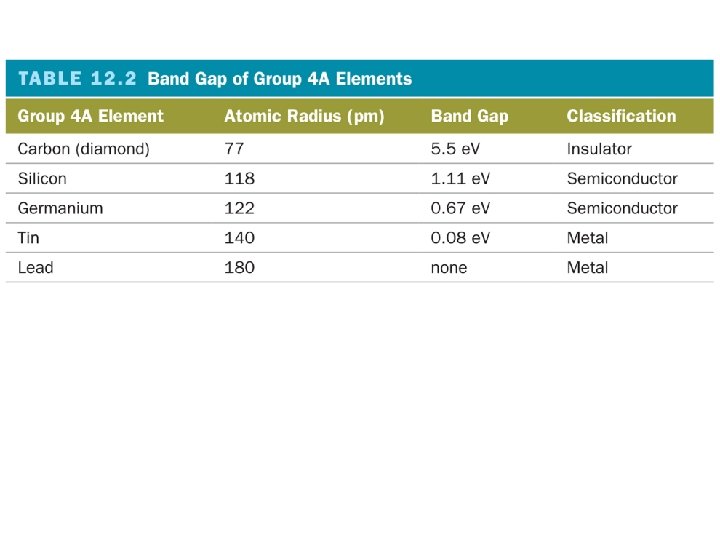
How does the band gap affect conduction?

How does molecular orbital theory relate to metallic bonding?

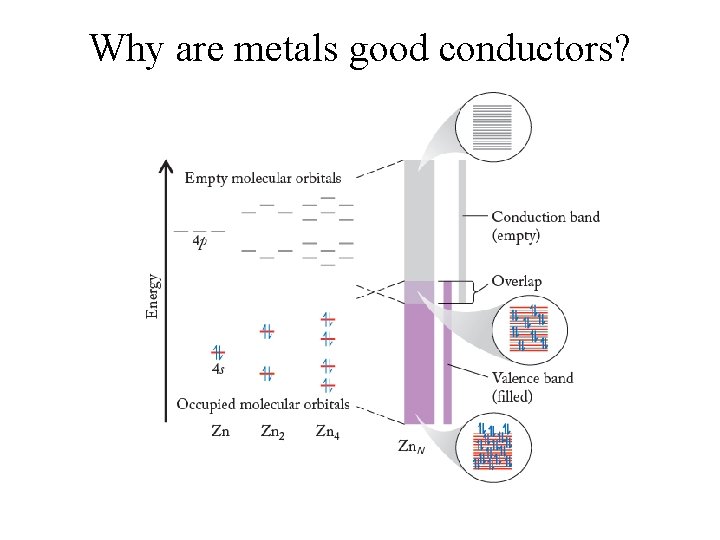
Why are metals good conductors?


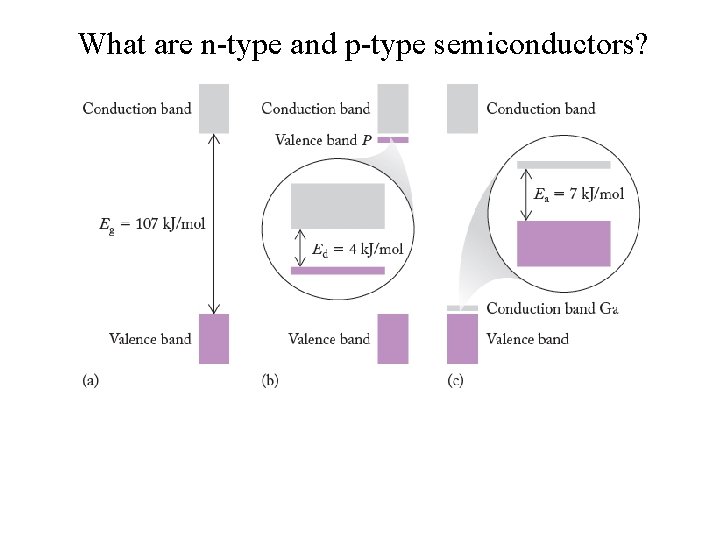
What are n-type and p-type semiconductors?

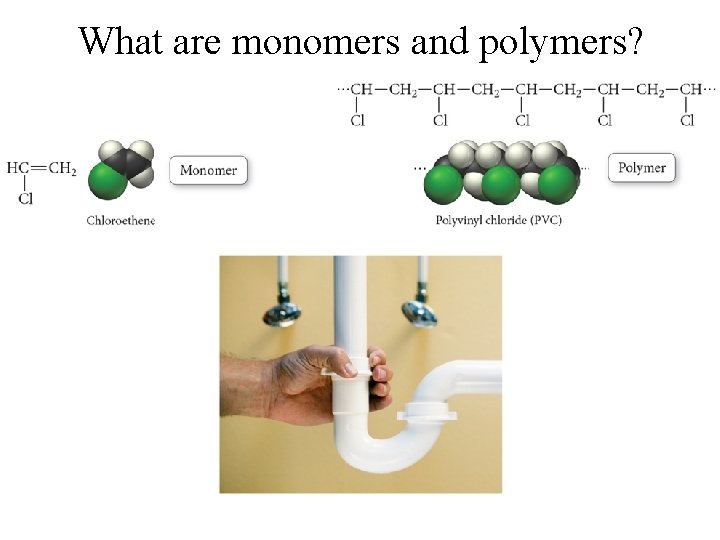
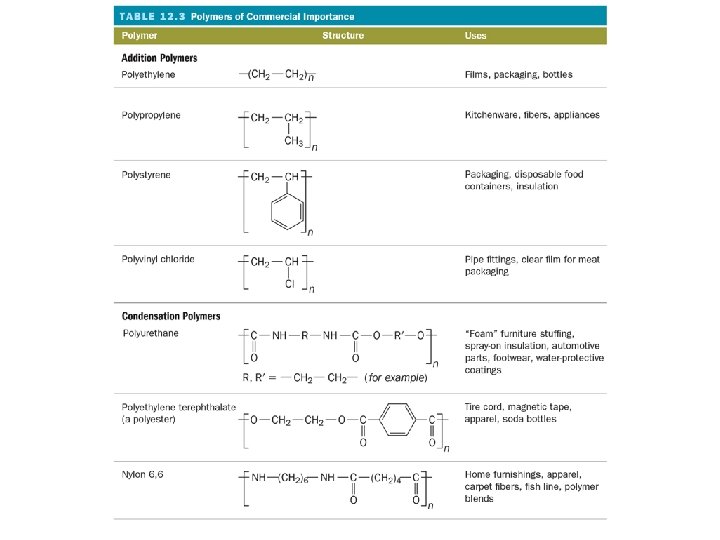
What are monomers and polymers?

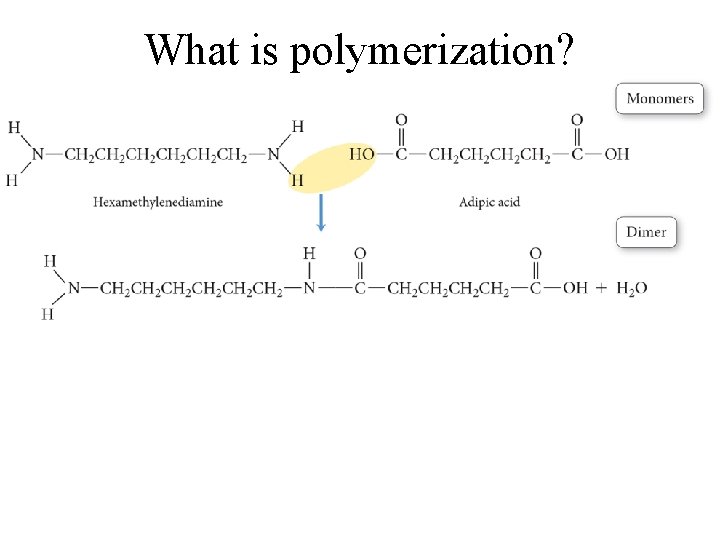
What is polymerization?
