Unit 12 Solids and Modern Materials Aim What





- Slides: 15

Unit 12: Solids and Modern Materials Aim: What are the four types of solids? Do Now: Which solid would have a higher boiling point? Covalent-Network Solids or Ionic Solids?

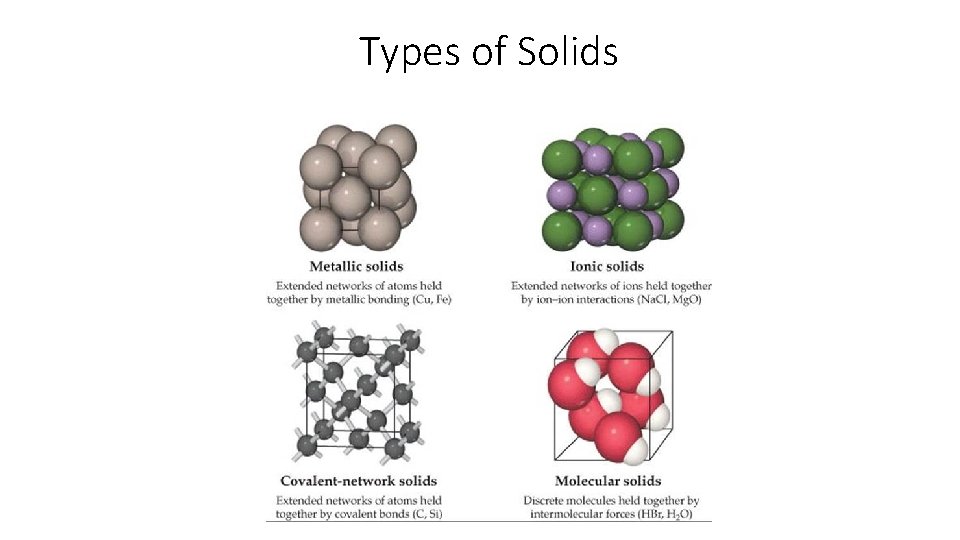
Types of Solids

Ionic Solids • Compounds of Metal Cations and Nonmetal Anions • Na. Cl, Al 2 O 3, K 2 SO 4, Fe. S • Crystal lattice of ions locked in place by relatively strong ionic bonds(electrostatic force) • Properties • Brittle, High melting non conductors in pure form, conductors in solution

Metallic Solids • Metal atoms ONLY! • Al, Fe, Cr, Ni • Metal ions surrounded by a uniform sea of delocalized(mobile) valence electrons • Properties • Good conductors of heat and electricity, malleable and ductile, mixtures of metals form alloys

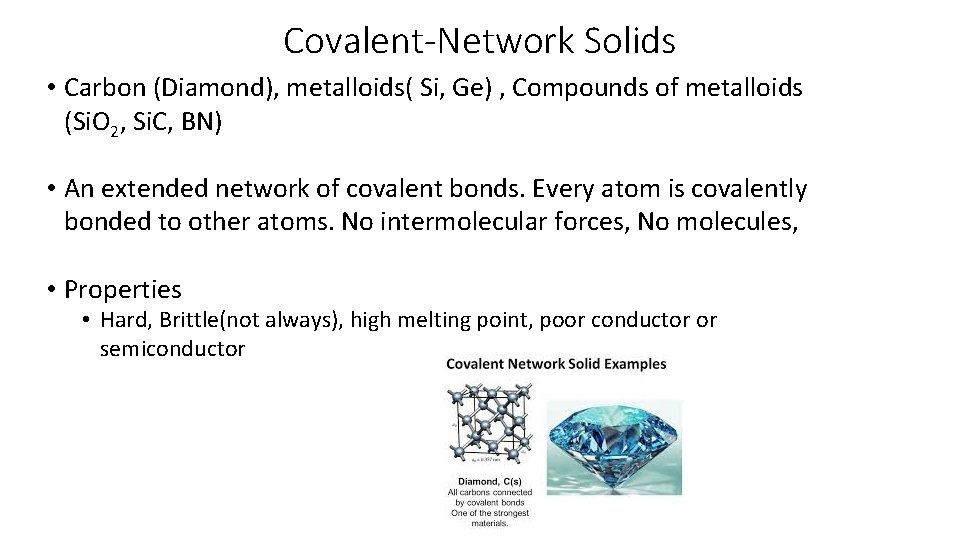
Covalent-Network Solids • Carbon (Diamond), metalloids( Si, Ge) , Compounds of metalloids (Si. O 2, Si. C, BN) • An extended network of covalent bonds. Every atom is covalently bonded to other atoms. No intermolecular forces, No molecules, • Properties • Hard, Brittle(not always), high melting point, poor conductor or semiconductor

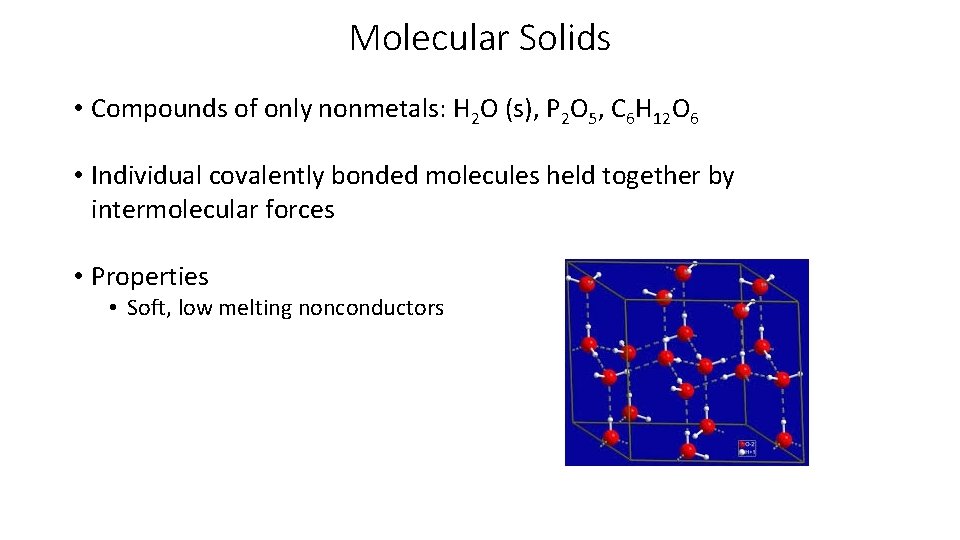
Molecular Solids • Compounds of only nonmetals: H 2 O (s), P 2 O 5, C 6 H 12 O 6 • Individual covalently bonded molecules held together by intermolecular forces • Properties • Soft, low melting nonconductors

Practice Classify each of the following chemical solids according to bonding type: CBr 4 (s), Ga. As, Na 3 PO 4, Mg

Metallic Bonding • Because the electrons in metals are delocalized, none of them belong to any metal cation. • High electrical conductivity in metals results from loosely held, mobile electrons. Electrical current easily moves through the electron sea • High thermal conductivity results because the mobile electrons can readily transfer kinetic energy(heat) through the solid • Alloys have properties of pure metal but contain two or more elements

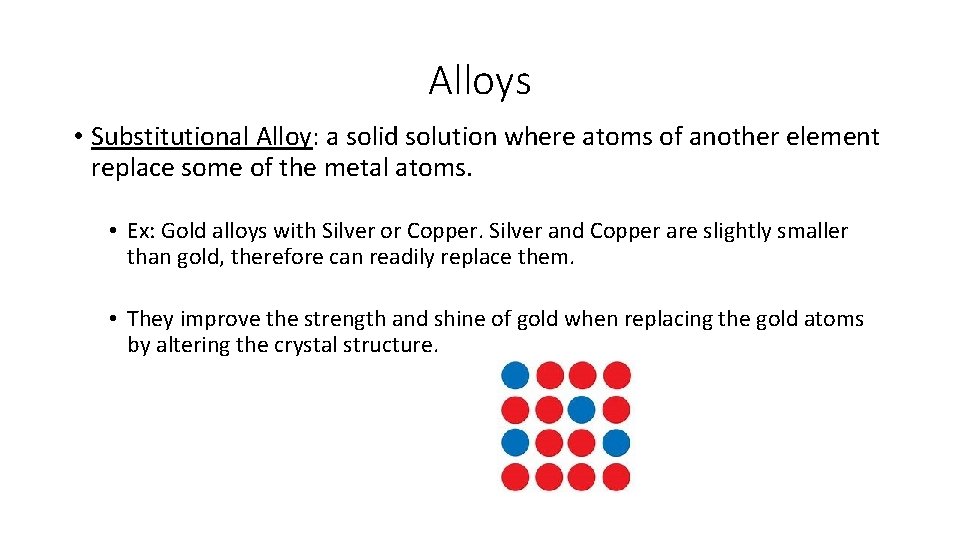
Alloys • Substitutional Alloy: a solid solution where atoms of another element replace some of the metal atoms. • Ex: Gold alloys with Silver or Copper. Silver and Copper are slightly smaller than gold, therefore can readily replace them. • They improve the strength and shine of gold when replacing the gold atoms by altering the crystal structure.

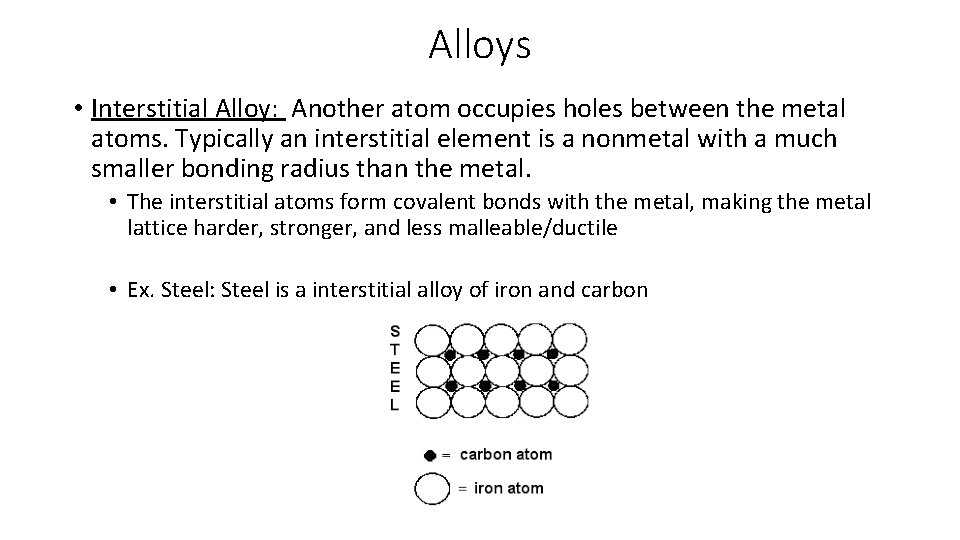
Alloys • Interstitial Alloy: Another atom occupies holes between the metal atoms. Typically an interstitial element is a nonmetal with a much smaller bonding radius than the metal. • The interstitial atoms form covalent bonds with the metal, making the metal lattice harder, stronger, and less malleable/ductile • Ex. Steel: Steel is a interstitial alloy of iron and carbon

Ionic Solids • Ionic Solids adhere to Coulomb’s Law • F=k. Q 1 Q 2/d 2 • Small ions with larger charges will produce strong forces of attraction. Larger ions with smaller charges will be less tightly bonded. • Ionic substances are insulators not conductors • Unless in the aqueous form. • Ionic substances are brittle because of repulsive forces of ions of like charge.

Molecular Solids • Molecular solids are held together by relatively weak dipole-dipole, London dispersion, and/or hydrogen bonds. • Electrons are confined to covalent bonds, therefore they are nonconductors. • Low molar mass molecular substances such as water and carbon dioxide tend to be liquids and gases at room temperature and form solids only at cold temperatures.

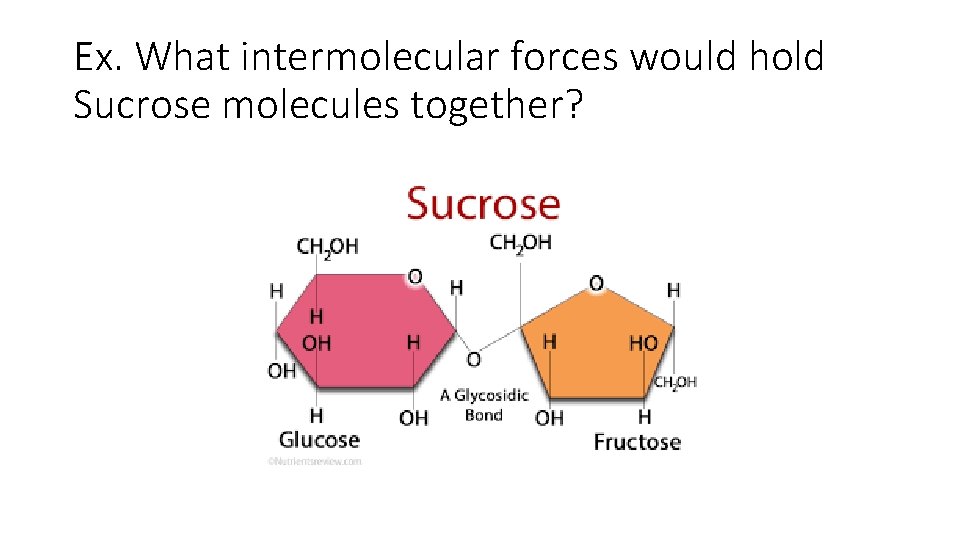
Ex. What intermolecular forces would hold Sucrose molecules together?

Covalent-Network Solids • Held together by chains or networks of covalent bonds • This makes them have higher melting points and hardness. • Graphite is composed of sheets that are bonded like network solids. Each sheet is called Graphene. The sheets are held together with intermolecular forces. • Diamond is held together strictly with covalent bonds

Doping and Semiconductors • Doping: The process of adding small amounts of impurities to the crystal lattice of a material to influence its electrical conductivity • n-type semiconductor: can carry a negative charge, by replacing a few atoms of pure silicon, each which has 4 valence electrons, with atoms of phosphorus, which contain five valence electrons. The ‘extra’ valence electrons greatly increase the conductivity of silicon. • p-type semiconductor: silicon doped with a Group 13 (3 A) element having three valence electrons. The resulting material has adeficiency of valence electrons called holes. A hole can be thought of as having a positive charge. Conductivity is increased because electrons can jump from hole to hole as they move through the material. • N and P type semiconductors are the basis for many electronic devices such as diodes, transistors, and solar cells.