Chapter 12 Solids and Modern Materials 2012 Pearson












- Slides: 62

Chapter 12 Solids and Modern Materials © 2012 Pearson Education, Inc.

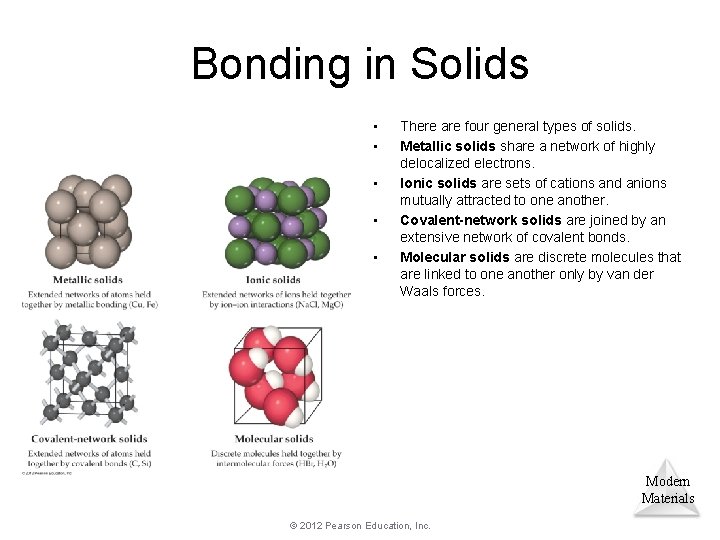
Bonding in Solids • • • There are four general types of solids. Metallic solids share a network of highly delocalized electrons. Ionic solids are sets of cations and anions mutually attracted to one another. Covalent-network solids are joined by an extensive network of covalent bonds. Molecular solids are discrete molecules that are linked to one another only by van der Waals forces. Modern Materials © 2012 Pearson Education, Inc.

Bonding in Solids • In crystalline solids atoms are arranged in a very regular pattern. • Amorphous solids are characterized by a distinct lack of order in the arrangement of atoms. Modern Materials © 2012 Pearson Education, Inc.

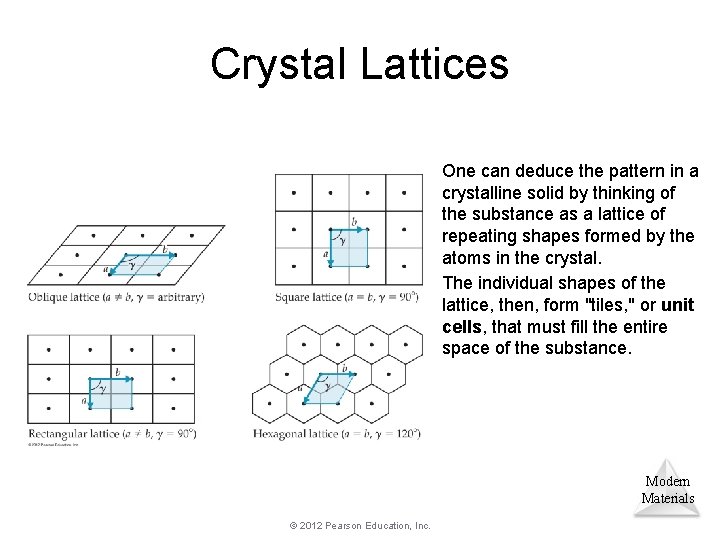
Crystal Lattices One can deduce the pattern in a crystalline solid by thinking of the substance as a lattice of repeating shapes formed by the atoms in the crystal. The individual shapes of the lattice, then, form "tiles, " or unit cells, that must fill the entire space of the substance. Modern Materials © 2012 Pearson Education, Inc.

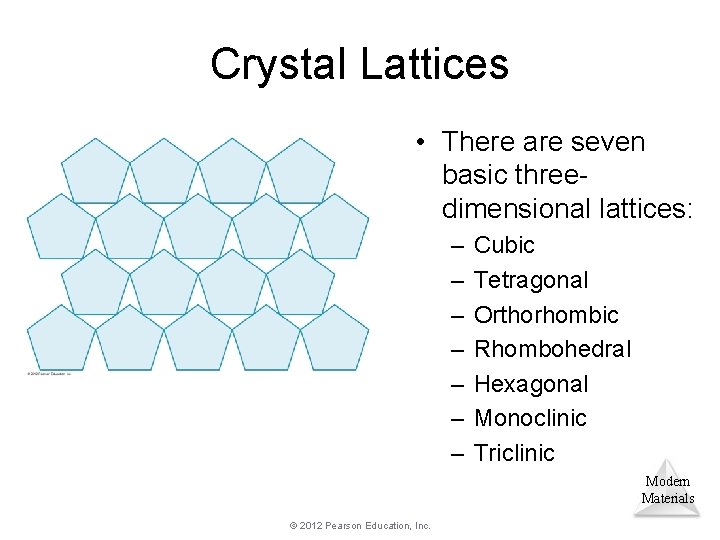
Crystal Lattices • There are seven basic threedimensional lattices: – – – – Cubic Tetragonal Orthorhombic Rhombohedral Hexagonal Monoclinic Triclinic Modern Materials © 2012 Pearson Education, Inc.

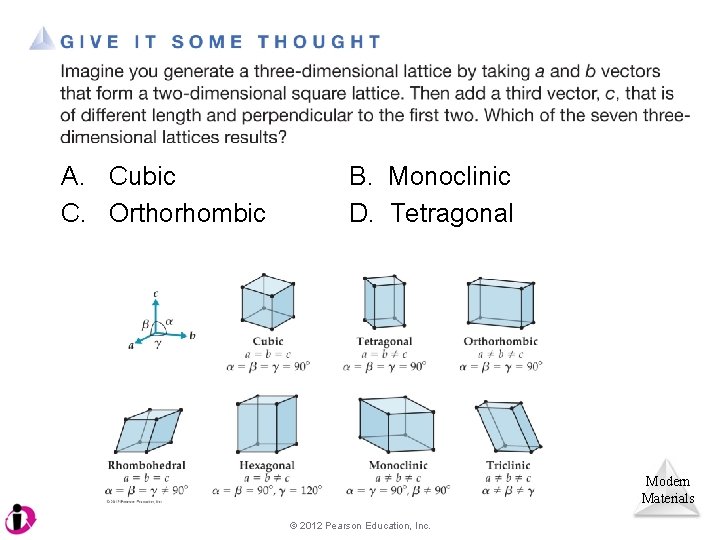
A. Cubic C. Orthorhombic B. Monoclinic D. Tetragonal Modern Materials © 2012 Pearson Education, Inc.

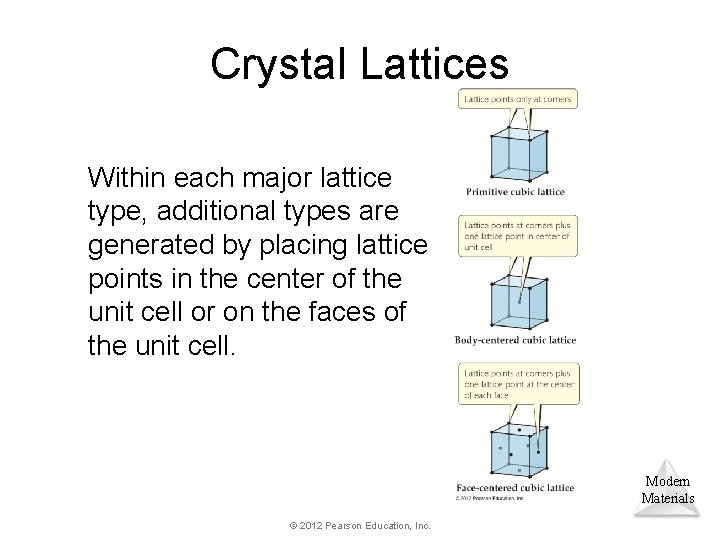
Crystal Lattices Within each major lattice type, additional types are generated by placing lattice points in the center of the unit cell or on the faces of the unit cell. Modern Materials © 2012 Pearson Education, Inc.

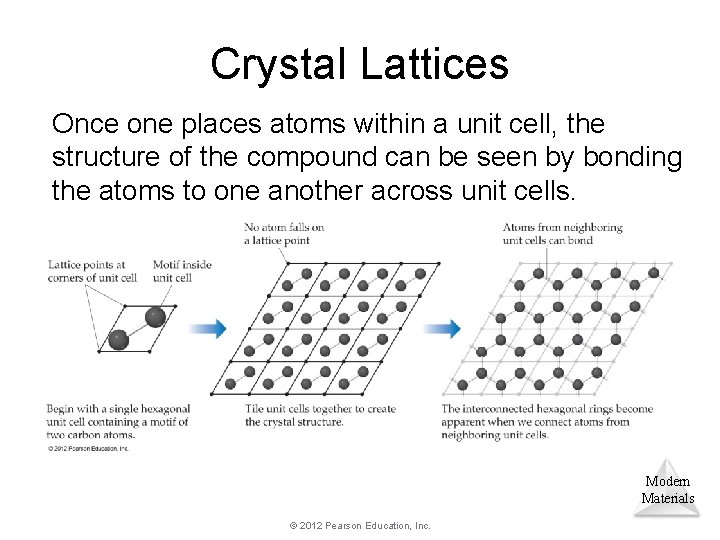
Crystal Lattices Once one places atoms within a unit cell, the structure of the compound can be seen by bonding the atoms to one another across unit cells. Modern Materials © 2012 Pearson Education, Inc.

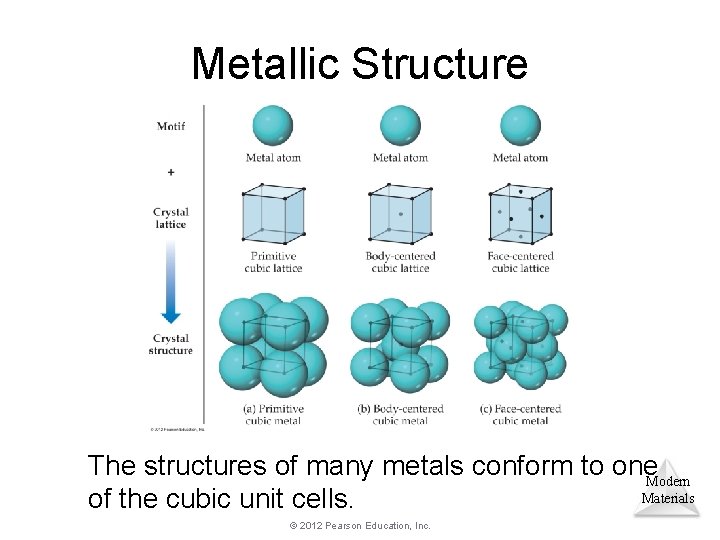
Metallic Structure The structures of many metals conform to one. Modern Materials of the cubic unit cells. © 2012 Pearson Education, Inc.

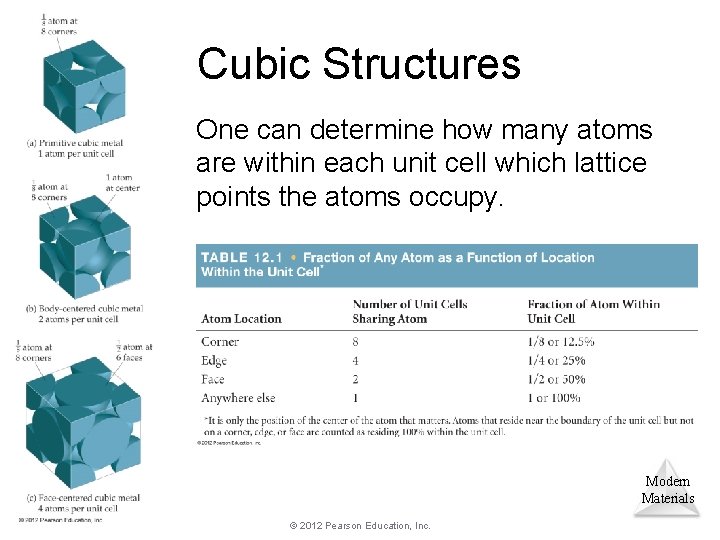
Cubic Structures One can determine how many atoms are within each unit cell which lattice points the atoms occupy. Modern Materials © 2012 Pearson Education, Inc.

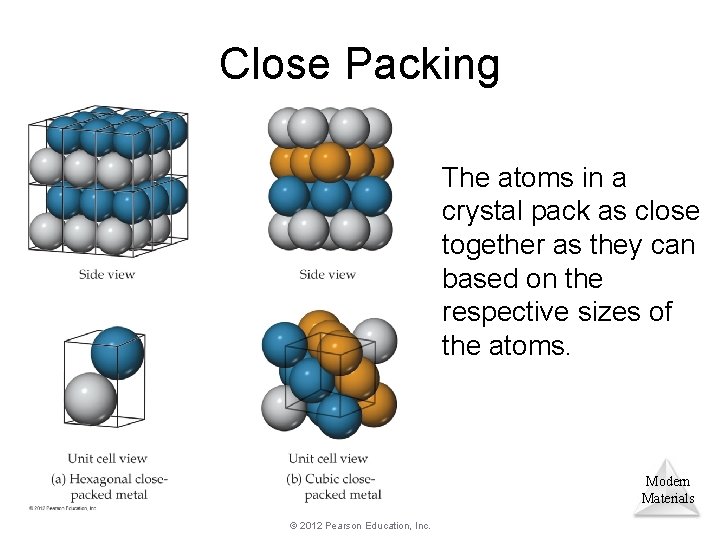
Close Packing The atoms in a crystal pack as close together as they can based on the respective sizes of the atoms. Modern Materials © 2012 Pearson Education, Inc.

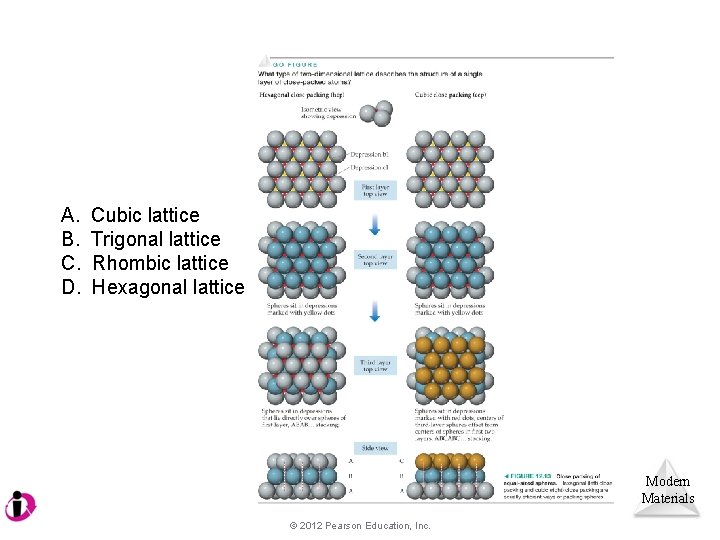
A. B. C. D. Cubic lattice Trigonal lattice Rhombic lattice Hexagonal lattice Modern Materials © 2012 Pearson Education, Inc.

Sample Exercise 12. 1 Calculating Packing Efficiency It is not possible to pack spheres together without leaving some void spaces between the spheres. Packing efficiency is the fraction of space in a crystal that is actually occupied by atoms. Determine the packing efficiency of a face-centered cubic metal. Practice Exercise Determine the packing efficiency by calculating the fraction of space occupied by atoms in a body-centered cubic metal. Modern Materials © 2012 Pearson Education, Inc.

A. Packing efficiency increases as the number of nearest neighbors decreases. B. Packing efficiency increases as the volume occupied by atoms decreases compared to the volume of the unit cell. C. Packing efficiency decreases as the number of nearest neighbors decreases. D. Packing efficiency decreases as the size of the nearest neighbors decreases. Modern Materials © 2012 Pearson Education, Inc.

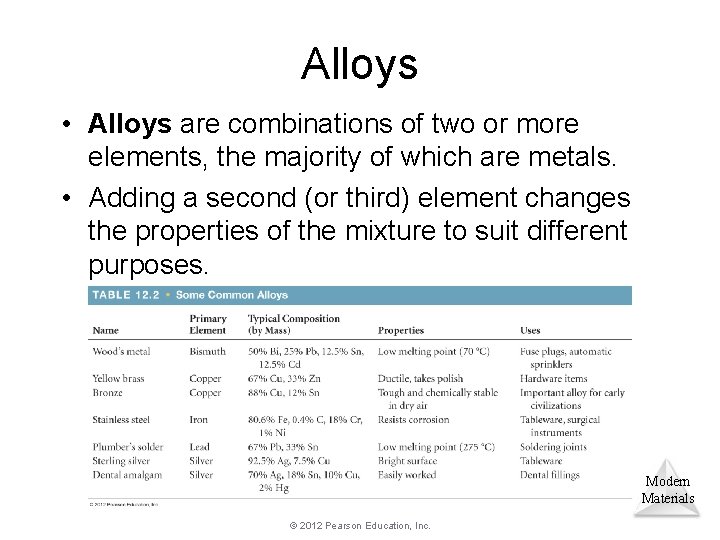
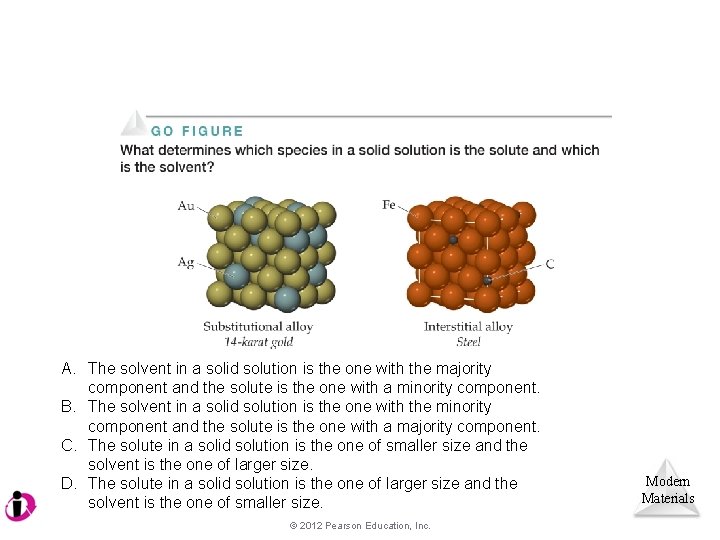
Alloys • Alloys are combinations of two or more elements, the majority of which are metals. • Adding a second (or third) element changes the properties of the mixture to suit different purposes. Modern Materials © 2012 Pearson Education, Inc.

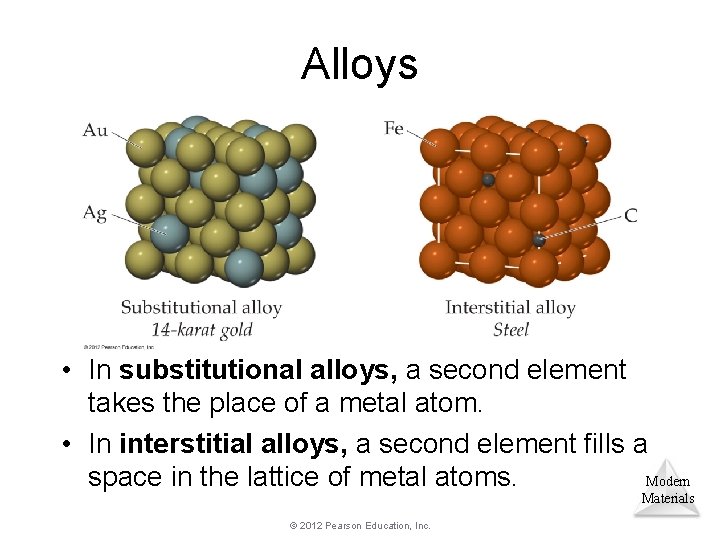
Alloys • In substitutional alloys, a second element takes the place of a metal atom. • In interstitial alloys, a second element fills a space in the lattice of metal atoms. Modern Materials © 2012 Pearson Education, Inc.

A. The solvent in a solid solution is the one with the majority component and the solute is the one with a minority component. B. The solvent in a solid solution is the one with the minority component and the solute is the one with a majority component. C. The solute in a solid solution is the one of smaller size and the solvent is the one of larger size. D. The solute in a solid solution is the one of larger size and the solvent is the one of smaller size. © 2012 Pearson Education, Inc. Modern Materials

A. Interstitial alloy, because B has a small size and can occupy “holes” between Pd atoms. B. Substitutional alloy, because B has a size that is sufficiently large to occupy positions held by Pd atoms. Modern Materials © 2012 Pearson Education, Inc.

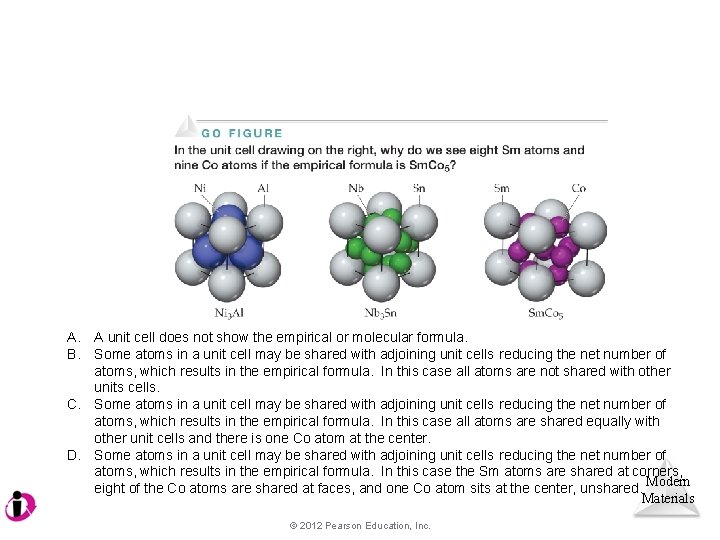
A. B. A unit cell does not show the empirical or molecular formula. Some atoms in a unit cell may be shared with adjoining unit cells reducing the net number of atoms, which results in the empirical formula. In this case all atoms are not shared with other units cells. C. Some atoms in a unit cell may be shared with adjoining unit cells reducing the net number of atoms, which results in the empirical formula. In this case all atoms are shared equally with other unit cells and there is one Co atom at the center. D. Some atoms in a unit cell may be shared with adjoining unit cells reducing the net number of atoms, which results in the empirical formula. In this case the Sm atoms are shared at corners, eight of the Co atoms are shared at faces, and one Co atom sits at the center, unshared. Modern Materials © 2012 Pearson Education, Inc.

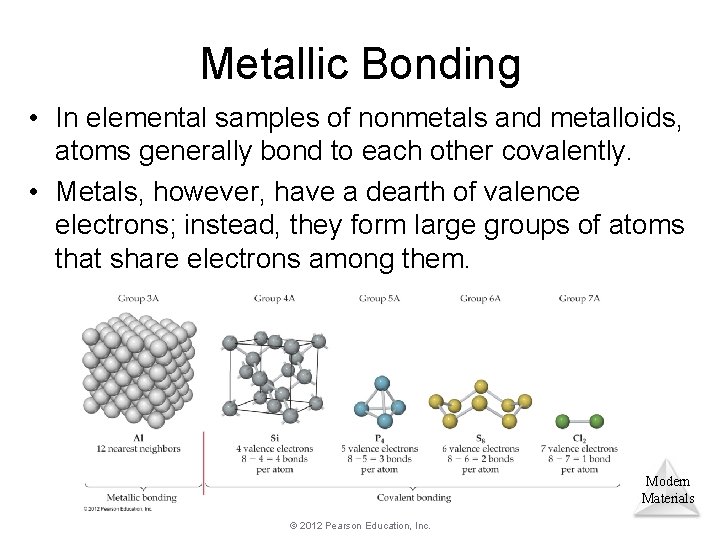
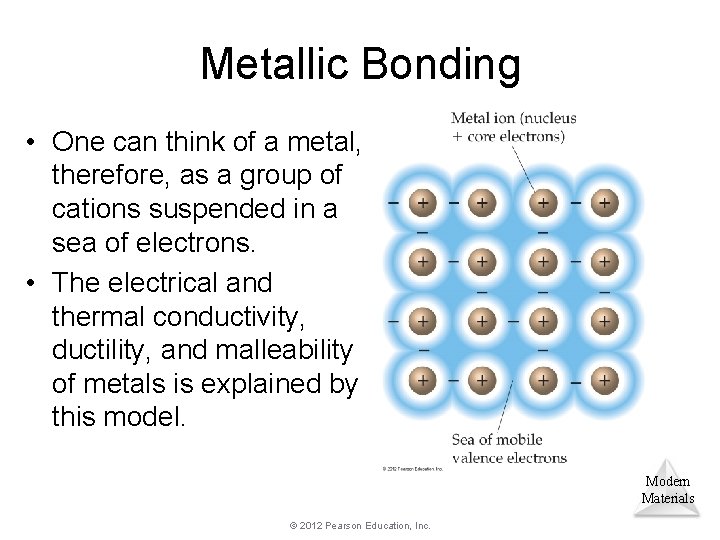
Metallic Bonding • In elemental samples of nonmetals and metalloids, atoms generally bond to each other covalently. • Metals, however, have a dearth of valence electrons; instead, they form large groups of atoms that share electrons among them. Modern Materials © 2012 Pearson Education, Inc.

A. Red gold B. Purple gold Modern Materials © 2012 Pearson Education, Inc.

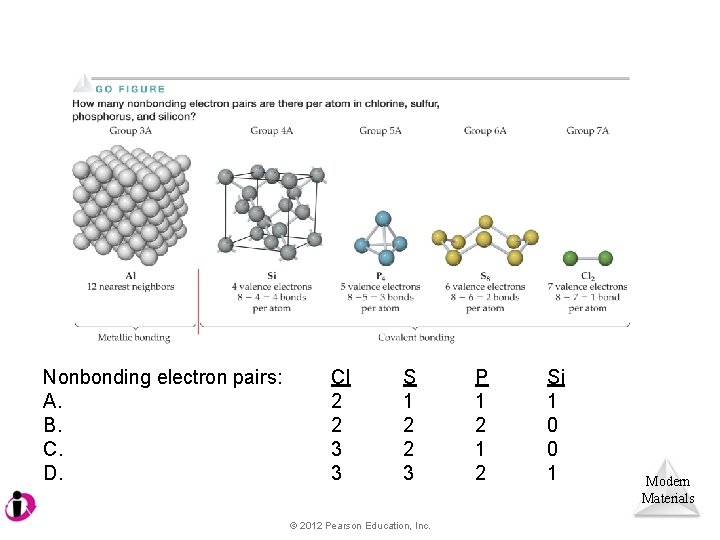
Nonbonding electron pairs: A. B. C. D. Cl 2 2 3 3 S 1 2 2 3 © 2012 Pearson Education, Inc. P 1 2 Si 1 0 0 1 Modern Materials

Metallic Bonding • One can think of a metal, therefore, as a group of cations suspended in a sea of electrons. • The electrical and thermal conductivity, ductility, and malleability of metals is explained by this model. Modern Materials © 2012 Pearson Education, Inc.

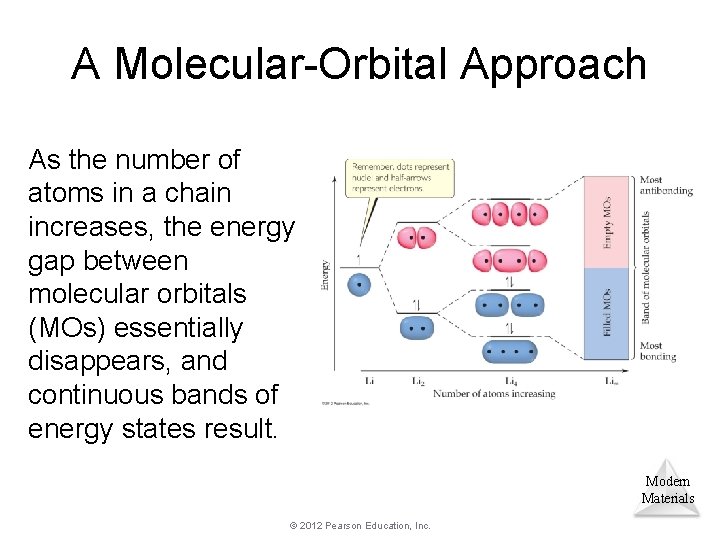
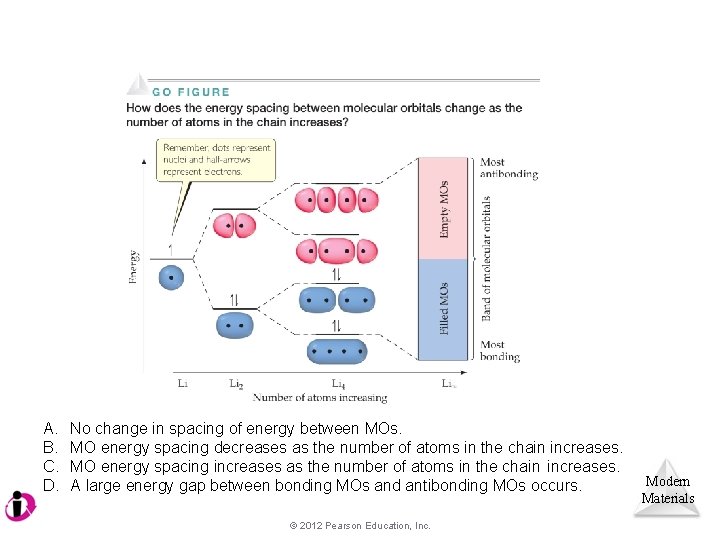
A Molecular-Orbital Approach As the number of atoms in a chain increases, the energy gap between molecular orbitals (MOs) essentially disappears, and continuous bands of energy states result. Modern Materials © 2012 Pearson Education, Inc.

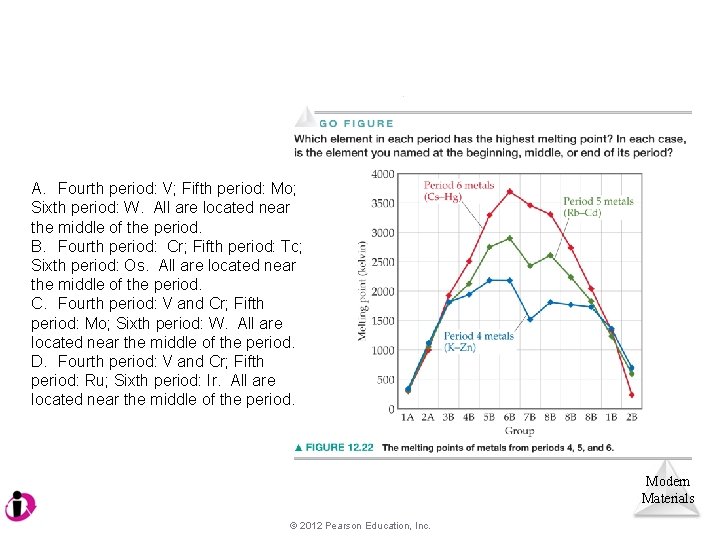
A. Fourth period: V; Fifth period: Mo; Sixth period: W. All are located near the middle of the period. B. Fourth period: Cr; Fifth period: Tc; Sixth period: Os. All are located near the middle of the period. C. Fourth period: V and Cr; Fifth period: Mo; Sixth period: W. All are located near the middle of the period. D. Fourth period: V and Cr; Fifth period: Ru; Sixth period: Ir. All are located near the middle of the period. Modern Materials © 2012 Pearson Education, Inc.

A. B. C. D. No change in spacing of energy between MOs. MO energy spacing decreases as the number of atoms in the chain increases. MO energy spacing increases as the number of atoms in the chain increases. A large energy gap between bonding MOs and antibonding MOs occurs. © 2012 Pearson Education, Inc. Modern Materials

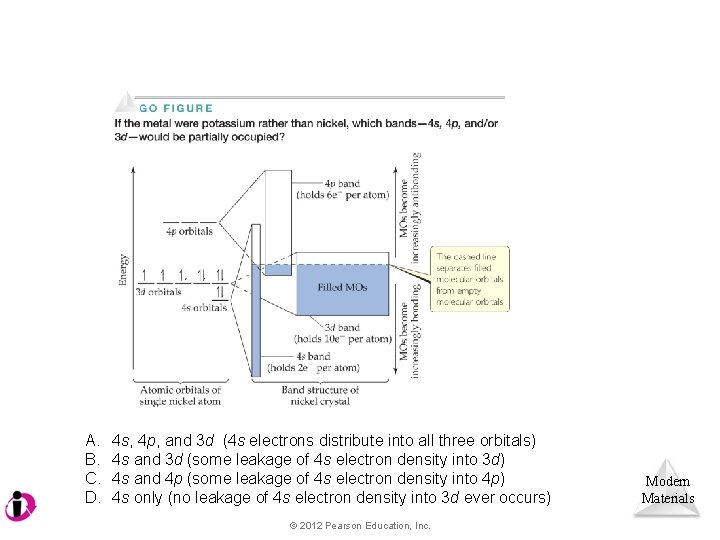
A. B. C. D. 4 s, 4 p, and 3 d (4 s electrons distribute into all three orbitals) 4 s and 3 d (some leakage of 4 s electron density into 3 d) 4 s and 4 p (some leakage of 4 s electron density into 4 p) 4 s only (no leakage of 4 s electron density into 3 d ever occurs) © 2012 Pearson Education, Inc. Modern Materials

A. Tungsten (W) has the greater number of electrons in antibonding orbitals and gold (Au) has the higher melting point. B. Au has the greater number of electrons in antibonding orbitals and W has the higher melting point. C. W has both the greater number of electrons in antibonding orbitals and higher melting point. Modern Materials © 2012 Pearson Education, Inc.

Ionic Solids • In ionic solids, the lattice comprises alternately charged ions. • Ionic solids have very high melting and boiling points and are quintessential crystals. Modern Materials © 2012 Pearson Education, Inc.

A. Metals cleave more readily because the atoms have zero charge. Ions in ionic substances are tightly bound to others of opposite charge and this limits cleavage. B. Metals don’t cleave readily as the atoms that have zero charge are strongly attracted to one another through metallic bonding. Nearest neighbor ions in ionic substances can move and rearrange themselves during cleavage. C. Metals contain atoms of the same size and thus the principles in Figure 12. 25 do not apply. D. Metal atoms are more electropositive than cations in ionic solids and thus only move with great force because of their greater attraction to other metal atoms. Modern Materials © 2012 Pearson Education, Inc.

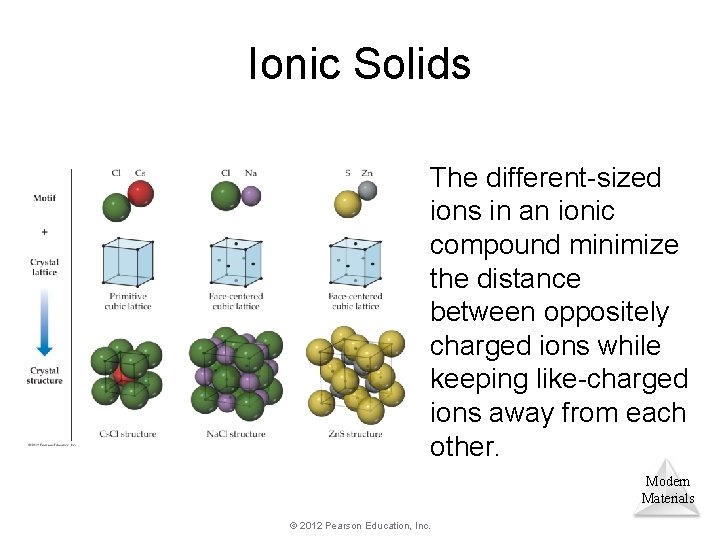
Ionic Solids The different-sized ions in an ionic compound minimize the distance between oppositely charged ions while keeping like-charged ions away from each other. Modern Materials © 2012 Pearson Education, Inc.

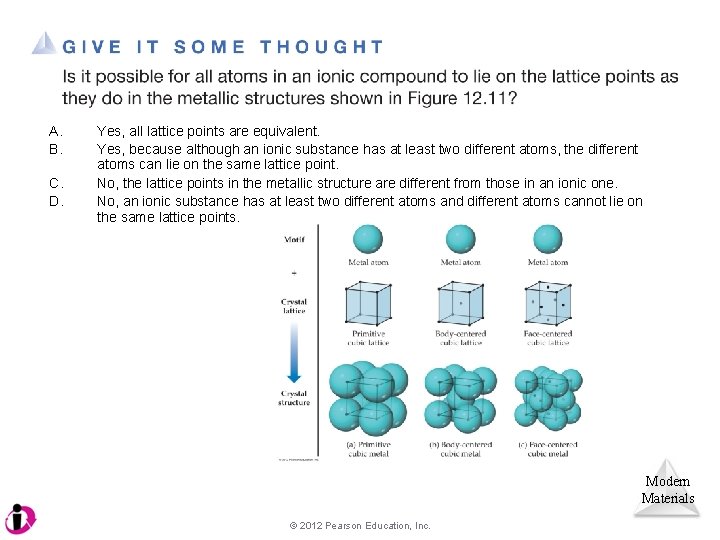
A. B. C. D. Yes, all lattice points are equivalent. Yes, because although an ionic substance has at least two different atoms, the different atoms can lie on the same lattice point. No, the lattice points in the metallic structure are different from those in an ionic one. No, an ionic substance has at least two different atoms and different atoms cannot lie on the same lattice points. Modern Materials © 2012 Pearson Education, Inc.

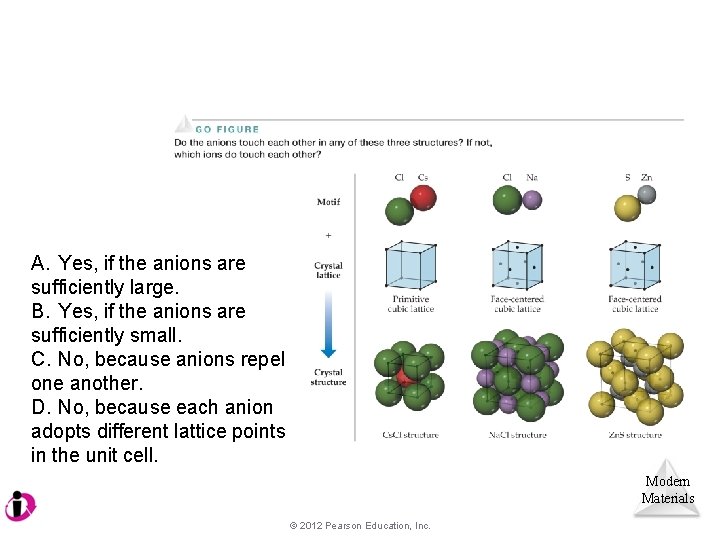
A. Yes, if the anions are sufficiently large. B. Yes, if the anions are sufficiently small. C. No, because anions repel one another. D. No, because each anion adopts different lattice points in the unit cell. Modern Materials © 2012 Pearson Education, Inc.

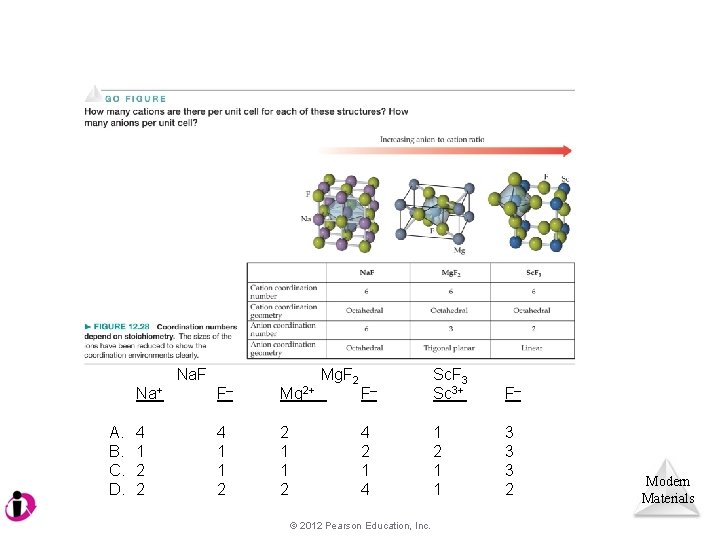
Na. F A. B. C. D. Na+ F– Mg 2+ 4 1 2 2 4 1 1 2 2 1 1 2 Mg. F 2 F– Sc. F 3 Sc 3+ F– 4 2 1 4 1 2 1 1 3 3 3 2 © 2012 Pearson Education, Inc. Modern Materials

A. B. C. D. 2 4 6 8 Modern Materials © 2012 Pearson Education, Inc.

Sample Exercise 12. 2 Calculating the Empirical Formula and Density of an Ionic Solid The unit cell of a binary compound of copper and oxygen is shown here. Given this image and the ionic radii r. Cu+ = 0. 74 Å and r. O 2 = 1. 26 Å, (a) determine the empirical formula of this compound, (b) determine the coordination numbers of copper and oxygen, (c) estimate the length of the edge of the cubic unit cell, and (d) estimate the density of the compound. Practice Exercise Estimate the length of the cubic unit cell edge and the density of Cs. Cl (Figure 12. 26) from the ionic radii of cesium, 1. 81 Å, and chloride, 1. 67 Å. Modern Materials © 2012 Pearson Education, Inc.

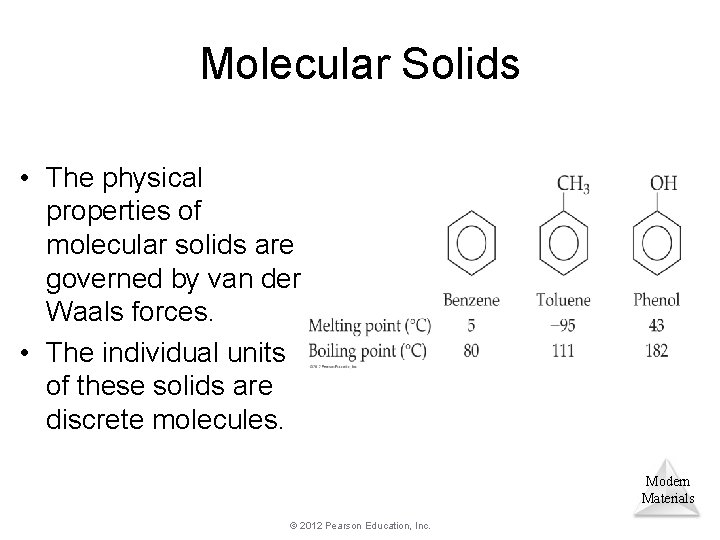
Molecular Solids • The physical properties of molecular solids are governed by van der Waals forces. • The individual units of these solids are discrete molecules. Modern Materials © 2012 Pearson Education, Inc.

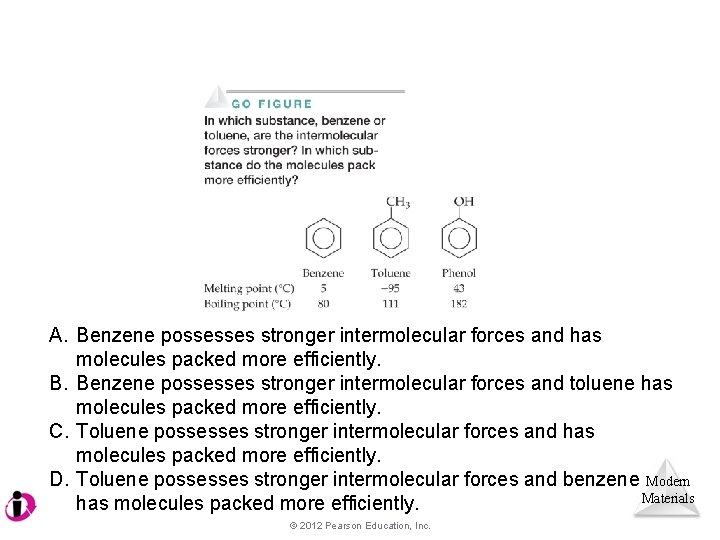
A. Benzene possesses stronger intermolecular forces and has molecules packed more efficiently. B. Benzene possesses stronger intermolecular forces and toluene has molecules packed more efficiently. C. Toluene possesses stronger intermolecular forces and has molecules packed more efficiently. D. Toluene possesses stronger intermolecular forces and benzene Modern Materials has molecules packed more efficiently. © 2012 Pearson Education, Inc.

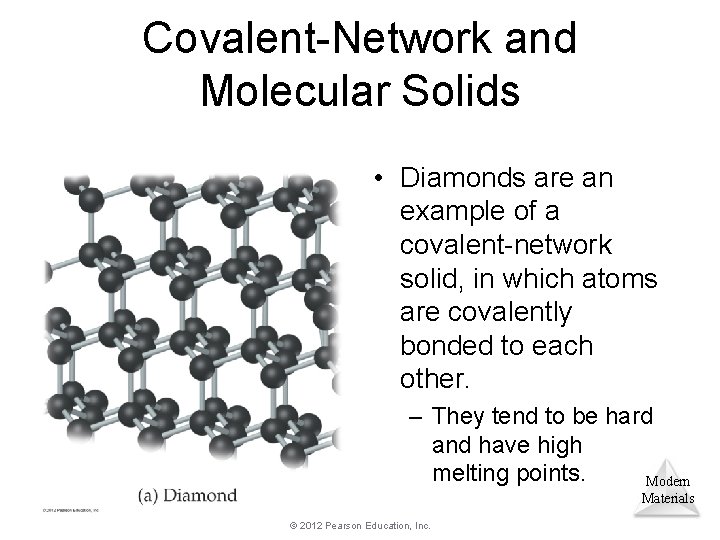
Covalent-Network and Molecular Solids • Diamonds are an example of a covalent-network solid, in which atoms are covalently bonded to each other. – They tend to be hard and have high melting points. Modern Materials © 2012 Pearson Education, Inc.

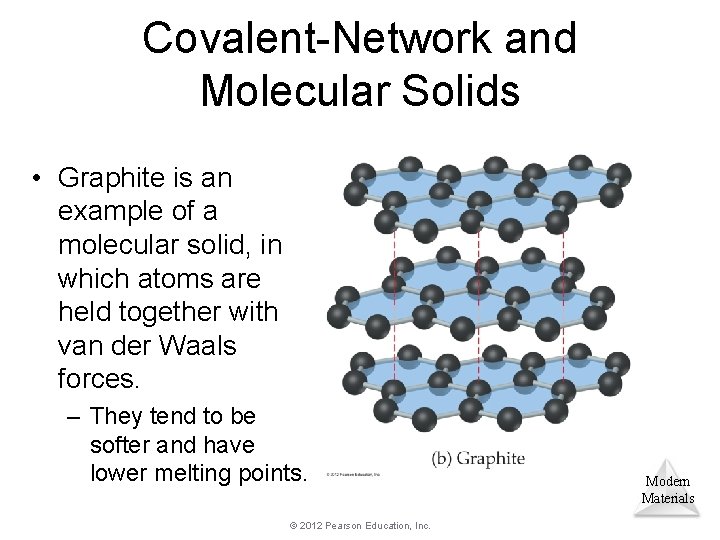
Covalent-Network and Molecular Solids • Graphite is an example of a molecular solid, in which atoms are held together with van der Waals forces. – They tend to be softer and have lower melting points. © 2012 Pearson Education, Inc. Modern Materials

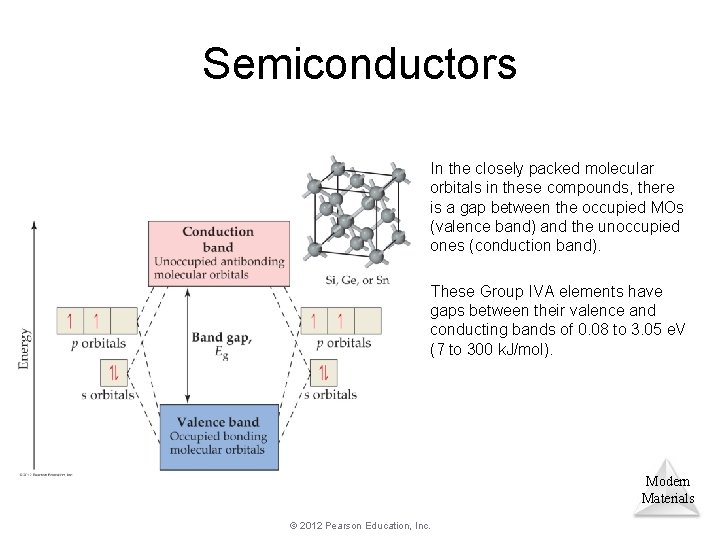
Semiconductors In the closely packed molecular orbitals in these compounds, there is a gap between the occupied MOs (valence band) and the unoccupied ones (conduction band). These Group IVA elements have gaps between their valence and conducting bands of 0. 08 to 3. 05 e. V (7 to 300 k. J/mol). Modern Materials © 2012 Pearson Education, Inc.

Semiconductors • Among elements, only Group IVA, all of which have 4 valence electrons, are semiconductors. • Inorganic semiconductors (like Ga. As) tend to have an average of 4 valence electrons (3 for Ga, 5 for As). Modern Materials © 2012 Pearson Education, Inc.

Sample Exercise 12. 3 Qualitative Comparison of Semiconductor Band Gaps Will Ga. P have a larger or smaller band gap than Zn. S? Will it have a larger or smaller band gap than Ga. N? Practice Exercise Will Zn. Se have a larger or smaller band gap than Zn. S? Modern Materials © 2012 Pearson Education, Inc.

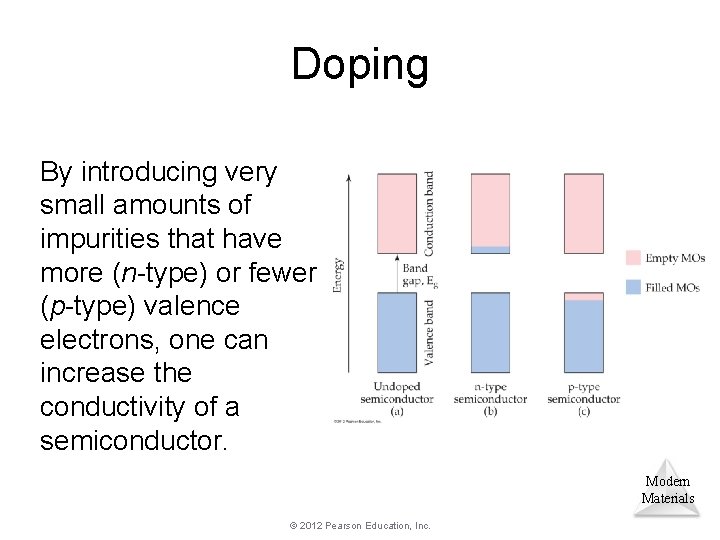
Doping By introducing very small amounts of impurities that have more (n-type) or fewer (p-type) valence electrons, one can increase the conductivity of a semiconductor. Modern Materials © 2012 Pearson Education, Inc.

Sample Exercise 12. 4 Identifying Types of Semiconductors Which of the following elements, if doped into silicon, would yield an n-type semiconductor: Ga, As, or C? Practice Exercise Suggest an element that could be used to dope silicon to yield a p-type material. Modern Materials © 2012 Pearson Education, Inc.

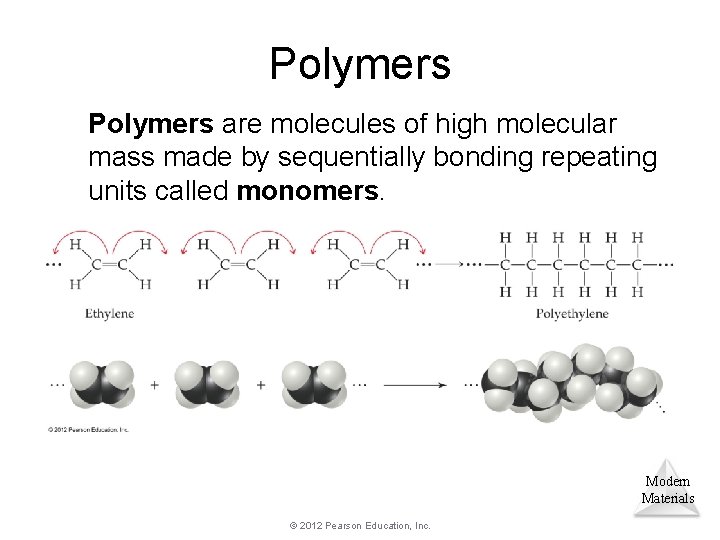
Polymers are molecules of high molecular mass made by sequentially bonding repeating units called monomers. Modern Materials © 2012 Pearson Education, Inc.

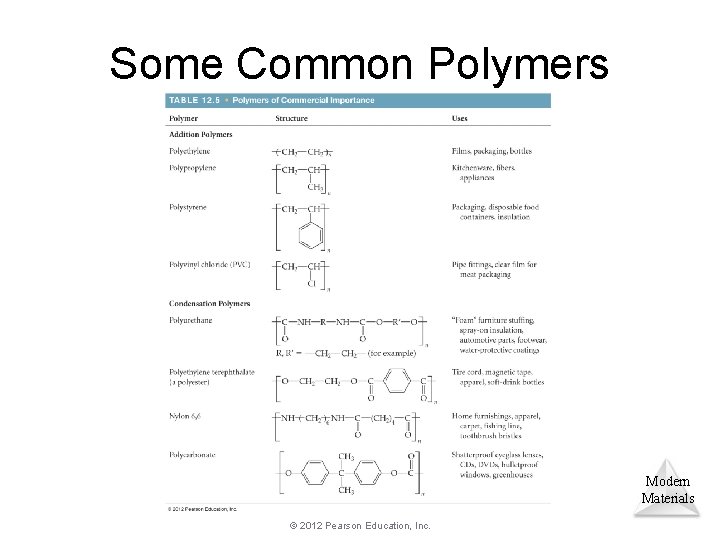
Some Common Polymers Modern Materials © 2012 Pearson Education, Inc.

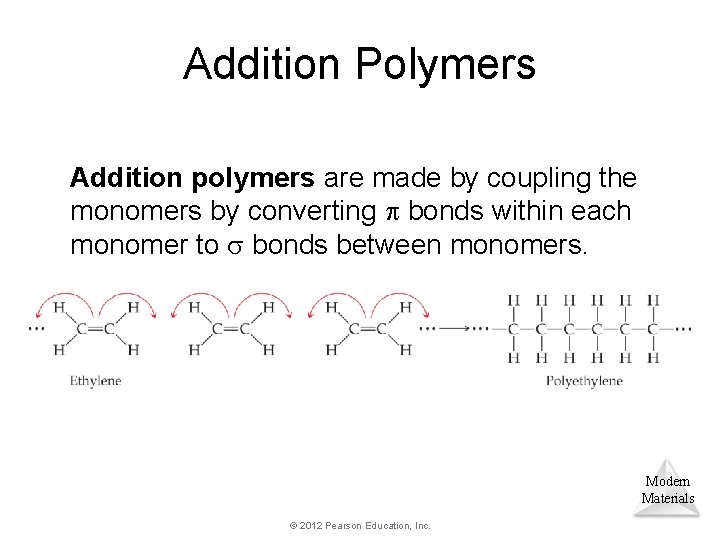
Addition Polymers Addition polymers are made by coupling the monomers by converting bonds within each monomer to bonds between monomers. Modern Materials © 2012 Pearson Education, Inc.

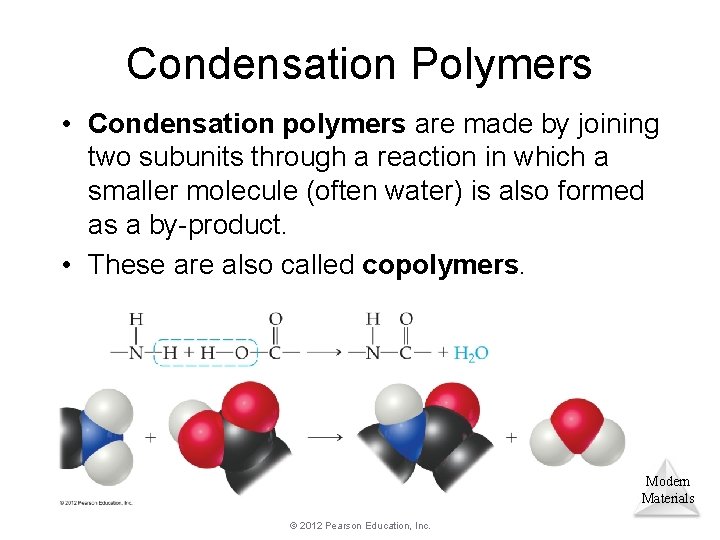
Condensation Polymers • Condensation polymers are made by joining two subunits through a reaction in which a smaller molecule (often water) is also formed as a by-product. • These are also called copolymers. Modern Materials © 2012 Pearson Education, Inc.

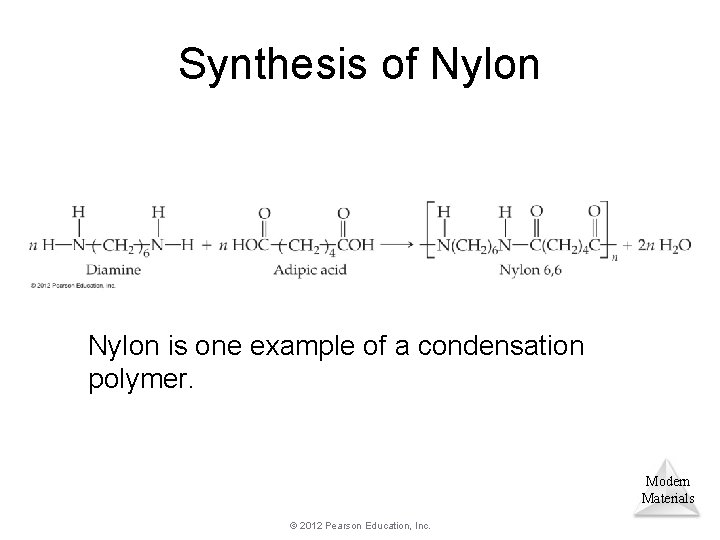
Synthesis of Nylon is one example of a condensation polymer. Modern Materials © 2012 Pearson Education, Inc.

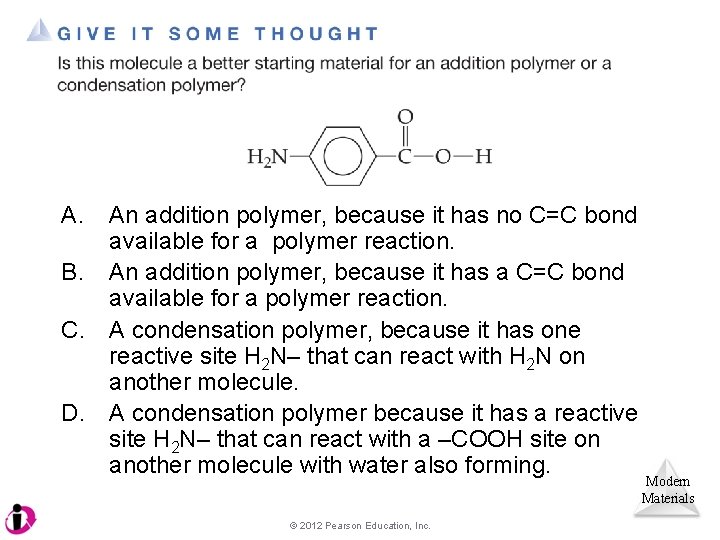
A. An addition polymer, because it has no C=C bond available for a polymer reaction. B. An addition polymer, because it has a C=C bond available for a polymer reaction. C. A condensation polymer, because it has one reactive site H 2 N– that can react with H 2 N on another molecule. D. A condensation polymer because it has a reactive site H 2 N– that can react with a –COOH site on another molecule with water also forming. © 2012 Pearson Education, Inc. Modern Materials

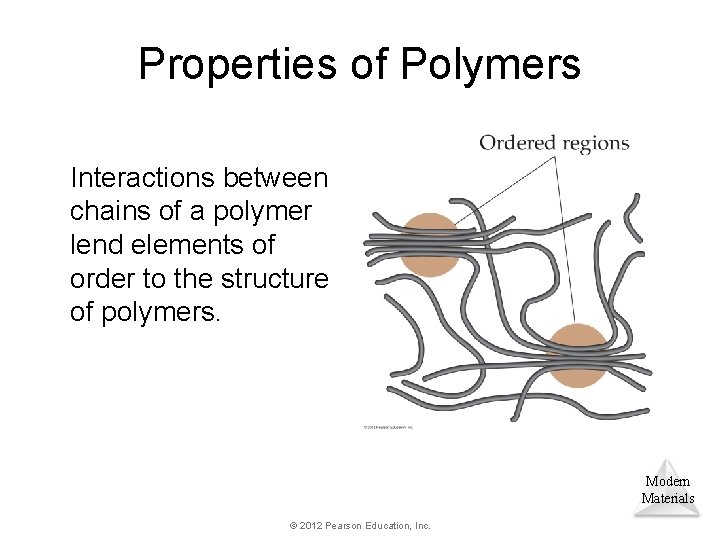
Properties of Polymers Interactions between chains of a polymer lend elements of order to the structure of polymers. Modern Materials © 2012 Pearson Education, Inc.

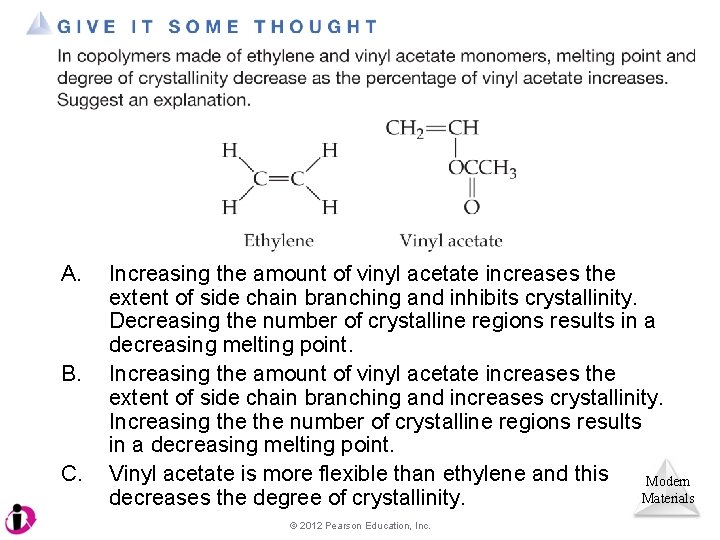
A. B. C. Increasing the amount of vinyl acetate increases the extent of side chain branching and inhibits crystallinity. Decreasing the number of crystalline regions results in a decreasing melting point. Increasing the amount of vinyl acetate increases the extent of side chain branching and increases crystallinity. Increasing the number of crystalline regions results in a decreasing melting point. Vinyl acetate is more flexible than ethylene and this Modern Materials decreases the degree of crystallinity. © 2012 Pearson Education, Inc.

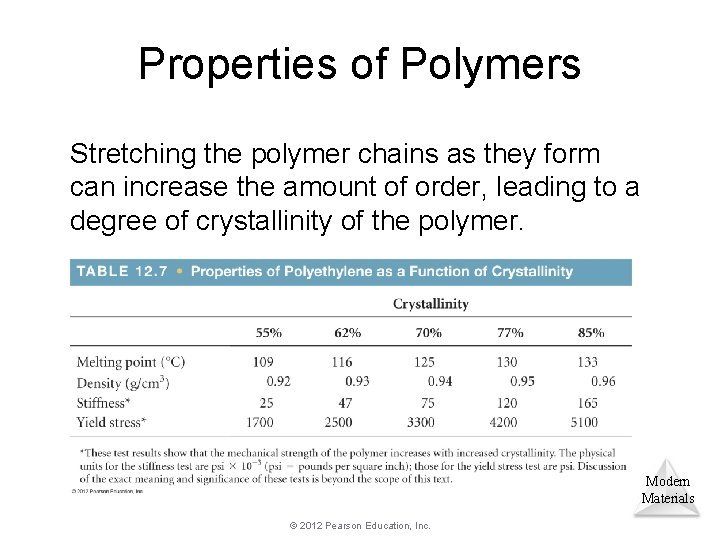
Properties of Polymers Stretching the polymer chains as they form can increase the amount of order, leading to a degree of crystallinity of the polymer. Modern Materials © 2012 Pearson Education, Inc.

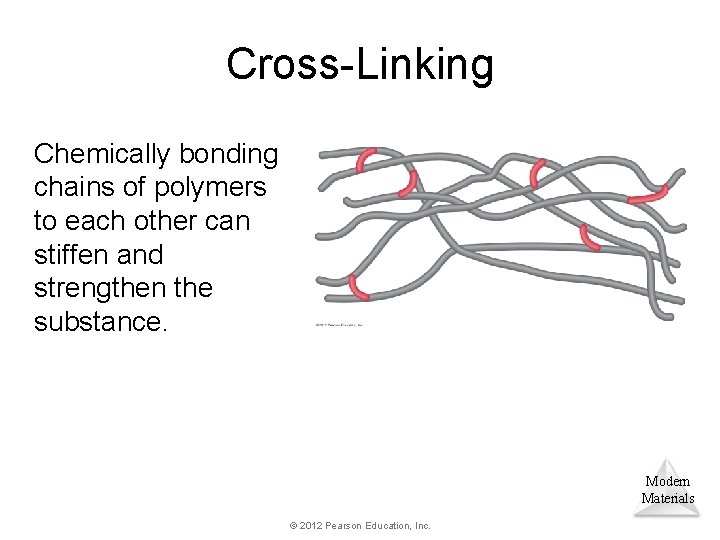
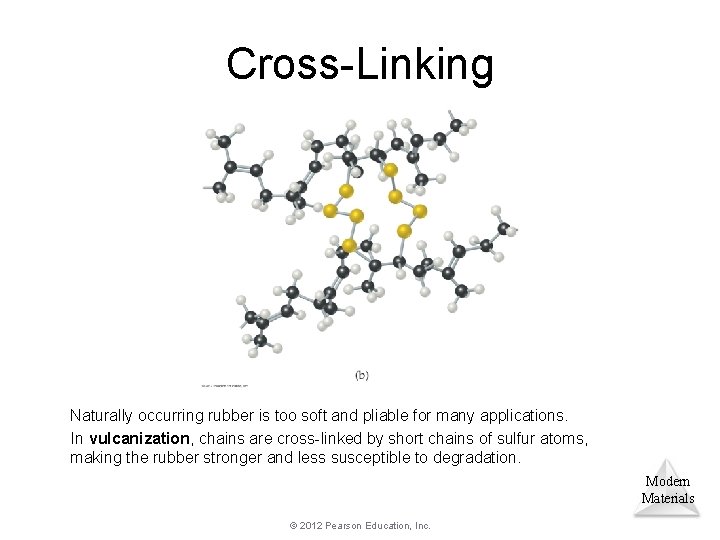
Cross-Linking Chemically bonding chains of polymers to each other can stiffen and strengthen the substance. Modern Materials © 2012 Pearson Education, Inc.

Cross-Linking Naturally occurring rubber is too soft and pliable for many applications. In vulcanization, chains are cross-linked by short chains of sulfur atoms, making the rubber stronger and less susceptible to degradation. Modern Materials © 2012 Pearson Education, Inc.

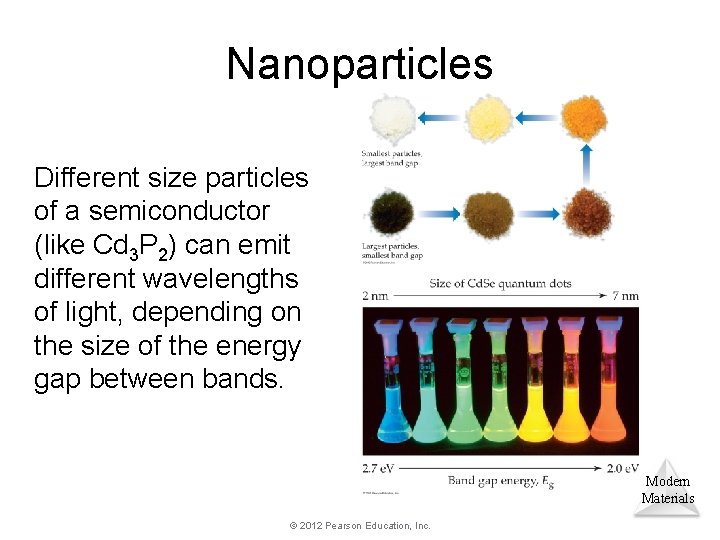
Nanoparticles Different size particles of a semiconductor (like Cd 3 P 2) can emit different wavelengths of light, depending on the size of the energy gap between bands. Modern Materials © 2012 Pearson Education, Inc.

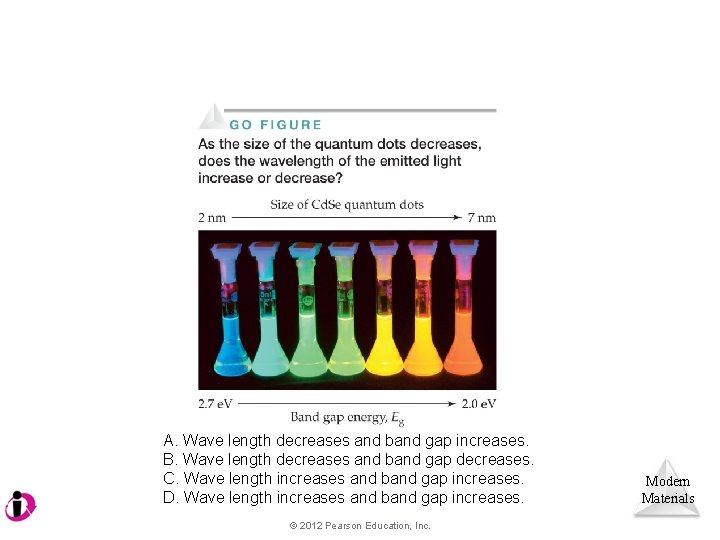
A. Wave length decreases and band gap increases. B. Wave length decreases and band gap decreases. C. Wave length increases and band gap increases. D. Wave length increases and band gap increases. © 2012 Pearson Education, Inc. Modern Materials

A. B. C. D. Yes, by decreasing size of the particles to form nanocrystals the band gap and wavelength will decrease resulting in a shift of emission to the visible region. Yes, by increasing size of the particles to form nanocrystals the band gap and wavelength will decrease resulting in a shift of emission to visible region. No, by decreasing the size of the particles to form nanocrystals the band gap and wavelength will decrease resulting in a shift of emission deeper into UV region. No, by increasing size of the particles to form nanocrystals the band gap and wavelength will decrease resulting in a shift of emission deeper into UV region. Modern Materials © 2012 Pearson Education, Inc.

Nanoparticles Finely divided metals can have quite different properties than larger samples of metals. Modern Materials © 2012 Pearson Education, Inc.

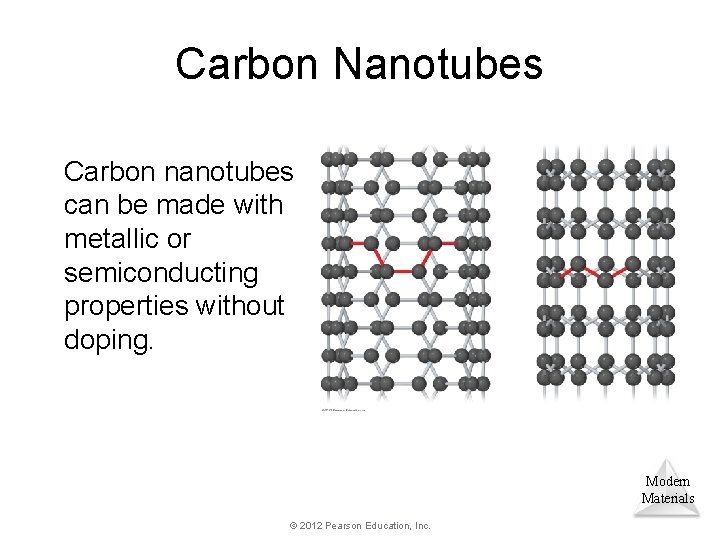
Carbon Nanotubes Carbon nanotubes can be made with metallic or semiconducting properties without doping. Modern Materials © 2012 Pearson Education, Inc.

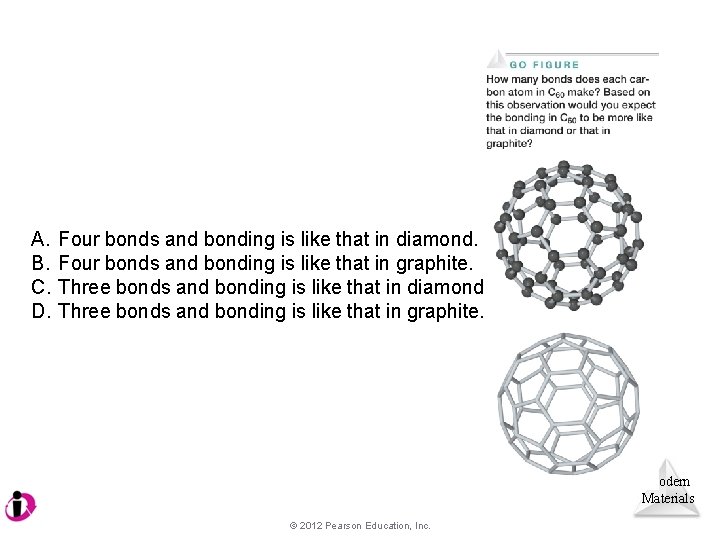
A. B. C. D. Four bonds and bonding is like that in diamond. Four bonds and bonding is like that in graphite. Three bonds and bonding is like that in diamond. Three bonds and bonding is like that in graphite. Modern Materials © 2012 Pearson Education, Inc.