Chapter 5 Defects in solids Defects in solids


- Slides: 13

Chapter 5 Defects in solids

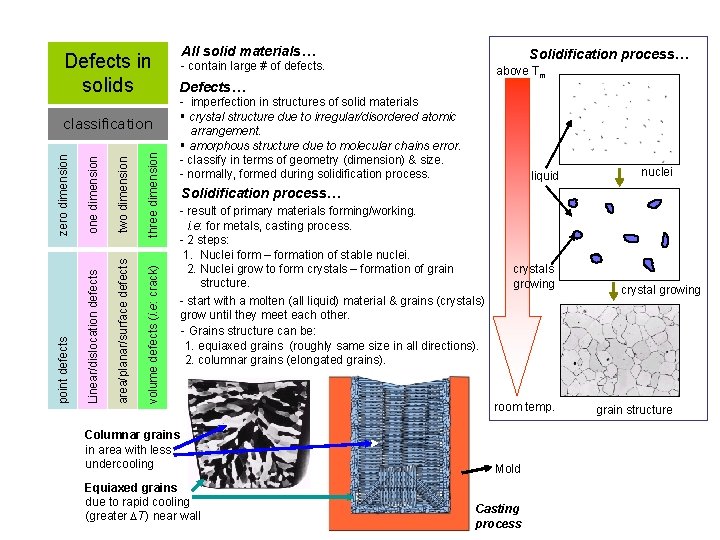
Defects in solids three dimension two dimension area/planar/surface defects volume defects (i. e: crack) one dimension Linear/dislocation defects point defects zero dimension classification All solid materials… Solidification process… - contain large # of defects. above Tm Defects… - imperfection in structures of solid materials § crystal structure due to irregular/disordered atomic arrangement. § amorphous structure due to molecular chains error. - classify in terms of geometry (dimension) & size. - normally, formed during solidification process. liquid nuclei Solidification process… - result of primary materials forming/working. i. e: for metals, casting process. - 2 steps: 1. Nuclei form – formation of stable nuclei. 2. Nuclei grow to form crystals – formation of grain structure. - start with a molten (all liquid) material & grains (crystals) grow until they meet each other. - Grains structure can be: 1. equiaxed grains (roughly same size in all directions). 2. columnar grains (elongated grains). Columnar grains in area with less undercooling Equiaxed grains due to rapid cooling (greater T) near wall crystals growing room temp. Mold Casting process crystal growing grain structure

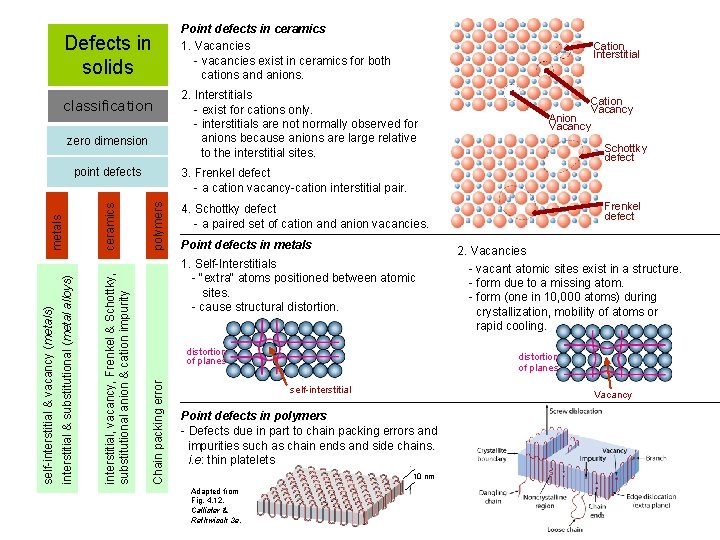
Defects in solids classification zero dimension Cation Interstitial 2. Interstitials - exist for cations only. - interstitials are not normally observed for anions because anions are large relative to the interstitial sites. Cation Vacancy Anion Vacancy Schottky defect polymers 3. Frenkel defect - a cation vacancy-cation interstitial pair. Frenkel defect 4. Schottky defect - a paired set of cation and anion vacancies. Point defects in metals 1. Self-Interstitials - "extra" atoms positioned between atomic sites. - cause structural distortion of planes Chain packing error ceramics interstitial, vacancy, Frenkel & Schottky, substitutional anion & cation impurity interstitial & substitutional (metal alloys) self-interstitial & vacancy (metals) metals point defects Point defects in ceramics 1. Vacancies - vacancies exist in ceramics for both cations and anions. distortion of planes self-interstitial Vacancy Point defects in polymers - Defects due in part to chain packing errors and impurities such as chain ends and side chains. i. e: thin platelets 10 nm Adapted from Fig. 4. 12, Callister & Rethwisch 3 e. 2. Vacancies - vacant atomic sites exist in a structure. - form due to a missing atom. - form (one in 10, 000 atoms) during crystallization, mobility of atoms or rapid cooling.

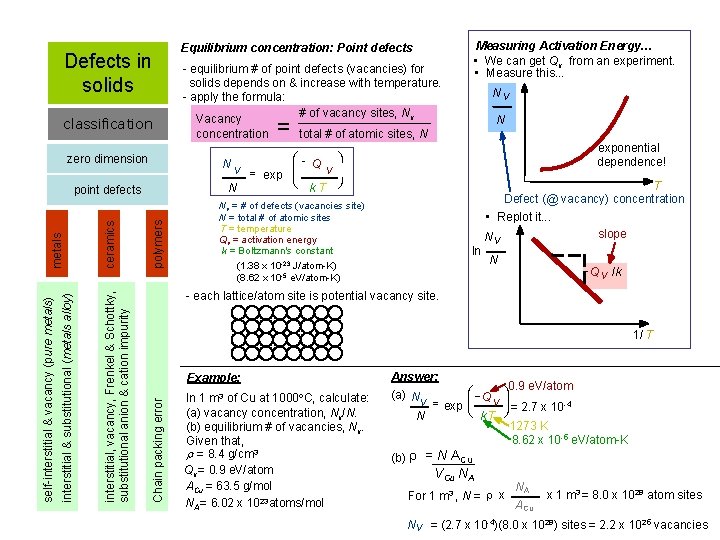
Defects in solids classification concentration = Nv N total # of atomic sites, N exponential dependence! polymers Nv = # of defects (vacancies site) N = total # of atomic sites T = temperature Qv = activation energy k = Boltzmann's constant (1. 38 x 10 -23 J/atom-K) (8. 62 x 10 -5 e. V/atom-K) ln T Defect (@ vacancy) concentration • Replot it. . . slope Nv N - Q v /k - each lattice/atom site is potential vacancy site. 1/ T Example: Chain packing error ceramics interstitial, vacancy, Frenkel & Schottky, substitutional anion & cation impurity metals point defects interstitial & substitutional (metals alloy) - equilibrium # of point defects (vacancies) for solids depends on & increase with temperature. - apply the formula: # of vacancy sites, Nv Vacancy æ ö N Q v = exp çç v è k. T ø N zero dimension self-interstitial & vacancy (pure metals) Measuring Activation Energy… • We can get Qv from an experiment. • Measure this. . . Equilibrium concentration: Point defects In 1 m 3 of Cu at 1000 C, calculate: (a) vacancy concentration, Nv/N. (b) equilibrium # of vacancies, Nv. Given that, r = 8. 4 g/cm 3 Qv = 0. 9 e. V/atom ACu = 63. 5 g/mol NA = 6. 02 x 1023 atoms/mol Answer: æ (a) N v = exp çç Q v è k. T N (b) = N ACu 0. 9 e. V/atom ö ÷÷ = 2. 7 x 10 -4 ø 1273 K 8. 62 x 10 -5 e. V/atom-K VCu NA For 1 m 3 , N = x NA A Cu x 1 m 3 = 8. 0 x 1028 atom sites Nv = (2. 7 x 10 -4)(8. 0 x 1028) sites = 2. 2 x 1025 vacancies

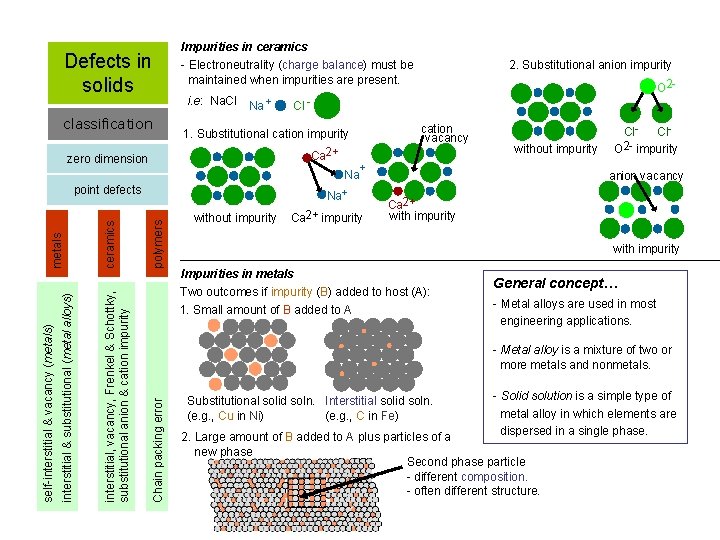
Defects in solids classification Impurities in ceramics - Electroneutrality (charge balance) must be maintained when impurities are present. i. e: Na. Cl Na + Ca 2+ polymers without impurity cation vacancy without impurity + Na Na+ Ca 2+ impurity Cl. O 2 - impurity anion vacancy Ca 2+ with impurity Impurities in metals Two outcomes if impurity (B) added to host (A): 1. Small amount of B added to A General concept… - Metal alloys are used in most engineering applications. - Metal alloy is a mixture of two or more metals and nonmetals. Chain packing error ceramics interstitial, vacancy, Frenkel & Schottky, substitutional anion & cation impurity interstitial & substitutional (metal alloys) self-interstitial & vacancy (metals) metals point defects O 2 - Cl - 1. Substitutional cation impurity zero dimension 2. Substitutional anion impurity Substitutional solid soln. Interstitial solid soln. (e. g. , Cu in Ni) (e. g. , C in Fe) - Solid solution is a simple type of metal alloy in which elements are dispersed in a single phase. 2. Large amount of B added to A plus particles of a new phase Second phase particle - different composition. - often different structure.

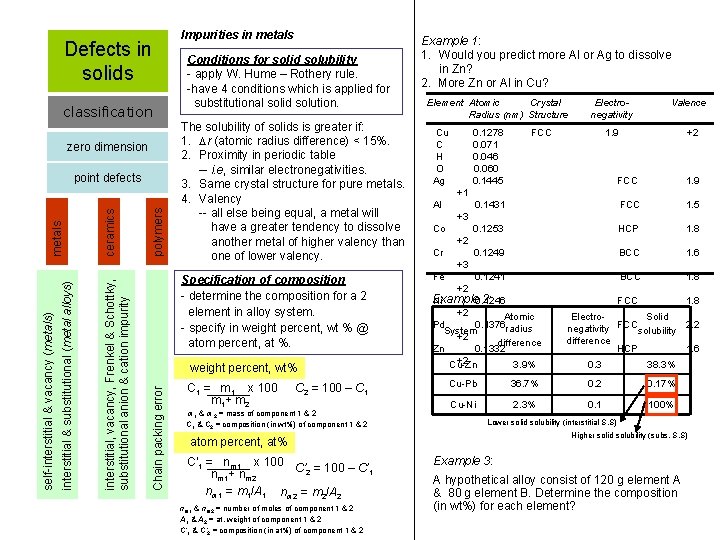
Defects in solids classification zero dimension polymers Conditions for solid solubility - apply W. Hume – Rothery rule. -have 4 conditions which is applied for substitutional solid solution. The solubility of solids is greater if: 1. r (atomic radius difference) < 15%. 2. Proximity in periodic table -- i. e, similar electronegativities. 3. Same crystal structure for pure metals. 4. Valency -- all else being equal, a metal will have a greater tendency to dissolve another metal of higher valency than one of lower valency. Specification of composition - determine the composition for a 2 element in alloy system. - specify in weight percent, wt % @ atom percent, at %. weight percent, wt% Chain packing error ceramics interstitial, vacancy, Frenkel & Schottky, substitutional anion & cation impurity interstitial & substitutional (metal alloys) self-interstitial & vacancy (metals) metals point defects Impurities in metals C 1 = m 1 x 100 m 1+ m 2 C 2 = 100 – C 1 m 1 & m 2 = mass of component 1 & 2 C 1 & C 2 = composition (in wt%) of component 1 & 2 Example 1: 1. Would you predict more Al or Ag to dissolve in Zn? 2. More Zn or Al in Cu? Element Atomic Crystal Radius (nm) Structure Cu C H O Ag 0. 1278 0. 071 0. 046 0. 060 0. 1445 nm 1 & nm 2 = number of moles of component 1 & 2 A 1 & A 2 = at. weight of component 1 & 2 C’ 1 & C’ 2 = composition (in at%) of component 1 & 2 FCC Valence 1. 9 +2 FCC 1. 9 0. 1431 FCC 1. 5 0. 1253 HCP 1. 8 0. 1249 BCC 1. 6 0. 1241 BCC 1. 8 Example 2: Ni 0. 1246 FCC 1. 8 +1 Al +3 Co +2 Cr +3 Fe +2 +2 Atomic Pd 0. 1376 radius System +2 difference Zn 0. 1332 +2 Cu-Zn 3. 9% Electro. Solid negativity FCCsolubility 2. 2 difference HCP 1. 6 0. 3 38. 3% Cu-Pb 36. 7% 0. 2 0. 17% Cu-Ni 2. 3% 0. 1 100% Lower solid solubility (interstitial S. S) Higher solid solubility (subs. S. S) atom percent, at% C’ 1 = nm 1 x 100 C’ = 100 – C’ 2 1 nm 1+ nm 2 nm 1 = m 1/A 1 nm 2 = m 2/A 2 Electronegativity Example 3: A hypothetical alloy consist of 120 g element A & 80 g element B. Determine the composition (in wt%) for each element?

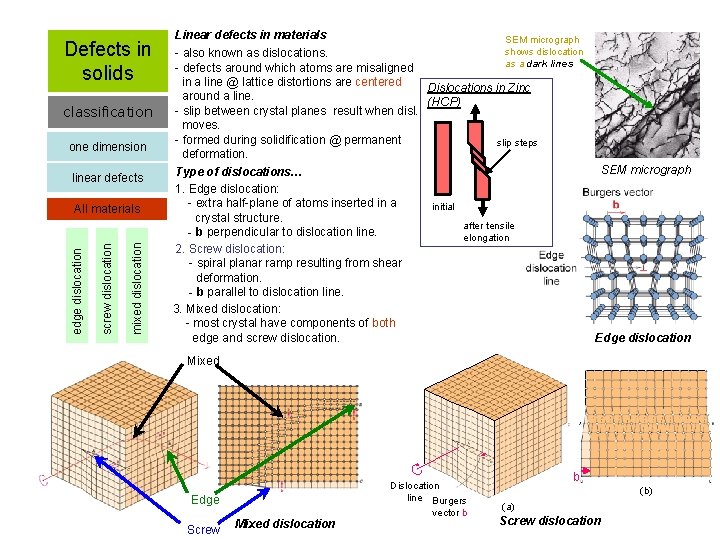
Defects in solids classification one dimension linear defects mixed dislocation screw dislocation edge dislocation All materials Linear defects in materials SEM micrograph shows dislocation - also known as dislocations. as a dark lines - defects around which atoms are misaligned in a line @ lattice distortions are centered Dislocations in Zinc around a line. (HCP) - slip between crystal planes result when disl. moves. - formed during solidification @ permanent slip steps deformation. SEM micrograph Type of dislocations… 1. Edge dislocation: - extra half-plane of atoms inserted in a initial crystal structure. after tensile - b perpendicular to dislocation line. elongation 2. Screw dislocation: - spiral planar ramp resulting from shear deformation. - b parallel to dislocation line. 3. Mixed dislocation: - most crystal have components of both Edge dislocation edge and screw dislocation. Mixed Screw Dislocation line Burgers Edge Screw Mixed dislocation vector b b (b) (a) Screw dislocation

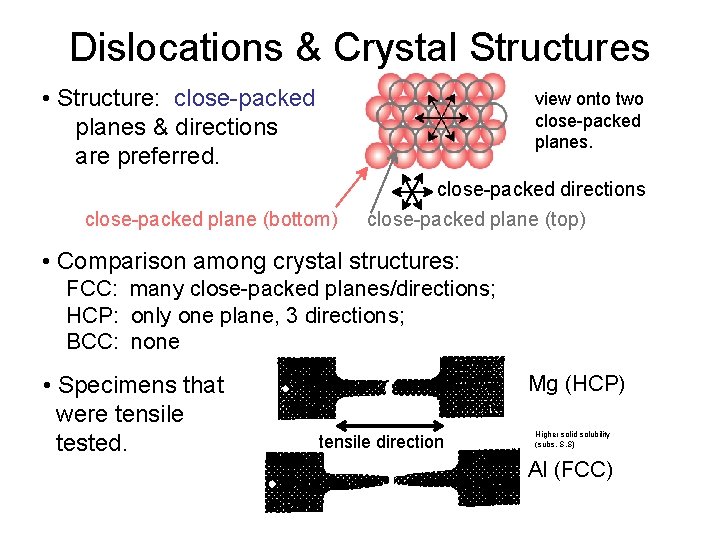
Dislocations & Crystal Structures • Structure: close-packed planes & directions are preferred. view onto two close-packed planes. close-packed plane (bottom) close-packed directions close-packed plane (top) • Comparison among crystal structures: FCC: many close-packed planes/directions; HCP: only one plane, 3 directions; BCC: none • Specimens that were tensile tested. Mg (HCP) tensile direction Higher solid solubility (subs. S. S) Al (FCC)

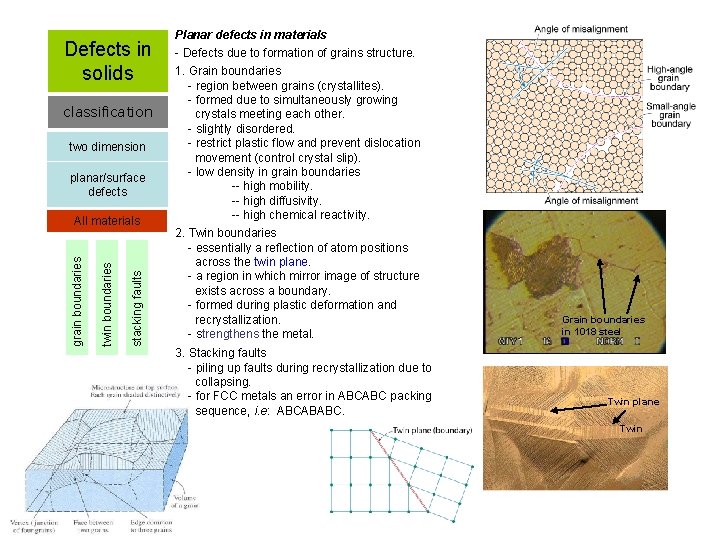
Defects in solids classification two dimension planar/surface defects stacking faults twin boundaries grain boundaries All materials Planar defects in materials - Defects due to formation of grains structure. 1. Grain boundaries - region between grains (crystallites). - formed due to simultaneously growing crystals meeting each other. - slightly disordered. - restrict plastic flow and prevent dislocation movement (control crystal slip). - low density in grain boundaries -- high mobility. -- high diffusivity. -- high chemical reactivity. 2. Twin boundaries - essentially a reflection of atom positions across the twin plane. - a region in which mirror image of structure exists across a boundary. - formed during plastic deformation and recrystallization. - strengthens the metal. 3. Stacking faults - piling up faults during recrystallization due to collapsing. - for FCC metals an error in ABCABC packing sequence, i. e: ABCABABC. Grain boundaries in 1018 steel Twin plane Twin

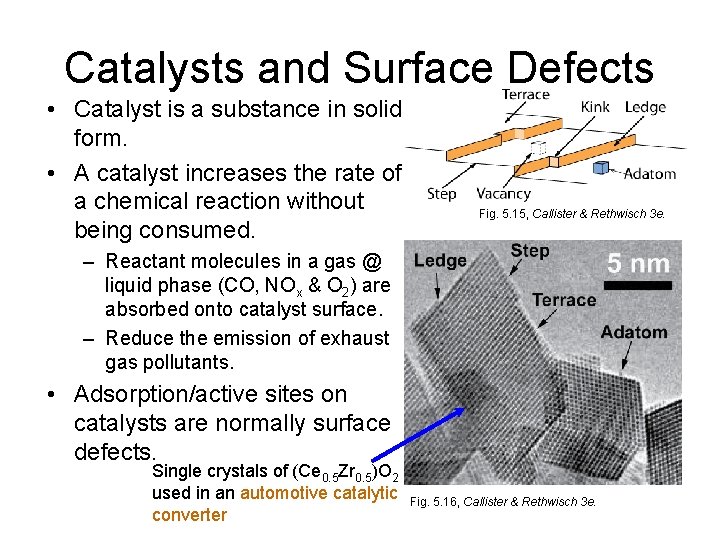
Catalysts and Surface Defects • Catalyst is a substance in solid form. • A catalyst increases the rate of a chemical reaction without being consumed. Fig. 5. 15, Callister & Rethwisch 3 e. – Reactant molecules in a gas @ liquid phase (CO, NOx & O 2) are absorbed onto catalyst surface. – Reduce the emission of exhaust gas pollutants. • Adsorption/active sites on catalysts are normally surface defects. Single crystals of (Ce 0. 5 Zr 0. 5)O 2 used in an automotive catalytic converter Fig. 5. 16, Callister & Rethwisch 3 e.

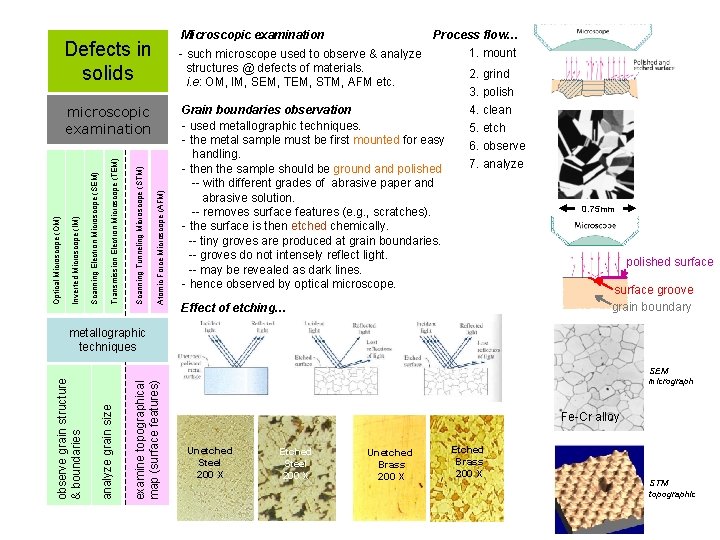
Microscopic examination Defects in solids Atomic Force Microscope (AFM) Scanning Tunneling Microscope (STM) Transmission Electron Microscope (TEM) Scanning Electron Microscope (SEM) Inverted Microscope (IM) Optical Microscope (OM) microscopic examination Process flow… 1. mount - such microscope used to observe & analyze structures @ defects of materials. 2. grind i. e: OM, IM, SEM, TEM, STM, AFM etc. 3. polish 4. clean Grain boundaries observation - used metallographic techniques. 5. etch - the metal sample must be first mounted for easy 6. observe handling. 7. analyze - then the sample should be ground and polished -- with different grades of abrasive paper and abrasive solution. -- removes surface features (e. g. , scratches). - the surface is then etched chemically. -- tiny groves are produced at grain boundaries. -- groves do not intensely reflect light. -- may be revealed as dark lines. - hence observed by optical microscope. Effect of etching… 0. 75 mm polished surface groove grain boundary examine topographical map (surface features) analyze grain size observe grain structure & boundaries metallographic techniques SEM micrograph Fe-Cr alloy Unetched Steel 200 X Etched Steel 200 X Unetched Brass 200 X Etched Brass 200 X STM topographic

Size of grains… - affects the mechanical properties of the material. - the smaller the grain size, more are the grain boundaries. - more grain boundaries means higher resistance to slip (plastic deformation occurs due to slip). - more grains means more uniform the mechanical properties are. Defects in solids Atomic Force Microscope (AFM) Scanning Tunneling Microscope (STM) Transmission Electron Microscope (TEM) Scanning Electron Microscope (SEM) Inverted Microscope (IM) Optical Microscope (OM) microscopic examination Measuring average grain diameter - Average grain diameter, d more directly represents grain size. - Random line of known length is drawn on photomicrograph. - Number of grains intersected is counted. - Ratio of number of grains intersected to length of line, n. L is determined. d = C/n. L(M) C = 1. 5 & M = magnification How to measure grain size? - use the formula: N = 2 n -1 n = ASTM grain size number. N = number of grains per square inch of a polished & etched specimen at 100 x magnification. - ASTM grain size number ‘n’ is a measure of grain size. n < 3 – Coarse grained 4 < n < 6 – Medium grained 7 < n < 9 – Fine grained n > 10 – ultrafine grained - If ASTM grain size #, n increase, -- size of grains decrease. -- # of grains/in 2, N increase. 1045 cold rolled steel, n=8 examine topographical map (surface features) analyze grain size observe grain structure & boundaries metallographic techniques 3 inches 5 grains Example: Determine the ASTM grain size number of a metal specimen if 45 grains per square inch are measured at a magnification of 100 x. 1018 cold rolled steel, n=10 log N = (n-1) log 2 n = log N +1 log 2 n = log 45 +1 log 2 n = 6. 5

Summary • Point, Line, and Area defects exist in solids. • The number and type of defects can be varied and controlled (e. g. , T controls vacancy conc. ) • Defects affect material properties (e. g. , grain boundaries control crystal slip). • Defects may be desirable or undesirable (e. g. , dislocations may be good or bad, depending on whether plastic deformation is desirable or not. )