Chapter 11 Intermolecular Forces The forces holding solids















- Slides: 39

Chapter 11

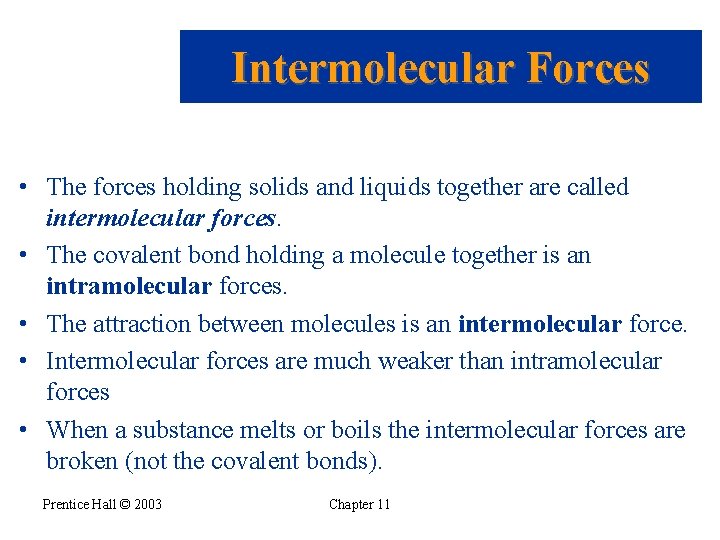
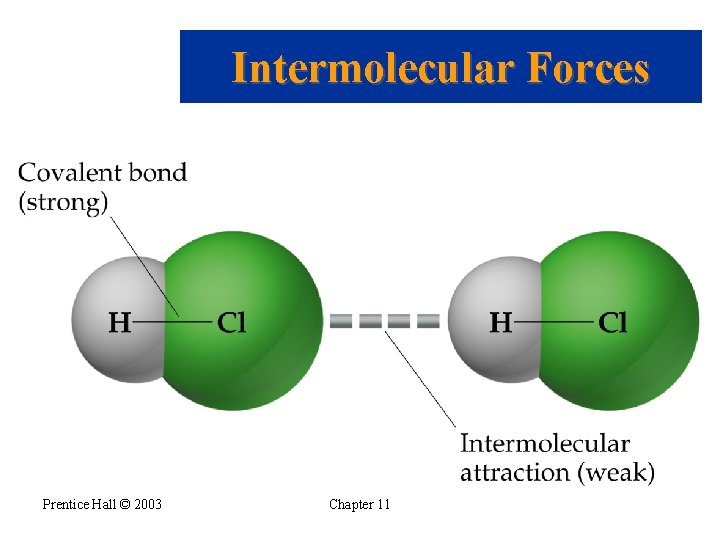
Intermolecular Forces • The forces holding solids and liquids together are called intermolecular forces. • The covalent bond holding a molecule together is an intramolecular forces. • The attraction between molecules is an intermolecular force. • Intermolecular forces are much weaker than intramolecular forces • When a substance melts or boils the intermolecular forces are broken (not the covalent bonds). Prentice Hall © 2003 Chapter 11

Intermolecular Forces Prentice Hall © 2003 Chapter 11

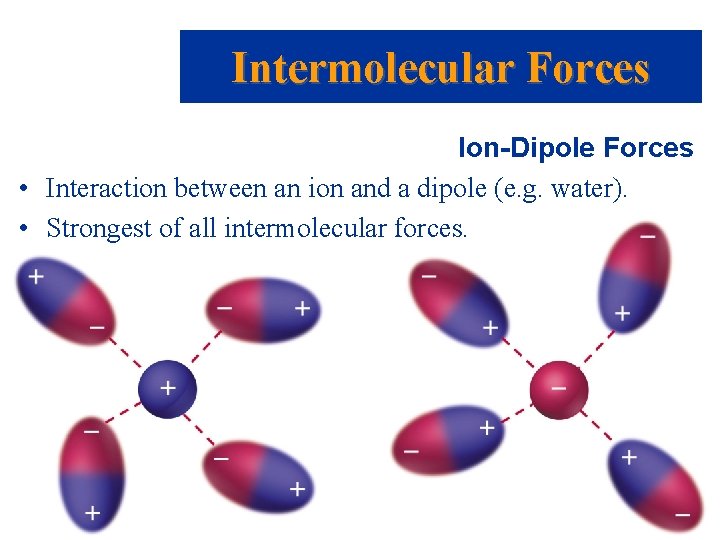
Intermolecular Forces Ion-Dipole Forces • Interaction between an ion and a dipole (e. g. water). • Strongest of all intermolecular forces. Prentice Hall © 2003 Chapter 11

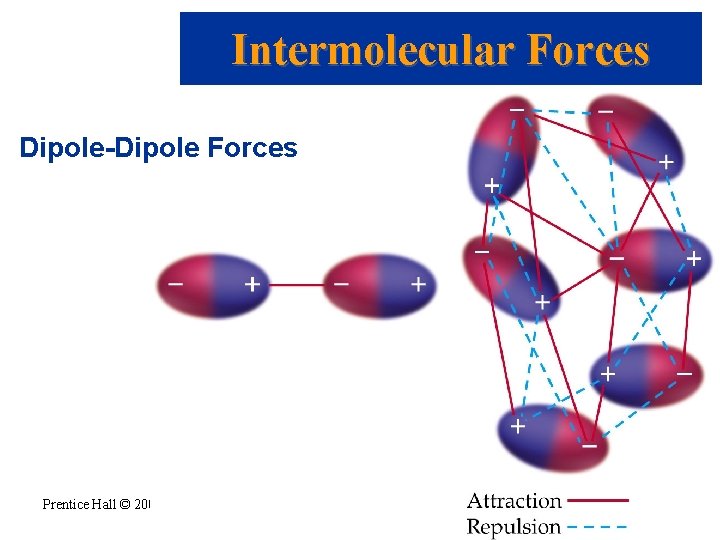
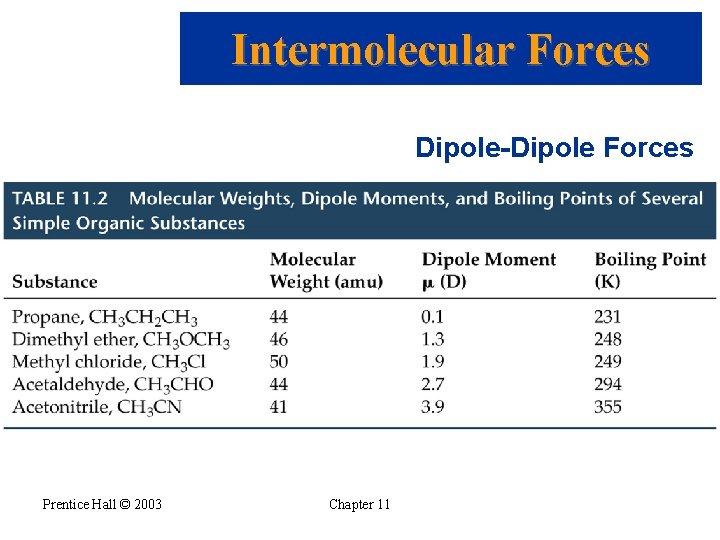
Intermolecular Forces • • • Dipole-Dipole Forces Exist between neutral polar molecules. Polar molecules need to be close together. Weaker than ion-dipole forces. There is a mix of attractive and repulsive dipole-dipole forces as the molecules tumble. If two molecules have about the same mass and size, then dipole-dipole forces increase with increasing polarity. Prentice Hall © 2003 Chapter 11

Intermolecular Forces Dipole-Dipole Forces Prentice Hall © 2003 Chapter 11

Intermolecular Forces Dipole-Dipole Forces Prentice Hall © 2003 Chapter 11

Intermolecular Forces • • • London Dispersion Forces Weakest of all intermolecular forces. It is possible for two adjacent neutral molecules to affect each other. The nucleus of one molecule (or atom) attracts the electrons of the adjacent molecule (or atom). For an instant, the electron clouds become distorted. In that instant a dipole is formed (called an instantaneous dipole). Prentice Hall © 2003 Chapter 11

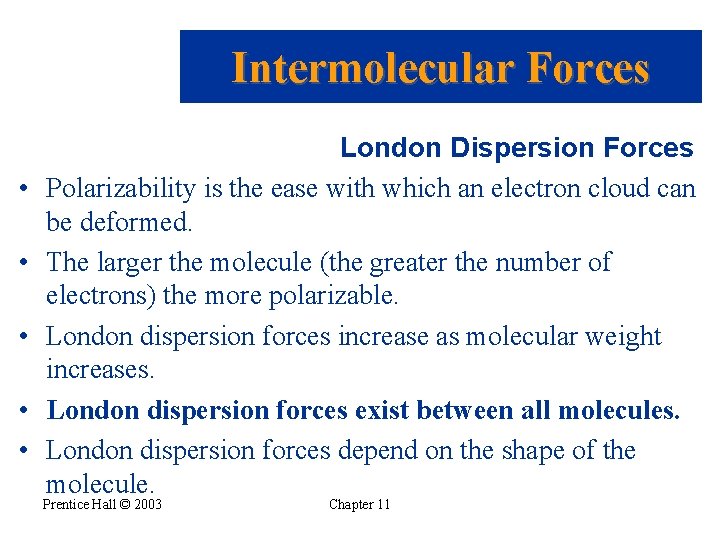
Intermolecular Forces • • • London Dispersion Forces Polarizability is the ease with which an electron cloud can be deformed. The larger the molecule (the greater the number of electrons) the more polarizable. London dispersion forces increase as molecular weight increases. London dispersion forces exist between all molecules. London dispersion forces depend on the shape of the molecule. Prentice Hall © 2003 Chapter 11

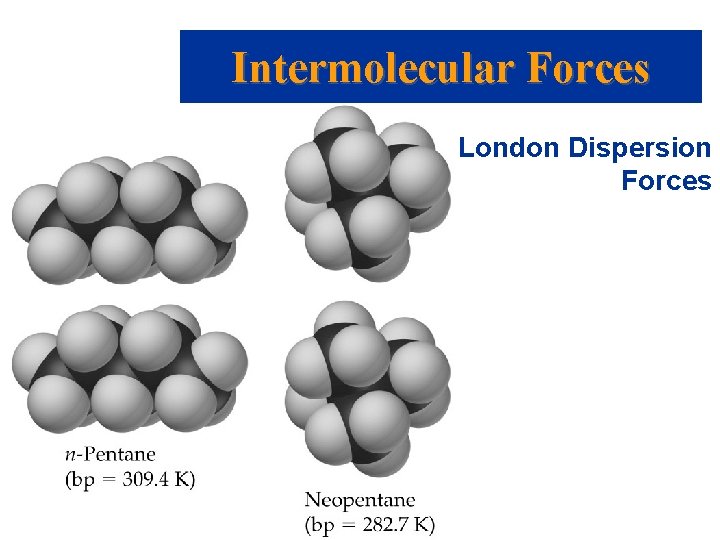
Intermolecular Forces London Dispersion Forces • The greater the surface area available for contact, the greater the dispersion forces. • London dispersion forces between spherical molecules are lower than between sausage-like molecules. Prentice Hall © 2003 Chapter 11

Intermolecular Forces London Dispersion Forces Prentice Hall © 2003 Chapter 11

Things to Remember • The more intermolecular forces present, the stronger the bonding between molecules; therefore, boiling pt. is higher and freezing pt. is lower • The stronger the intermolecular forces, the less volatile (does not want to evaporate). • The less volatile a compound is, the lower the vapor pressure. The more volatile a compound is, the higher the vapor pressure. • If # of intermolecular forces are the same, the higher the molar mass, the higher the boiling pt and the lower the freezing pt.

True or False • A. CBr 4 is more volatile than CCl 4. • B. CBr 4 has a higher boiling point than CCl 4. • C. CBr 4 has weaker intermolecular forces than CCl 4. • D. CBr 4 has a higher vapor pressure at the same temperature than CCl 4.

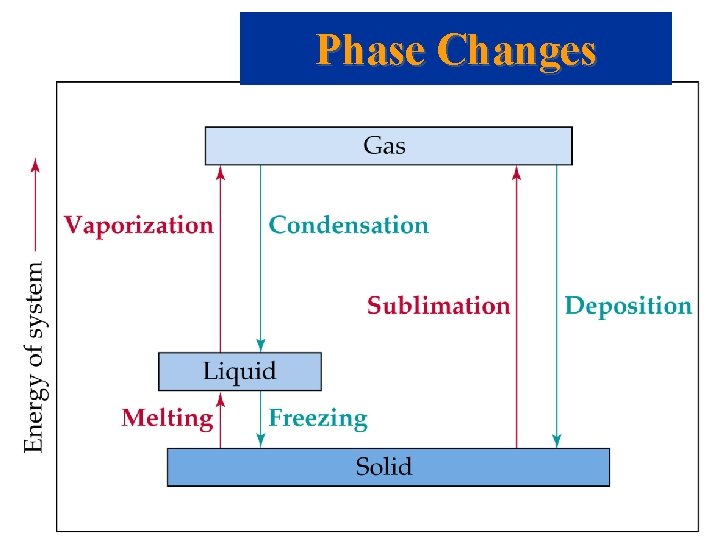
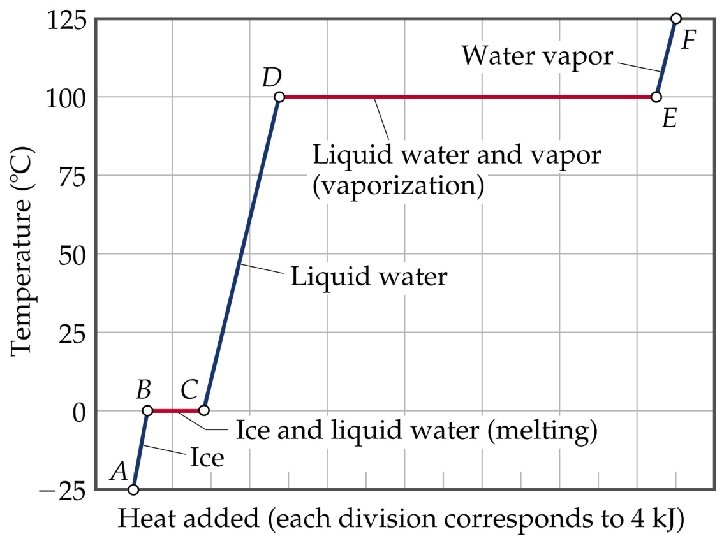
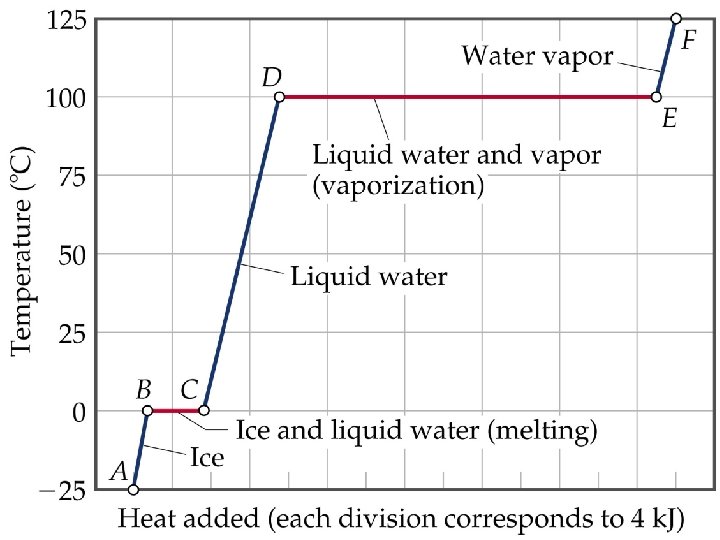
Phase Changes • • • Sublimation: solid gas. Vaporization: liquid gas. Melting or fusion: solid liquid. Deposition: gas solid. Condensation: gas liquid. Freezing: liquid solid.

Phase Changes


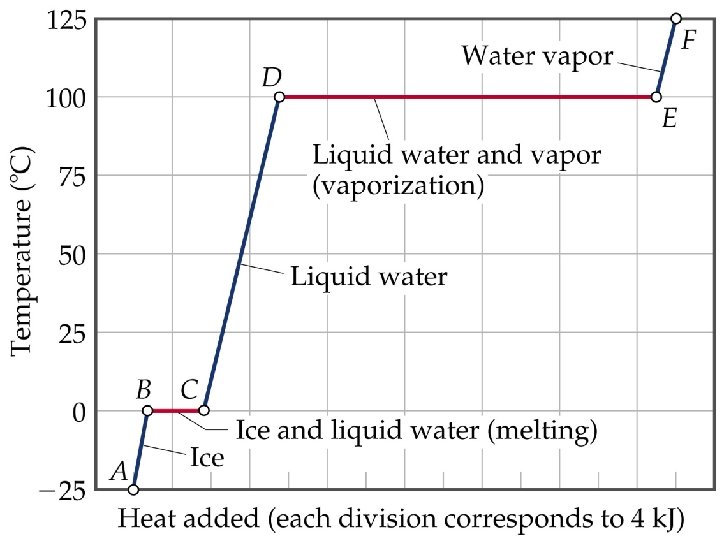
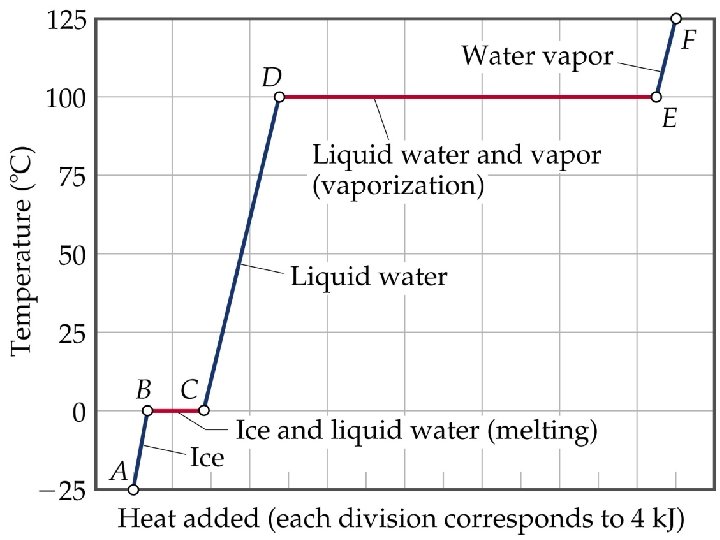
• ENERGY ASSOCIATED WITH HEATING CURVES

Topics • • • Vapor Pressure Normal Boiling Point Normal Freezing Specific Heat Enthalpy (Heat) of Vaporization Enthalpy (Heat) of Fusion

Vapor Pressure • THE PRESSURE OF A VAPOR IN EQUILIBRIUM WITH ITS LIQUID (OR ITS SOLID)

NORMAL BOILING POINT & FREEZING POINTS • NORMAL BOILING PT. - THE TEMPERATURE @WHICH VAPOR PRESSURE = 1 atm • NORMAL FREEZING PT. – THE TEMPERATURE @ WHICH THE VAPOR PRESSURE OF THE SOLID AND THE LIQUID ARE THE SAME


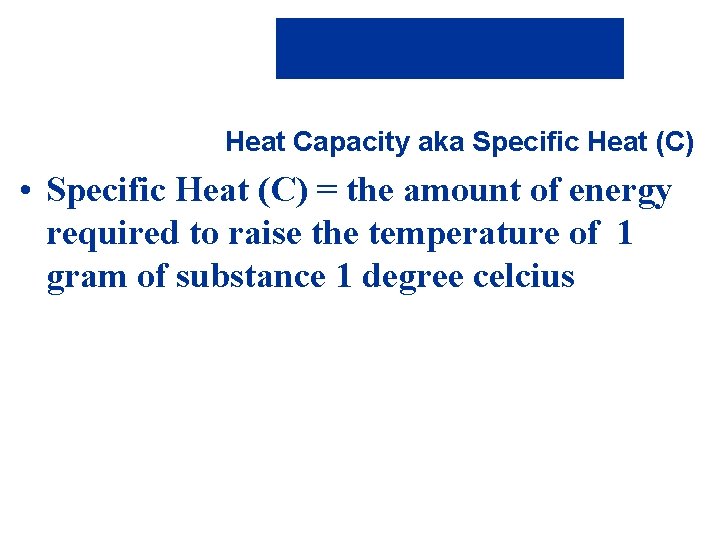
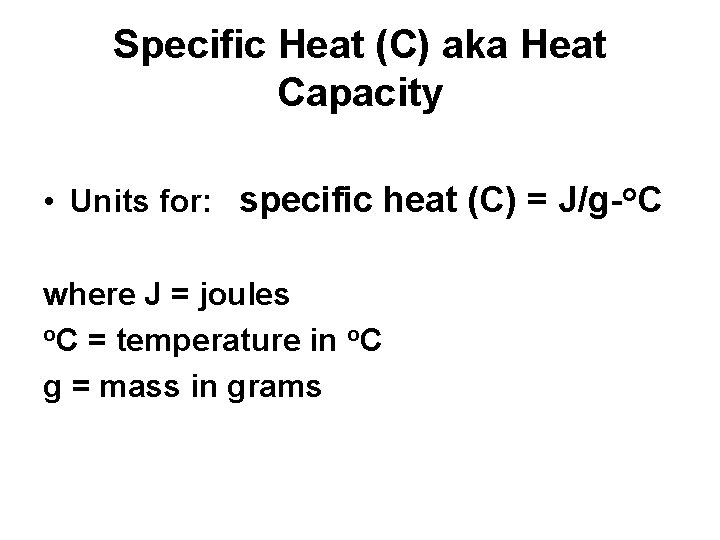
Heat Capacity aka Specific Heat (C) • Specific Heat (C) = the amount of energy required to raise the temperature of 1 gram of substance 1 degree celcius

Specific Heat (C) aka Heat Capacity • Units for: specific heat (C) = J/g-o. C where J = joules o. C = temperature in o. C g = mass in grams

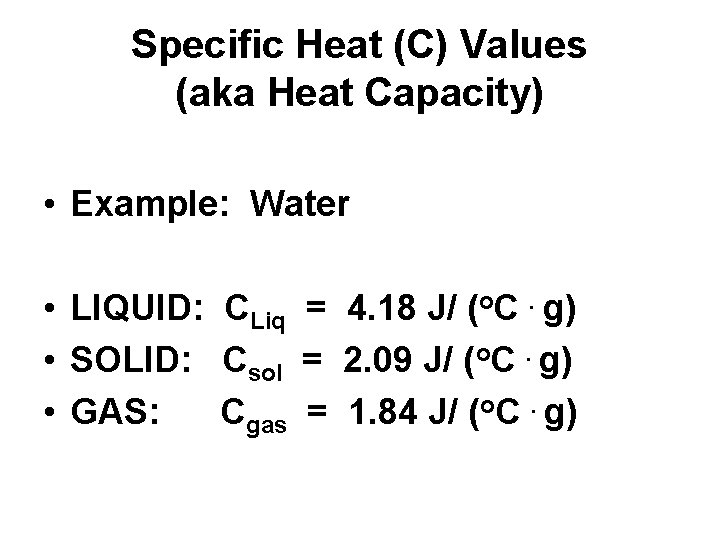
Specific Heat (C) Values (aka Heat Capacity) • Example: Water • LIQUID: CLiq = 4. 18 J/ (o. C. g) • SOLID: Csol = 2. 09 J/ (o. C. g) • GAS: Cgas = 1. 84 J/ (o. C. g)

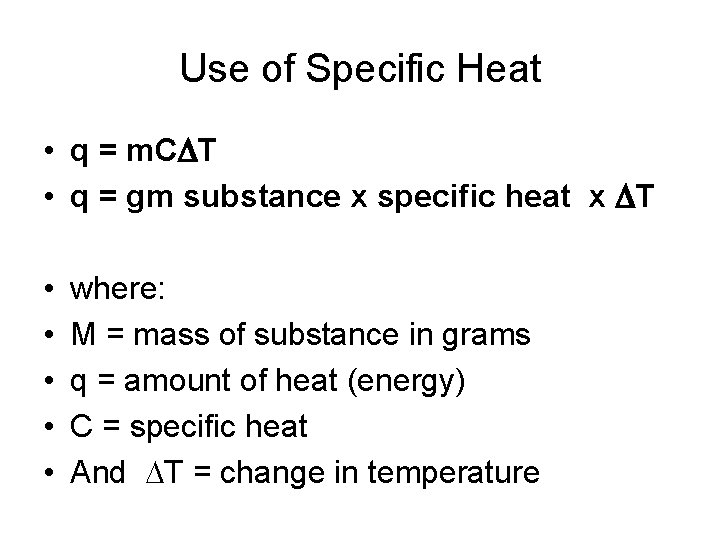
Use of Specific Heat • q = m. CDT • q = gm substance x specific heat x DT • • • where: M = mass of substance in grams q = amount of heat (energy) C = specific heat And DT = change in temperature

Enthalpy of Vaporization aka heat of vaporization (DHvap) • Is the amount of heat needed to convert a liquid to a vapor at its normal boiling point

Enthalpy of Fusion aka heat of fusion (DHfus) • Is the amount of heat needed to convert a solid to a liquid at its normal melting (freezing) point

Units for DHvap, DHfus and heat(q) Heat of fusion DHvap = k. J/mol Heat of vaporization DHfus = k. J/mol Heat (q) = Joules

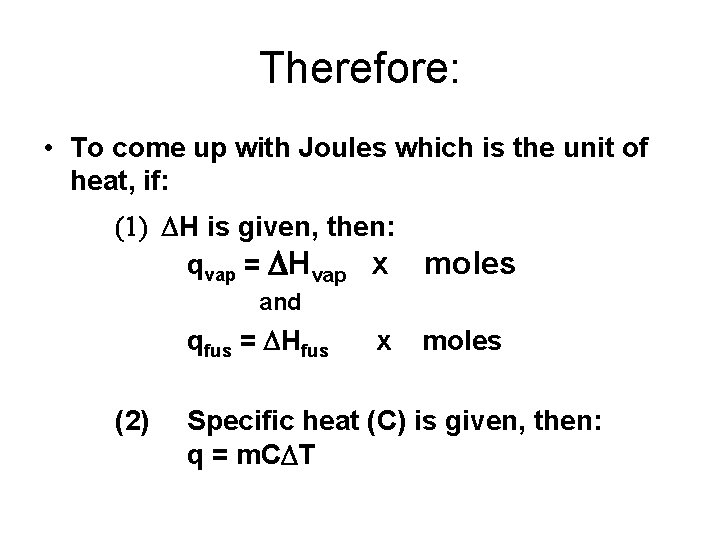
Therefore: • To come up with Joules which is the unit of heat, if: (1) DH is given, then: qvap = DHvap x moles and qfus = DHfus (2) x moles Specific heat (C) is given, then: q = m. CDT

For Water Heat of fusion: DHfus = 6. 02 k. J/mol Heat of vaporization: DHvap = 40. 7 k. J/mol


Sample Problem • Calculate the enthalpy change upon converting 1 mole of ice at -25 o. C to steam at 125 o. C under a constant pressure of 1 atm? The specific heats are of ice, water and steam 2. 09 J/g-K for ice, 4. 18 J/g-K for water and 1. 84 J/g-K for steam. For water, DHfus= 6. 01 k. J/mol, and DHvap = 40. 67 k. J/mol. • Note: The total enthalpy change is the sum of the changes of the individual steps.

HEATING CURVES • ENERGY ASSOCIATED WITH HEATING CURVES • During a phase change, adding heat causes no temperature change.


Phase Changes Critical Temperature and Pressure • Gases liquefied by increasing pressure at some temperature. • Critical temperature: the minimum temperature for liquefaction of a gas using pressure. • Critical pressure: pressure required for liquefaction.

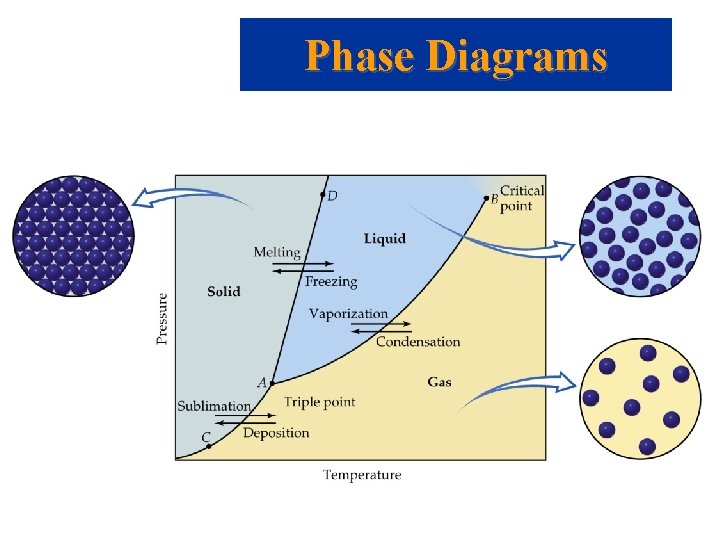
Phase Diagrams • Phase diagram: plot of pressure vs. Temperature summarizing all equilibria between phases. • Given a temperature and pressure, phase diagrams tell us which phase will exist. • Any temperature and pressure combination not on a curve represents a single phase.

Phase Diagrams

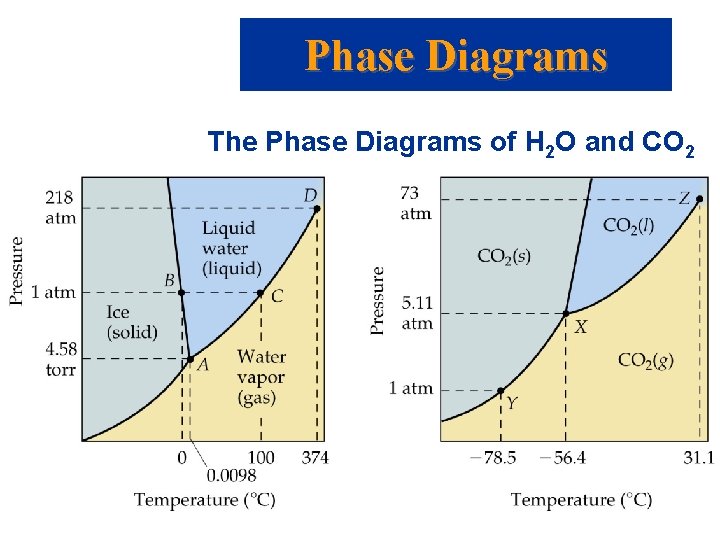
Phase Diagrams The Phase Diagrams of H 2 O and CO 2

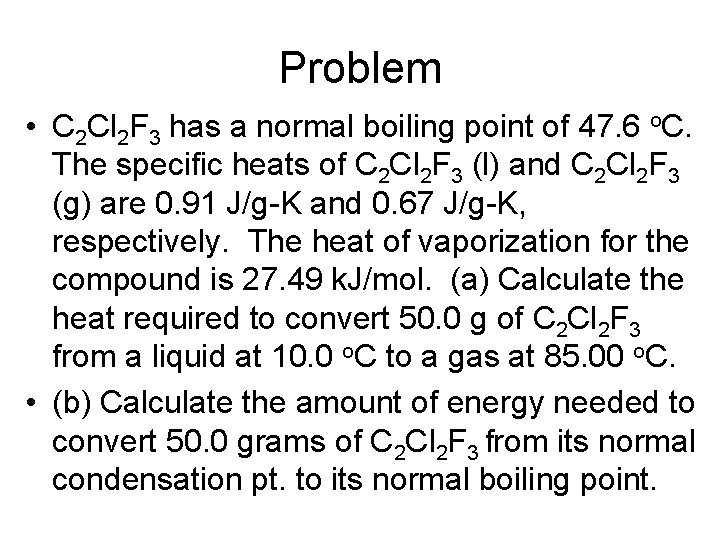
Problem • C 2 Cl 2 F 3 has a normal boiling point of 47. 6 o. C. The specific heats of C 2 Cl 2 F 3 (l) and C 2 Cl 2 F 3 (g) are 0. 91 J/g-K and 0. 67 J/g-K, respectively. The heat of vaporization for the compound is 27. 49 k. J/mol. (a) Calculate the heat required to convert 50. 0 g of C 2 Cl 2 F 3 from a liquid at 10. 0 o. C to a gas at 85. 00 o. C. • (b) Calculate the amount of energy needed to convert 50. 0 grams of C 2 Cl 2 F 3 from its normal condensation pt. to its normal boiling point.