Alkynes C C Synthesis of Acetylene Heating coke



























- Slides: 61

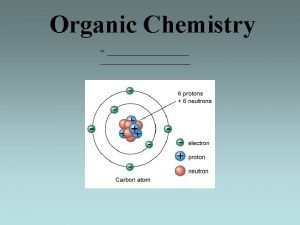
Alkynes C C

Synthesis of Acetylene Heating coke with lime in an electric furnace to forms calcium carbide. Then drip water on the calcium carbide. coke lime * *This reaction was used to produce light for miners’ lamps and for the stage.

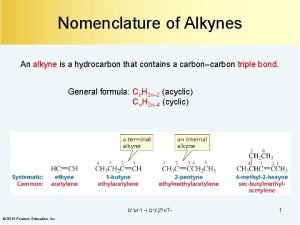
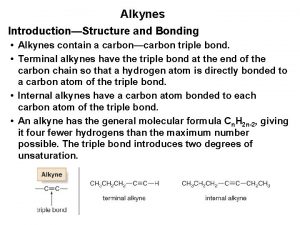
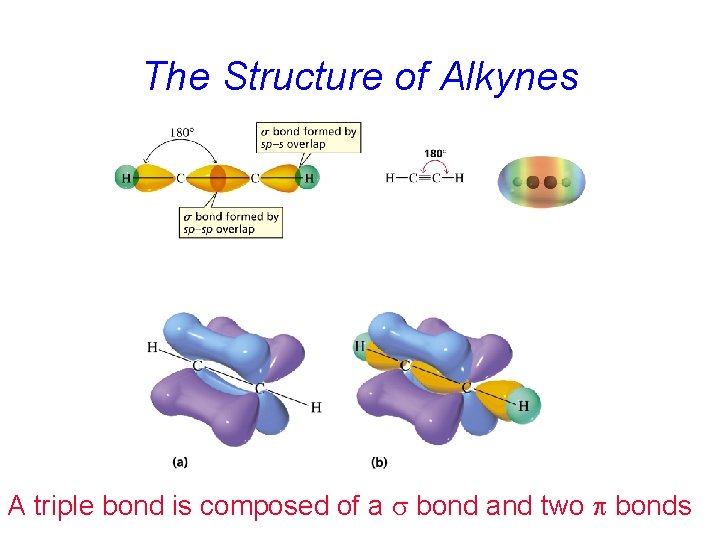
The Structure of Alkynes A triple bond is composed of a s bond and two p bonds

Acidity of Acetylene and Terminal Alkynes H C C

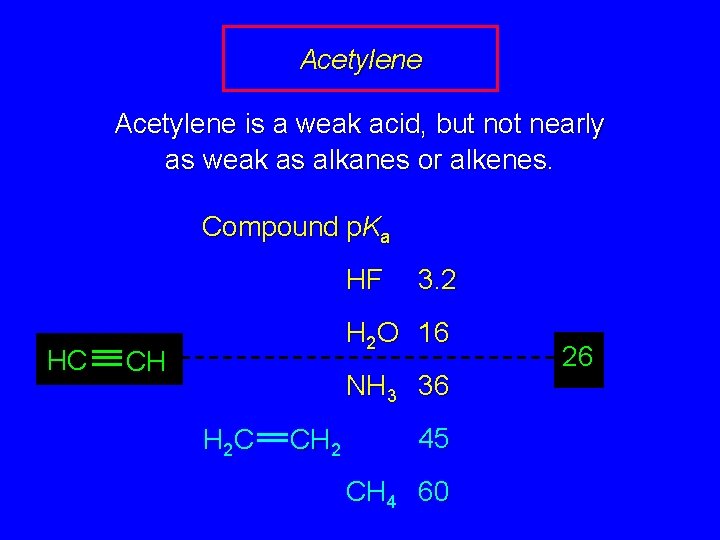
Acidity of Hydrocarbons In general, hydrocarbons are excedingly weak acids Compound p. Ka HF 3. 2 H 2 O 16 NH 3 36 H 2 C CH 2 45 CH 4 60

Acetylene is a weak acid, but not nearly as weak as alkanes or alkenes. Compound p. Ka HF HC 3. 2 H 2 O 16 CH NH 3 36 H 2 C CH 2 45 CH 4 60 26


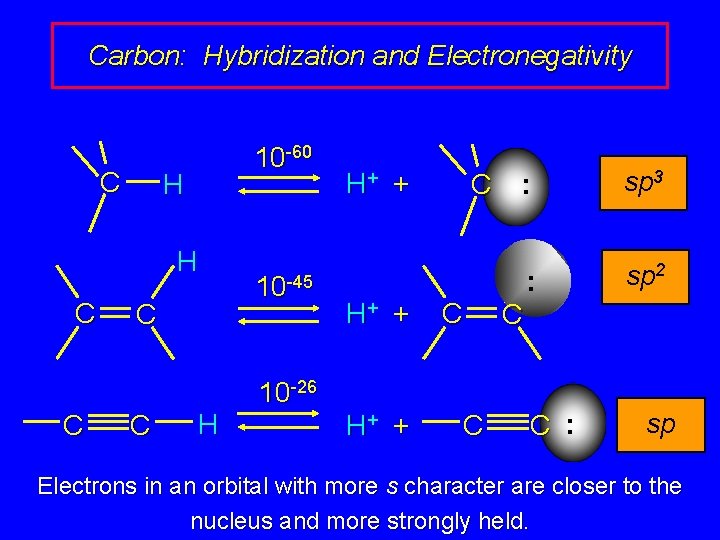
Carbon: Hybridization and Electronegativity C 10 -60 H H C C H 10 -45 H+ + C C 10 -26 H+ + C : sp 3 : sp 2 C C : sp Electrons in an orbital with more s character are closer to the nucleus and more strongly held.

The stronger the acid, the weaker its conjugate base top 252

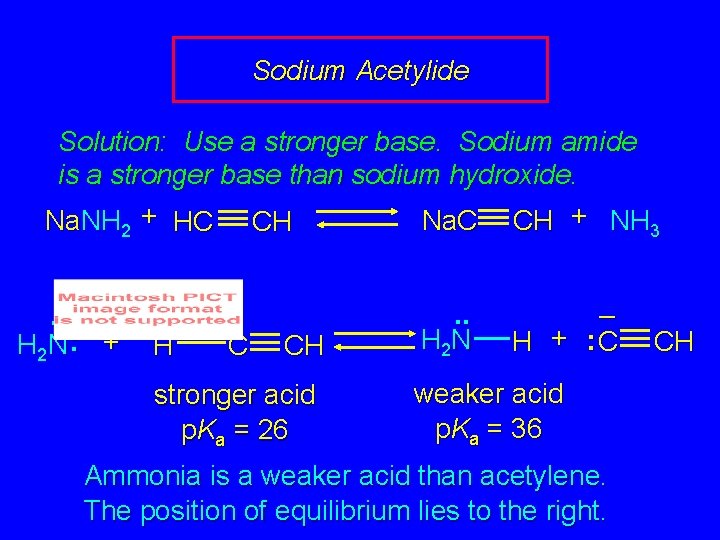
Sodium Acetylide Solution: Use a stronger base. Sodium amide is a stronger base than sodium hydroxide. Na. NH 2 + HC CH Na. C CH + NH 3. . – H 2 N : + H C CH stronger acid p. Ka = 26 . . H 2 N – H + : C weaker acid p. Ka = 36 Ammonia is a weaker acid than acetylene. The position of equilibrium lies to the right. CH

Preparation of Various Alkynes by alkylation reactions with Acetylide or Terminal Alkynes

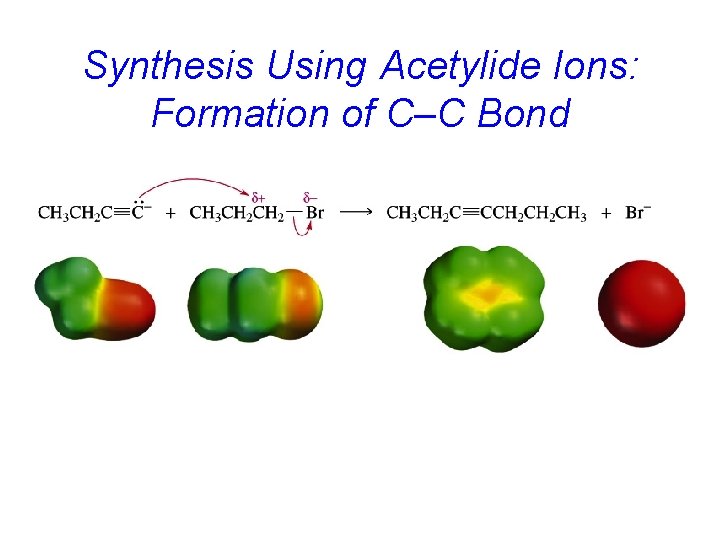
Synthesis Using Acetylide Ions: Formation of C–C Bond

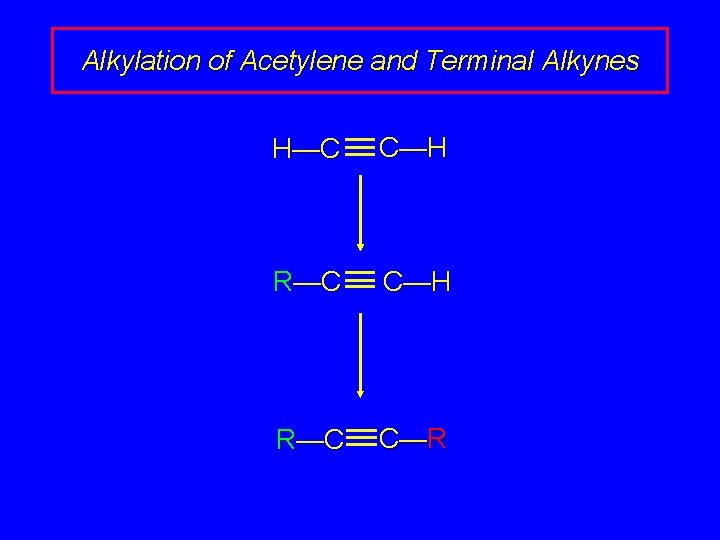
Alkylation of Acetylene and Terminal Alkynes H—C C—H R—C C—R

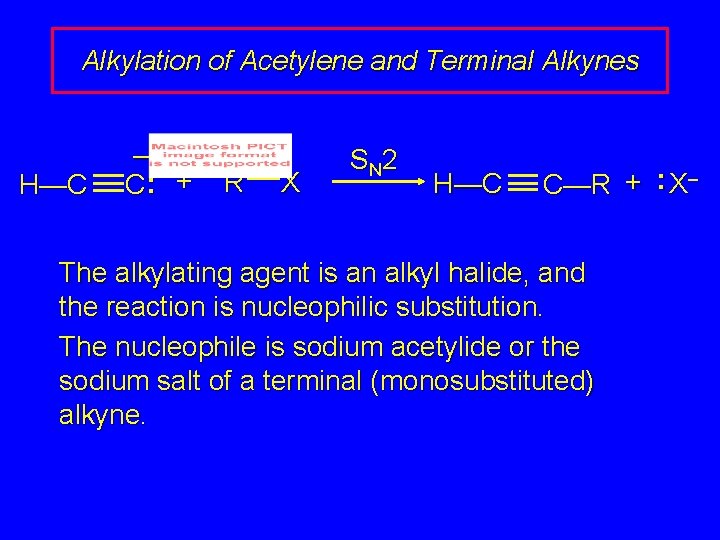
Alkylation of Acetylene and Terminal Alkynes H—C – C: + R X S N 2 H—C C—R + : X– The alkylating agent is an alkyl halide, and the reaction is nucleophilic substitution. The nucleophile is sodium acetylide or the sodium salt of a terminal (monosubstituted) alkyne.

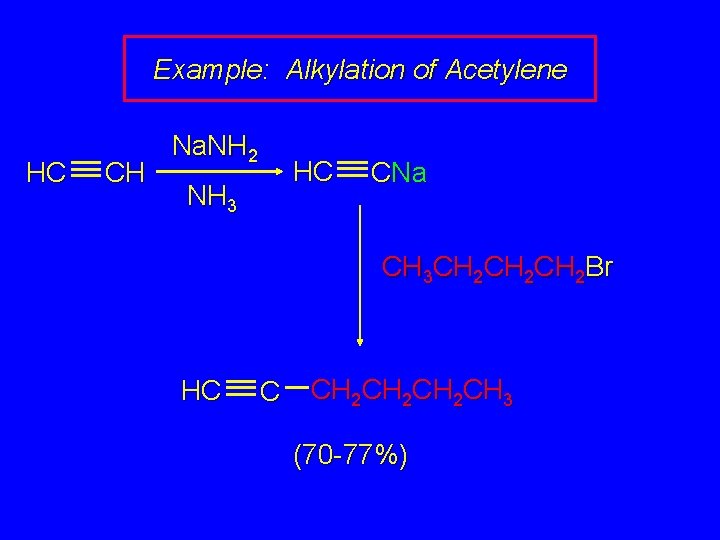
Example: Alkylation of Acetylene HC CH Na. NH 2 HC NH 3 CNa CH 3 CH 2 CH 2 Br HC C CH 2 CH 2 CH 3 (70 -77%)

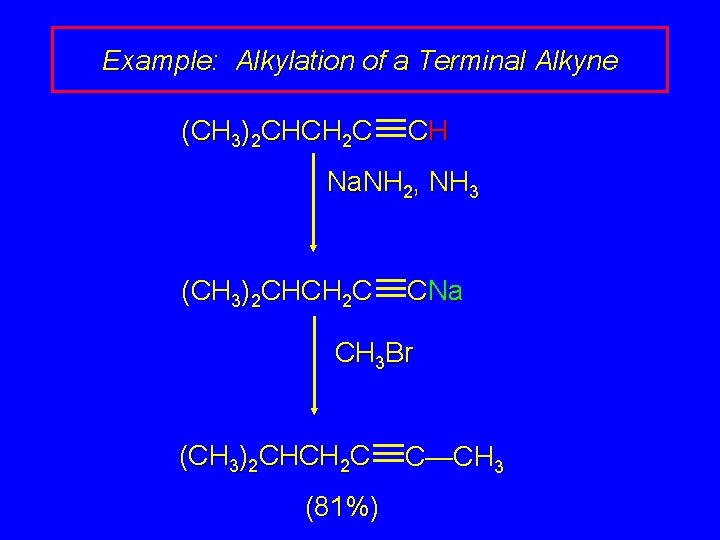
Example: Alkylation of a Terminal Alkyne (CH 3)2 CHCH 2 C CH Na. NH 2, NH 3 (CH 3)2 CHCH 2 C CNa CH 3 Br (CH 3)2 CHCH 2 C (81%) C—CH 3

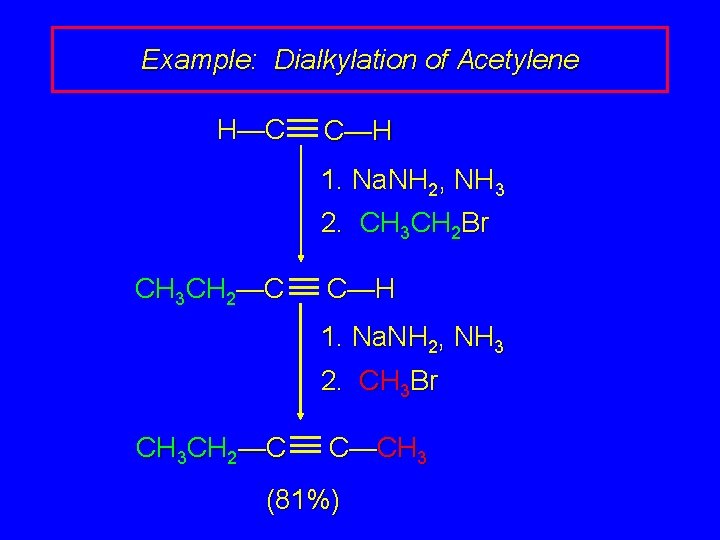
Example: Dialkylation of Acetylene H—C C—H 1. Na. NH 2, NH 3 2. CH 3 CH 2 Br CH 3 CH 2—C C—H 1. Na. NH 2, NH 3 2. CH 3 Br CH 3 CH 2—C C—CH 3 (81%)

Limitation Effective only with primary alkyl halides Secondary and tertiary alkyl halides undergo elimination

Reactions of Alkynes

Reactions of Alkynes Acidity Hydrogenation Metal-Ammonia Reduction Addition of Hydrogen Halides Hydration

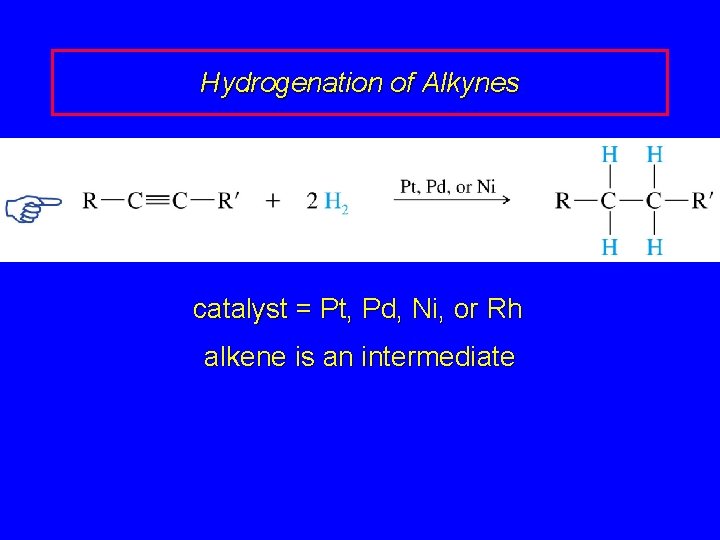
Hydrogenation of Alkynes

Hydrogenation of Alkynes RC CR' + 2 H 2 cat RCH 2 R' catalyst = Pt, Pd, Ni, or Rh alkene is an intermediate

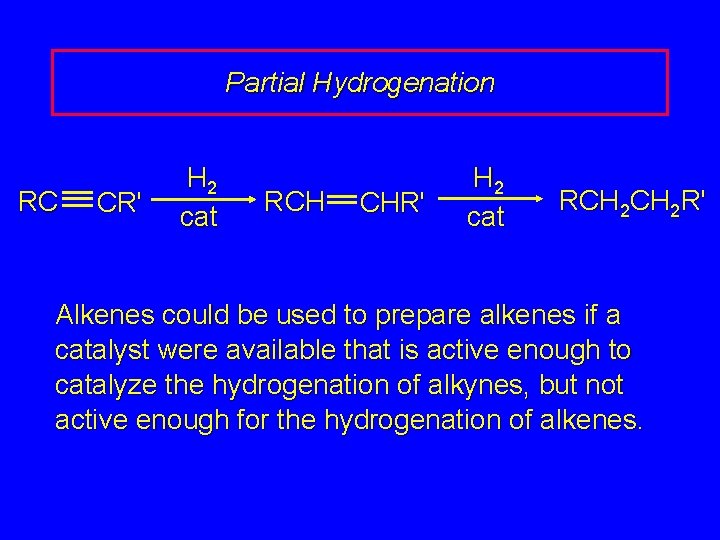
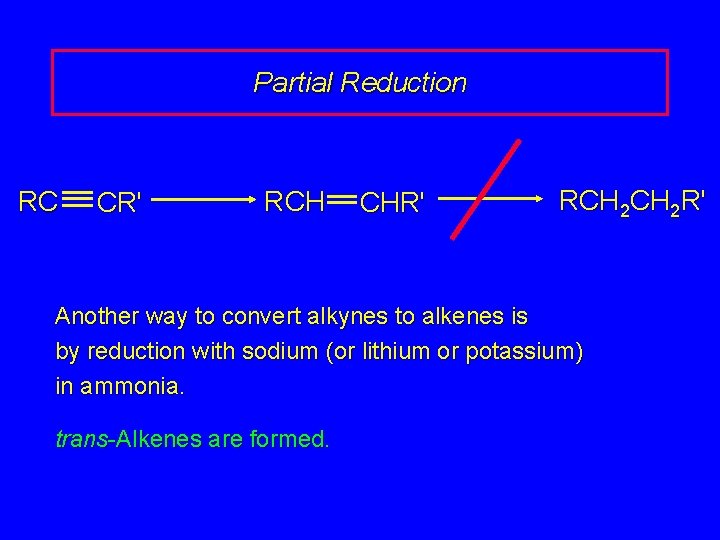
Partial Hydrogenation RC CR' H 2 cat RCH CHR' H 2 cat RCH 2 R' Alkenes could be used to prepare alkenes if a catalyst were available that is active enough to catalyze the hydrogenation of alkynes, but not active enough for the hydrogenation of alkenes.

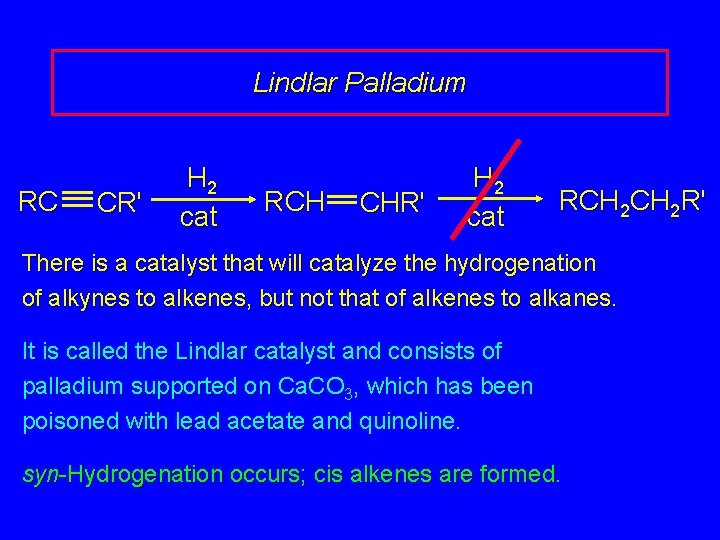
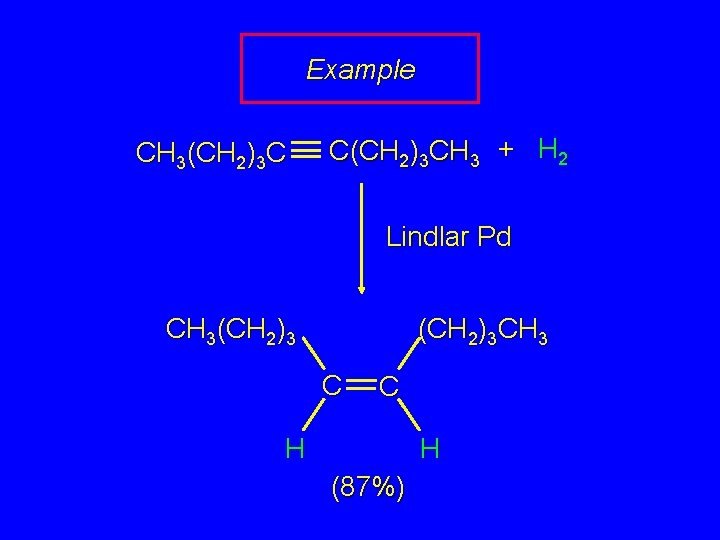
Lindlar Palladium RC CR' H 2 cat RCH CHR' H 2 cat RCH 2 R' There is a catalyst that will catalyze the hydrogenation of alkynes to alkenes, but not that of alkenes to alkanes. It is called the Lindlar catalyst and consists of palladium supported on Ca. CO 3, which has been poisoned with lead acetate and quinoline. syn-Hydrogenation occurs; cis alkenes are formed.

Example CH 3(CH 2)3 C C(CH 2)3 CH 3 + H 2 Lindlar Pd CH 3(CH 2)3 CH 3 C C H H (87%)



Metal-Ammonia Reduction of Alkynes trans-Alkenes

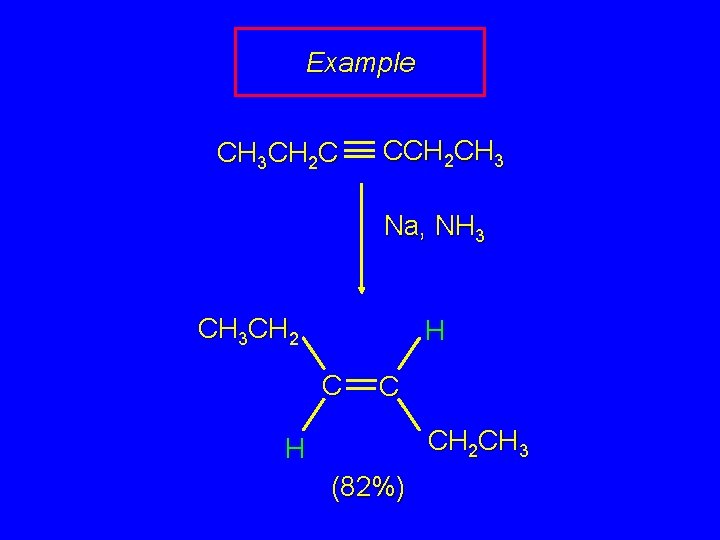
Partial Reduction RC CR' RCH CHR' RCH 2 R' Another way to convert alkynes to alkenes is by reduction with sodium (or lithium or potassium) in ammonia. trans-Alkenes are formed.


Example CH 3 CH 2 C CCH 2 CH 3 Na, NH 3 CH 3 CH 2 H C C CH 2 CH 3 H (82%)


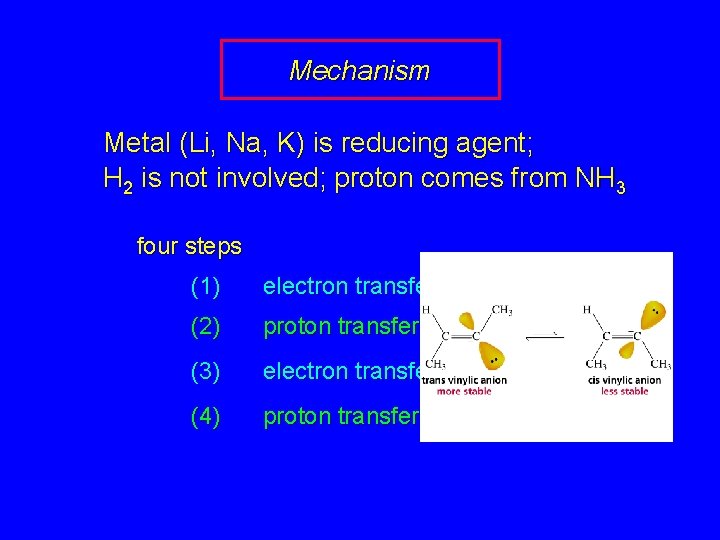
Mechanism Metal (Li, Na, K) is reducing agent; H 2 is not involved; proton comes from NH 3 four steps (1) electron transfer (2) proton transfer (3) electron transfer (4) proton transfer

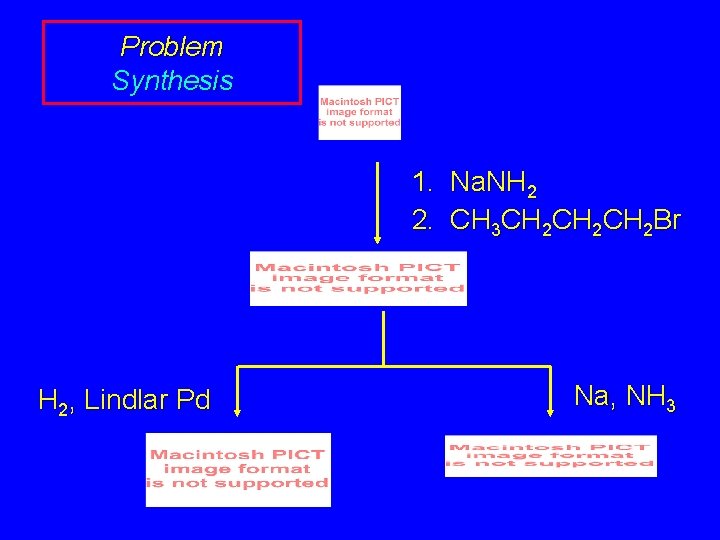
Problem Suggest an efficient syntheses of (E)- and (Z)-2 heptene from propyne and any necessary organic or inorganic reagents.

Problem Strategy

Problem Strategy

Problem Synthesis 1. Na. NH 2 2. CH 3 CH 2 CH 2 Br H 2, Lindlar Pd Na, NH 3

Addition of Hydrogen Halides to Alkynes

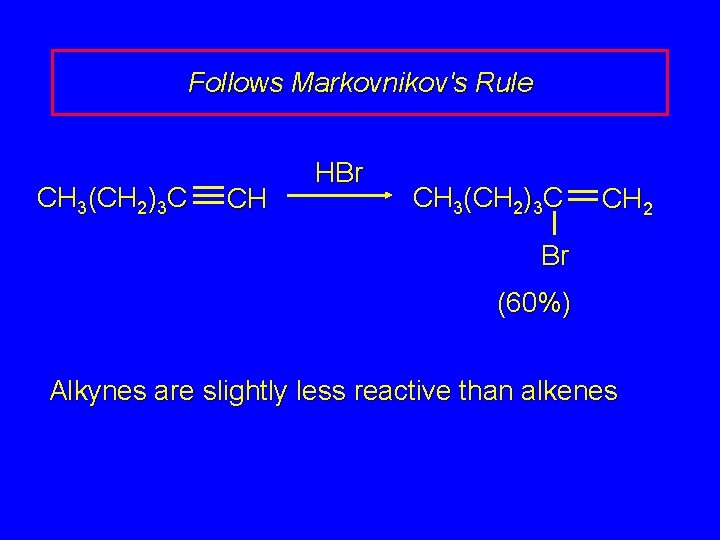
Follows Markovnikov's Rule CH 3(CH 2)3 C CH HBr CH 3(CH 2)3 C CH 2 Br (60%) Alkynes are slightly less reactive than alkenes

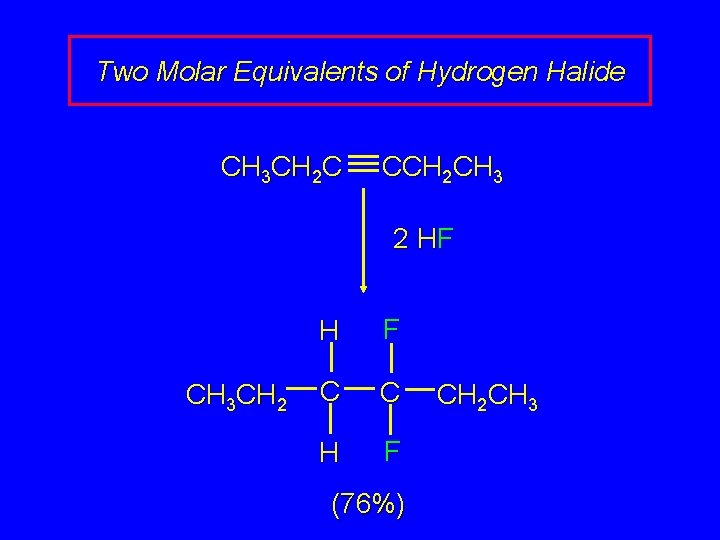
Two Molar Equivalents of Hydrogen Halide CH 3 CH 2 C CCH 2 CH 3 2 HF CH 3 CH 2 H F C C H F (76%) CH 2 CH 3

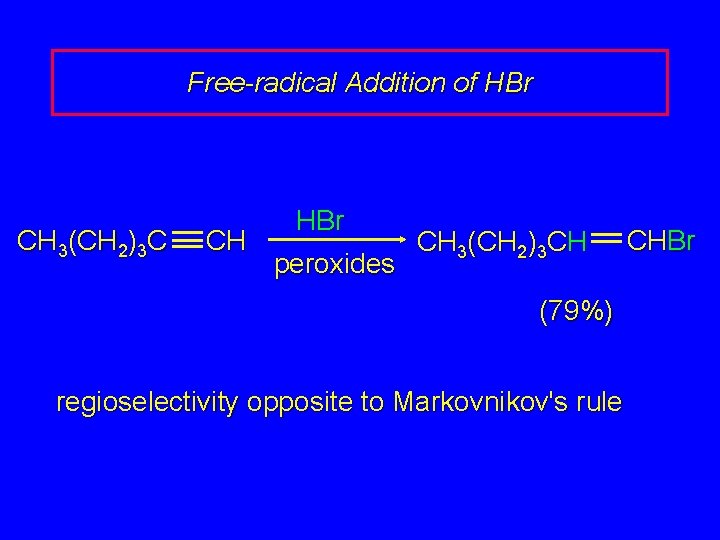
Free-radical Addition of HBr CH 3(CH 2)3 C CH HBr peroxides CH 3(CH 2)3 CH (79%) regioselectivity opposite to Markovnikov's rule CHBr

Hydration of Alkynes


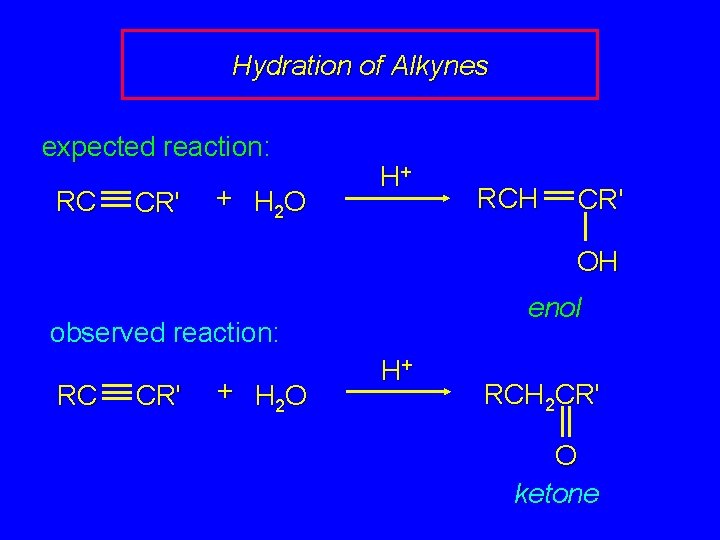
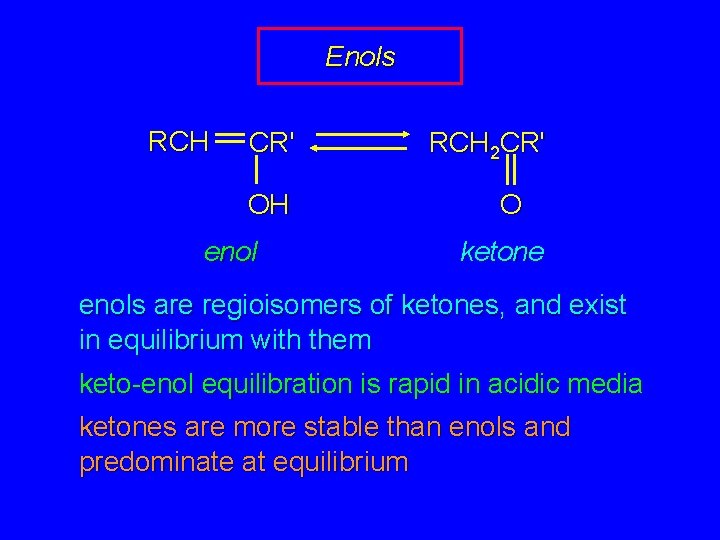
Hydration of Alkynes expected reaction: RC CR' + H 2 O H+ RCH CR' OH enol observed reaction: RC CR' + H 2 O H+ RCH 2 CR' O ketone

Enols RCH CR' OH enol RCH 2 CR' O ketone enols are regioisomers of ketones, and exist in equilibrium with them keto-enol equilibration is rapid in acidic media ketones are more stable than enols and predominate at equilibrium


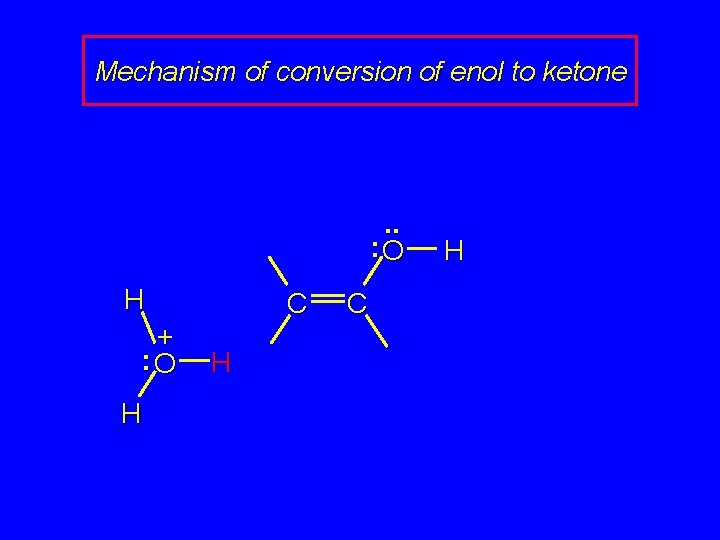
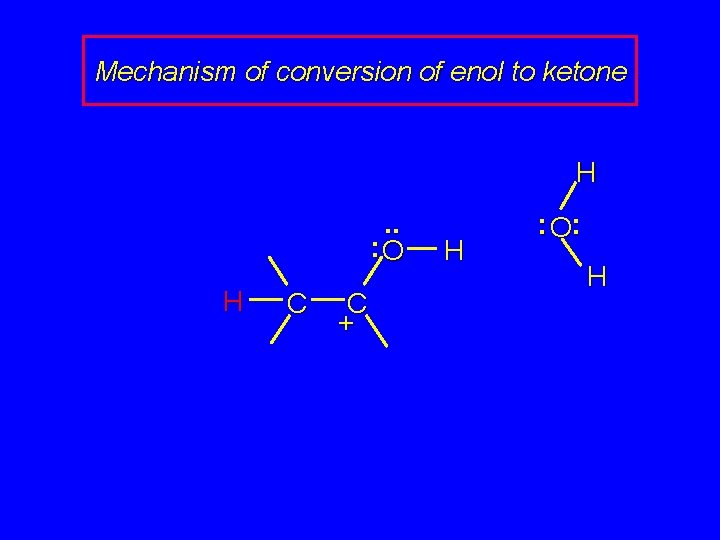
Mechanism of conversion of enol to ketone . . : O H + : O H C H

Mechanism of conversion of enol to ketone . . : O H + : O H C H

Mechanism of conversion of enol to ketone . . : O H : O: H H C C + H

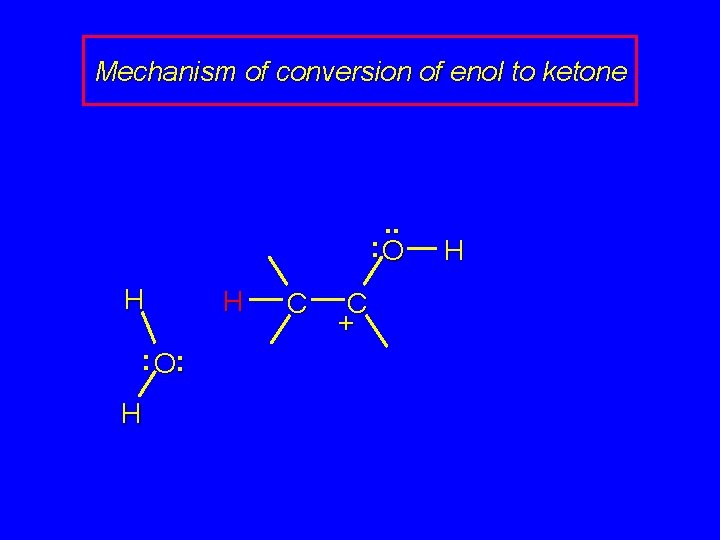
Mechanism of conversion of enol to ketone H. . : O H C C + H : O: H

Mechanism of conversion of enol to ketone H. . : O H C C + H : O: H

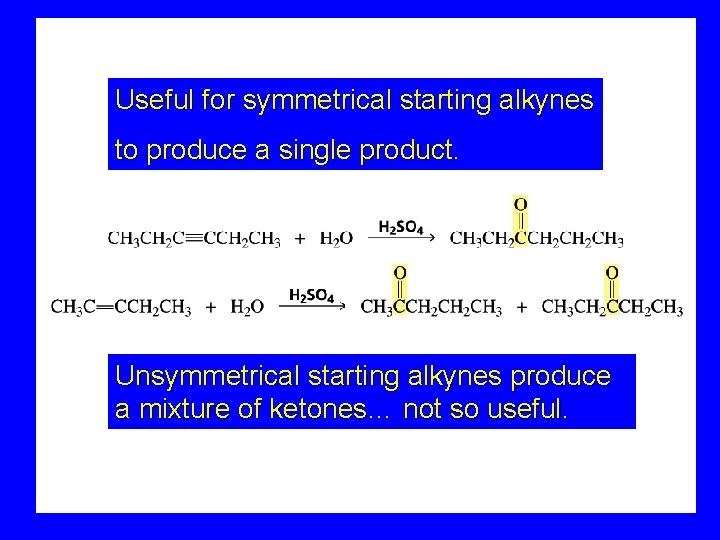
Useful for symmetrical starting alkynes to produce a single product. Unsymmetrical starting alkynes produce a mixture of ketones… not so useful.

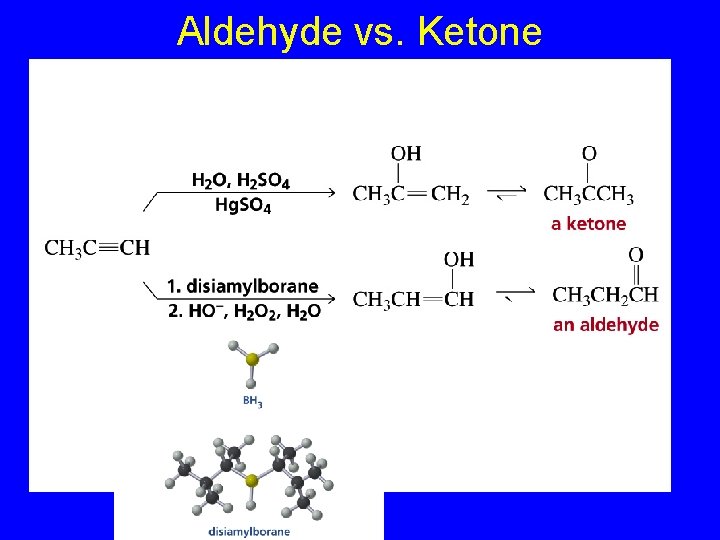
Aldehyde vs. Ketone

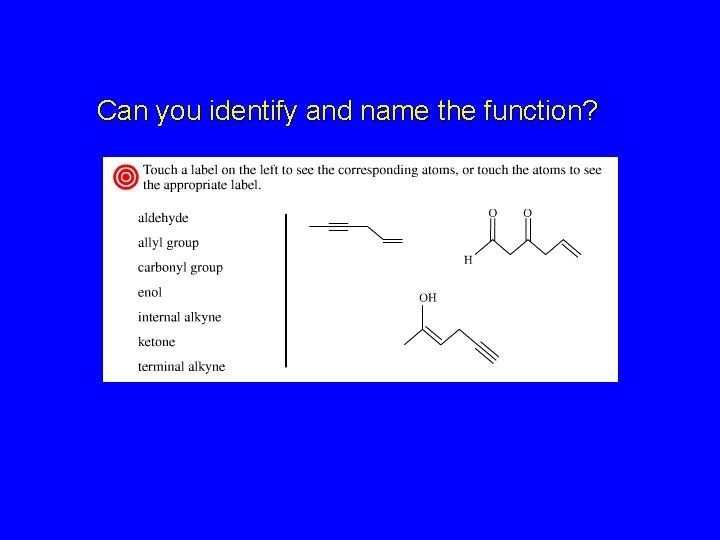
Can you identify and name the function?





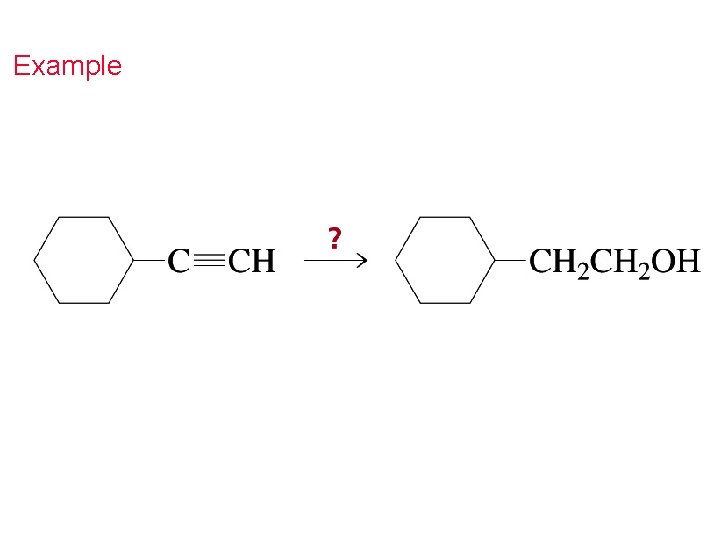
Example


 Coke vs diet coke density lab report
Coke vs diet coke density lab report Difference between induction heating and dielectric heating
Difference between induction heating and dielectric heating Killer coke
Killer coke Pepsi 4ps marketing mix
Pepsi 4ps marketing mix Write countable and uncountable nouns
Write countable and uncountable nouns Post colonial literature
Post colonial literature Thomas coke 8th earl of leicester
Thomas coke 8th earl of leicester Was diet coke
Was diet coke Share a coke with lisa
Share a coke with lisa Apples are countable or uncountable
Apples are countable or uncountable Coke coffee
Coke coffee Otto hoffman by product oven diagram
Otto hoffman by product oven diagram Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Combustion of alkynes
Combustion of alkynes What are the first 10 alkynes
What are the first 10 alkynes Lindlar pd
Lindlar pd Dissolving metal reduction
Dissolving metal reduction Alkynes
Alkynes Alkynes
Alkynes Syn addition
Syn addition Addition of hydrogen halides to alkynes
Addition of hydrogen halides to alkynes First 10 members of alkynes
First 10 members of alkynes Alkanes alkenes alkynes
Alkanes alkenes alkynes Alkynes
Alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Aldol condensation
Aldol condensation Ozonolysis of alkynes
Ozonolysis of alkynes Halogenation of alkynes
Halogenation of alkynes Alkynes
Alkynes 29 cfr 1910 subpart q
29 cfr 1910 subpart q Oxy acetylene gas flame
Oxy acetylene gas flame Oxygen/acetylene ratio for brazing
Oxygen/acetylene ratio for brazing Oxy acetylene ppe
Oxy acetylene ppe Oaw welding
Oaw welding Oxy-acetylene safety checklist
Oxy-acetylene safety checklist Oxy-acetylene brazing pressure settings chart
Oxy-acetylene brazing pressure settings chart Hexahydropyridin
Hexahydropyridin Trimerisation cyclique de l'acétylène
Trimerisation cyclique de l'acétylène Morgan balabanoff
Morgan balabanoff Acetylene cylinder cutaway
Acetylene cylinder cutaway Oxy acetylene safety rules
Oxy acetylene safety rules When was the oxy acetylene torch invented
When was the oxy acetylene torch invented Gas welding definition
Gas welding definition A regulator diaphragm is often made from ____
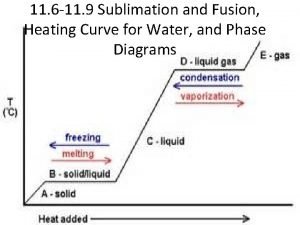
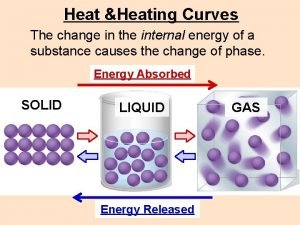
A regulator diaphragm is often made from ____ Sublimation fusion
Sublimation fusion Sieb heating and cooling
Sieb heating and cooling This operates simply by heating air in an enclosed space.
This operates simply by heating air in an enclosed space. Heating curve
Heating curve Thermal energy equation
Thermal energy equation Heating of the earth's surface causes
Heating of the earth's surface causes Used for holding hot objects especially crucible and cover
Used for holding hot objects especially crucible and cover Ken brady
Ken brady Heating curve
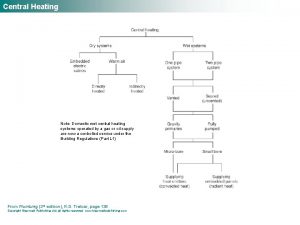
Heating curve Ise plumbing & heating
Ise plumbing & heating Central boiler heat exchanger
Central boiler heat exchanger Advanced heating
Advanced heating 7 steps of critical thinking
7 steps of critical thinking Tidal heating
Tidal heating On heating solids
On heating solids Induction heating comsol
Induction heating comsol Termis district heating software
Termis district heating software Karen heating and cooling
Karen heating and cooling