Infrared Spectroscopy of Deuterated Acetylene in Solid Parahydrogen

![Gas-phase d 2 -Acetylene FTIR Spectrum (>99%*) [*] Purity; C. D. N. Isotopes n Gas-phase d 2 -Acetylene FTIR Spectrum (>99%*) [*] Purity; C. D. N. Isotopes n](https://slidetodoc.com/presentation_image/343726f699bfe3a64fc1ce795cb8c036/image-9.jpg)


- Slides: 27

Infrared Spectroscopy of Deuterated Acetylene in Solid Parahydrogen and the Helium Recovery Initiative Aaron I. Strom Mentor: Dr. David T. Anderson Department of Chemistry, University of Wyoming Laramie, WY 82071

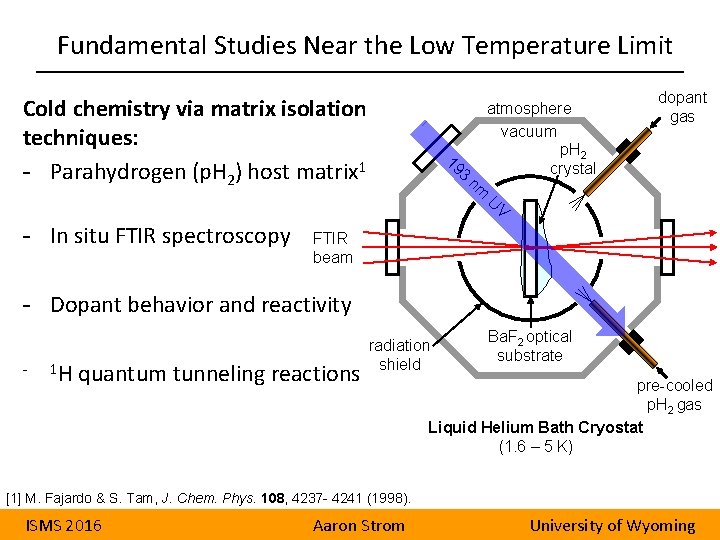
Fundamental Studies Near the Low Temperature Limit Cold chemistry via matrix isolation techniques: - Parahydrogen (p. H 2) host matrix 1 19 3 nm atmosphere vacuum p. H 2 crystal dopant gas U V - In situ FTIR spectroscopy FTIR beam - Dopant behavior and reactivity - 1 H quantum tunneling reactions radiation shield Ba. F 2 optical substrate pre-cooled p. H 2 gas Liquid Helium Bath Cryostat (1. 6 – 5 K) [1] M. Fajardo & S. Tam, J. Chem. Phys. 108, 4237 - 4241 (1998). ISMS 2016 Aaron Strom University of Wyoming

The Group Dr. David Anderson Left - Right: Myself, Wes Gates and Morgan Balabanoff ISMS 2016 Dr. Fred Mutunga Aaron Strom University of Wyoming

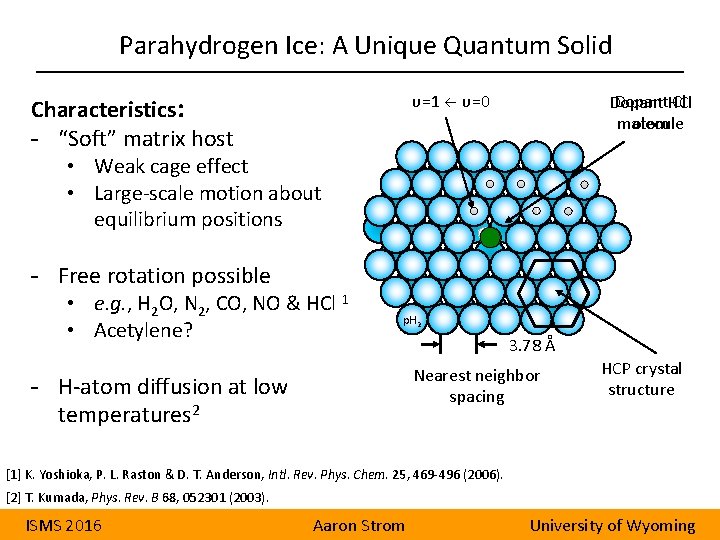
Parahydrogen Ice: A Unique Quantum Solid Characteristics: - “Soft” matrix host υ=1 υ=0 Dopant Cl Dopant HCl atom molecule • Weak cage effect • Large-scale motion about equilibrium positions - Free rotation possible • e. g. , H 2 O, N 2, CO, NO & HCl 1 • Acetylene? p. H 2 3. 78 Å Nearest neighbor spacing - H-atom diffusion at low temperatures 2 HCP crystal structure [1] K. Yoshioka, P. L. Raston & D. T. Anderson, Intl. Rev. Phys. Chem. 25, 469 -496 (2006). [2] T. Kumada, Phys. Rev. B 68, 052301 (2003). ISMS 2016 Aaron Strom University of Wyoming

Motivation I. Helium: A Nonrenewable Resource – 2 nd most abundant element in the Universe • Produced within Earth’s crust and upper mantle – e. g. , 23892 Ur 23490 Th + 42 He 2+ (α particle) – Atmospheric He is very short-lived II. d 2 -Acetylene Research – Prototype triple bond – Interstellar chemistry – Combustion chemistry [1] M. Herman, A. Campargue, M. I. El Idrissi & J. Vander Auwera, J. Phys. Chem. Ref. Data, 32, 921(2003). ISMS 2016 Aaron Strom University of Wyoming

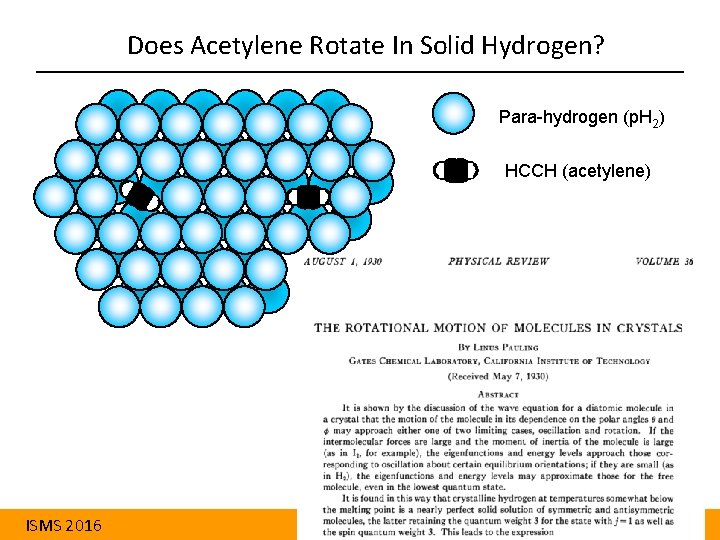
Does Acetylene Rotate In Solid Hydrogen? Para-hydrogen (p. H 2) HCCH (acetylene) ISMS 2016 Aaron Strom University of Wyoming

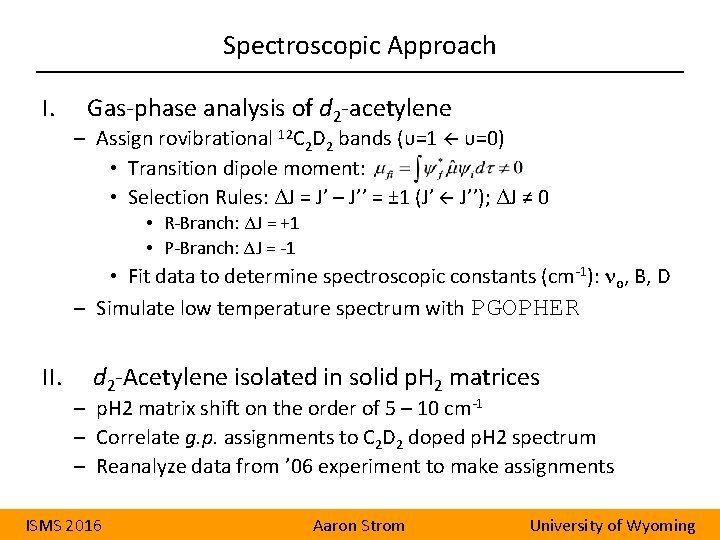
Spectroscopic Approach I. Gas-phase analysis of d 2 -acetylene – Assign rovibrational 12 C 2 D 2 bands (υ=1 υ=0) • Transition dipole moment: • Selection Rules: ΔJ = J’ – J’’ = ± 1 (J’ J’’); ΔJ ≠ 0 • R-Branch: ΔJ = +1 • P-Branch: ΔJ = -1 • Fit data to determine spectroscopic constants (cm-1): no, B, D – Simulate low temperature spectrum with PGOPHER II. d 2 -Acetylene isolated in solid p. H 2 matrices – p. H 2 matrix shift on the order of 5 – 10 cm-1 – Correlate g. p. assignments to C 2 D 2 doped p. H 2 spectrum – Reanalyze data from ’ 06 experiment to make assignments ISMS 2016 Aaron Strom University of Wyoming

The Lab: Gas-phase Experiments
![Gasphase d 2 Acetylene FTIR Spectrum 99 Purity C D N Isotopes n Gas-phase d 2 -Acetylene FTIR Spectrum (>99%*) [*] Purity; C. D. N. Isotopes n](https://slidetodoc.com/presentation_image/343726f699bfe3a64fc1ce795cb8c036/image-9.jpg)
Gas-phase d 2 -Acetylene FTIR Spectrum (>99%*) [*] Purity; C. D. N. Isotopes n 3(C 2 D 2) 300 K d=10 cm 0. 5 Torr n 3(C 2 HD) ν(CD) ISMS 2016 Aaron Strom n 1(C 2 HD) ν(CH) University of Wyoming

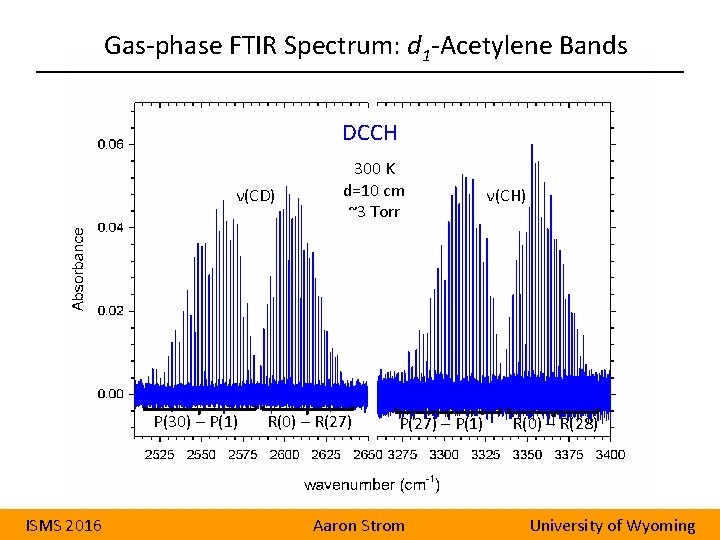
Gas-phase FTIR Spectrum: d 1 -Acetylene Bands DCCH ν(CD) P(30) – P(1) ISMS 2016 300 K d=10 cm ~3 Torr R(0) – R(27) P(27) – P(1) Aaron Strom ν(CH) R(0) – R(28) University of Wyoming

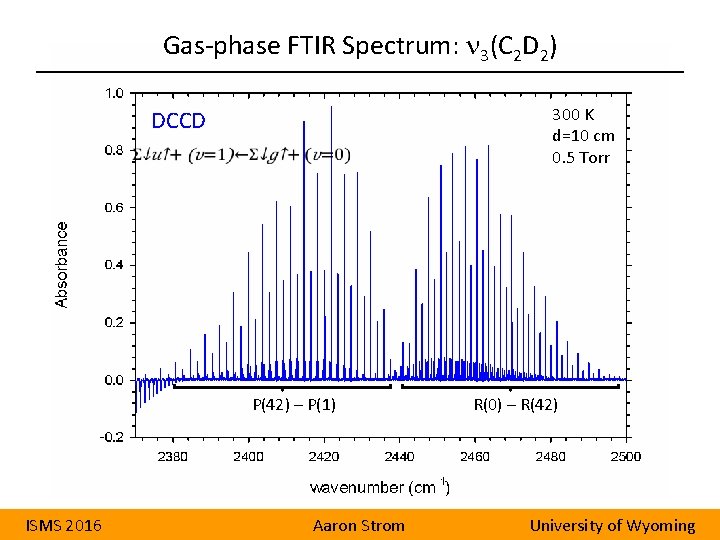
Gas-phase FTIR Spectrum: n 3(C 2 D 2) 300 K d=10 cm 0. 5 Torr DCCD P(42) – P(1) ISMS 2016 Aaron Strom R(0) – R(42) University of Wyoming

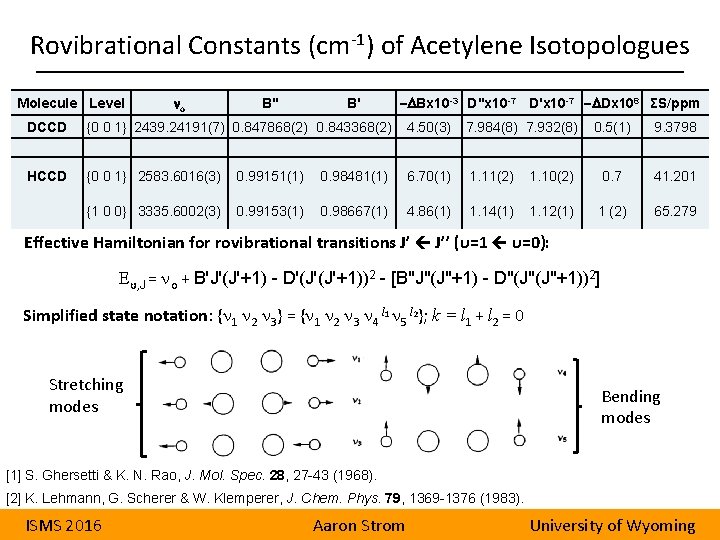
Rovibrational Constants (cm-1) of Acetylene Isotopologues Molecule Level no B'' B' -DBx 10 -3 D''x 10 -7 D'x 10 -7 -DDx 108 ΣS/ppm DCCD {0 0 1} 2439. 24191(7) 0. 847868(2) 0. 843368(2) 4. 50(3) 7. 984(8) 7. 932(8) 0. 5(1) 9. 3798 HCCD {0 0 1} 2583. 6016(3) 0. 99151(1) 0. 98481(1) 6. 70(1) 1. 11(2) 1. 10(2) 0. 7 41. 201 {1 0 0} 3335. 6002(3) 0. 99153(1) 0. 98667(1) 4. 86(1) 1. 14(1) 1. 12(1) 1 (2) 65. 279 Effective Hamiltonian for rovibrational transitions J’ J’’ (υ=1 υ=0): Eυ, J = no + BʹJʹ(Jʹ+1) - Dʹ(Jʹ(Jʹ+1))2 - [B"J"(J"+1) - D"(J"(J"+1))2] Simplified state notation: {n 1 n 2 n 3} = {n 1 n 2 n 3 n 4 l 1 n 5 l 2}; k = l 1 + l 2 = 0 Stretching modes Bending modes [1] S. Ghersetti & K. N. Rao, J. Mol. Spec. 28, 27 -43 (1968). [2] K. Lehmann, G. Scherer & W. Klemperer, J. Chem. Phys. 79, 1369 -1376 (1983). ISMS 2016 Aaron Strom University of Wyoming

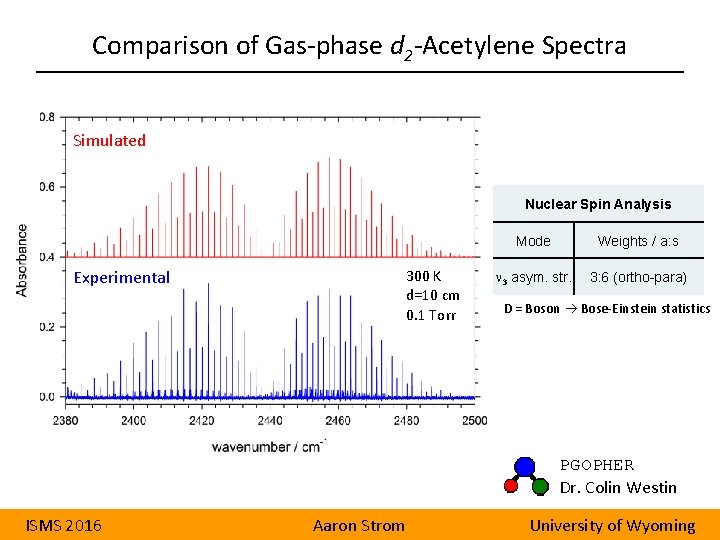
Comparison of Gas-phase d 2 -Acetylene Spectra Simulated Nuclear Spin Analysis Experimental 300 K d=10 cm 0. 1 Torr Mode Weights / a: s n 3 asym. str. 3: 6 (ortho-para) D = Boson Bose-Einstein statistics PGOPHER Dr. Colin Westin ISMS 2016 Aaron Strom University of Wyoming

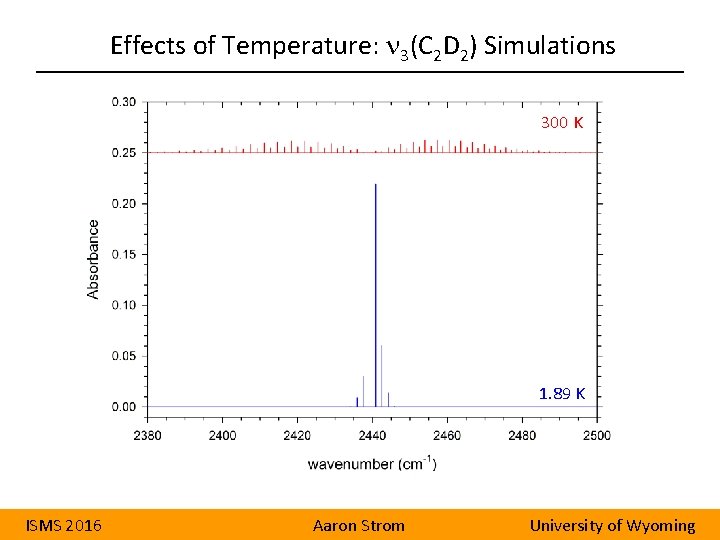
Effects of Temperature: n 3(C 2 D 2) Simulations 300 K 1. 89 K ISMS 2016 Aaron Strom University of Wyoming

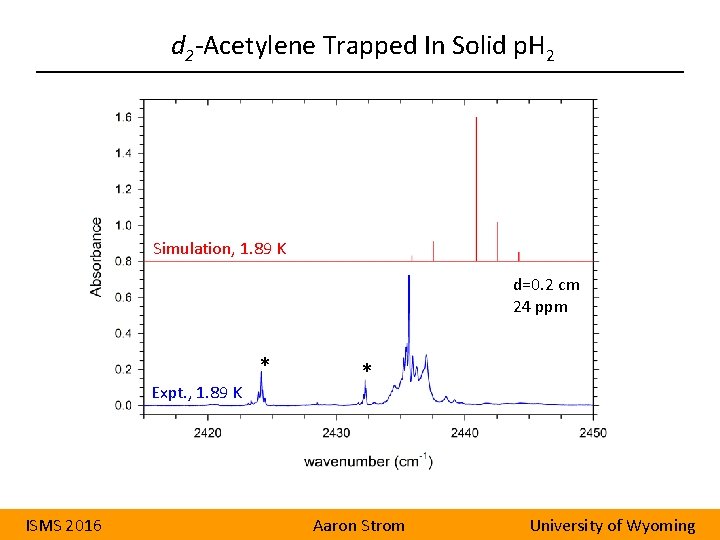
d 2 -Acetylene Trapped In Solid p. H 2 Simulation, 1. 89 K d=0. 2 cm 24 ppm * Expt. , 1. 89 K ISMS 2016 * Aaron Strom University of Wyoming

Interlude: History of Old C 2 D 2 Data I. Sample synthesized back in ’ 04 by A. Oman Ca. C 2 + D 2 O C 2 D 2 + Ca. O II. Gas-phase data remains uncharacterized – High pressure conditions – Sample is likely wet – Ca. C 2 is only 80% pure III. C 2 D 2/p. H 2 assignments remain tentative until spectral features can be reproduced ISMS 2016 Aaron Strom University of Wyoming

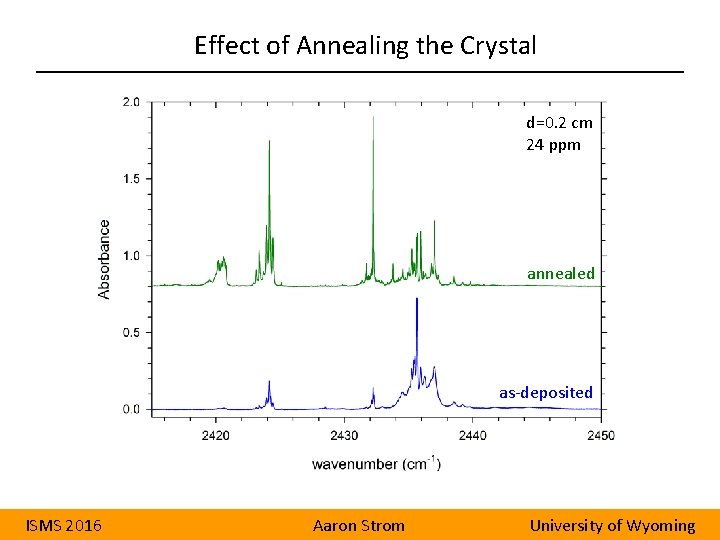
Effect of Annealing the Crystal d=0. 2 cm 24 ppm annealed as-deposited ISMS 2016 Aaron Strom University of Wyoming

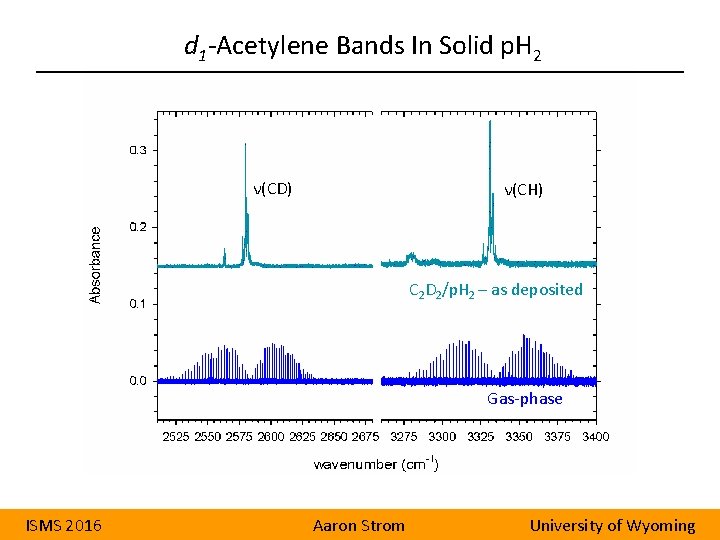
d 1 -Acetylene Bands In Solid p. H 2 ν(CD) ν(CH) C 2 D 2/p. H 2 – as deposited Gas-phase ISMS 2016 Aaron Strom University of Wyoming

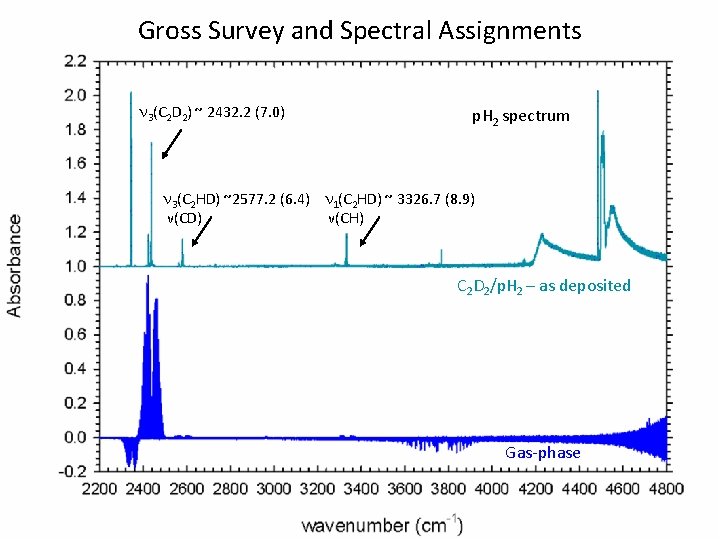
Gross Survey and Spectral Assignments n 3(C 2 D 2) ~ 2432. 2 (7. 0) p. H 2 spectrum n 3(C 2 HD) ~2577. 2 (6. 4) n 1(C 2 HD) ~ 3326. 7 (8. 9) ν(CD) ν(CH) C 2 D 2/p. H 2 – as deposited Gas-phase

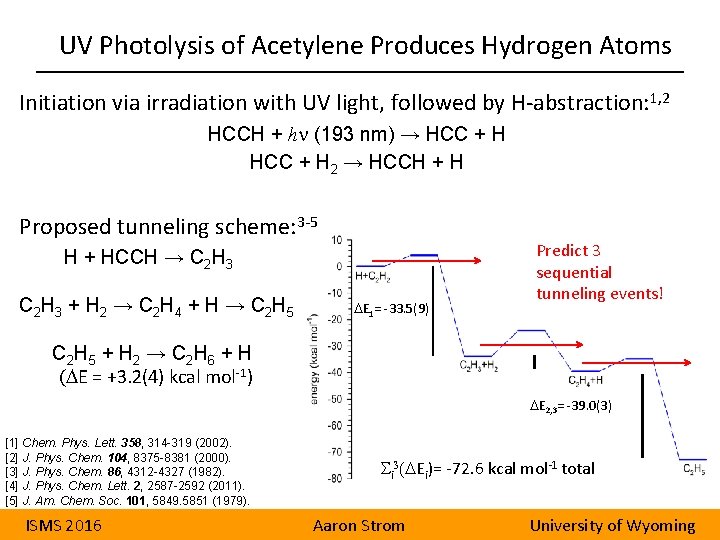
UV Photolysis of Acetylene Produces Hydrogen Atoms Initiation via irradiation with UV light, followed by H-abstraction: 1, 2 HCCH + hn (193 nm) → HCC + H 2 → HCCH + H Proposed tunneling scheme: 3 -5 H + HCCH → C 2 H 3 C 2 H 3 + H 2 → C 2 H 4 + H → C 2 H 5 DE 1= -33. 5(9) Predict 3 sequential tunneling events! C 2 H 5 + H 2 → C 2 H 6 + H (DE = +3. 2(4) kcal mol-1) DE 2, 3= -39. 0(3) [1] Chem. Phys. Lett. 358, 314 -319 (2002). [2] J. Phys. Chem. 104, 8375 -8381 (2000). [3] J. Phys. Chem. 86, 4312 -4327 (1982). [4] J. Phys. Chem. Lett. 2, 2587 -2592 (2011). [5] J. Am. Chem. Soc. 101, 5849. 5851 (1979). ISMS 2016 i 3(DEi)= -72. 6 kcal mol-1 total Aaron Strom University of Wyoming

The Helium Recovery Initiative: β Model Introducing: The He Recovery System w/ recovery platform @ Capacity ~8300 L gas ≈11 L l. He Empty ISMS 2016 Aaron Strom University of Wyoming

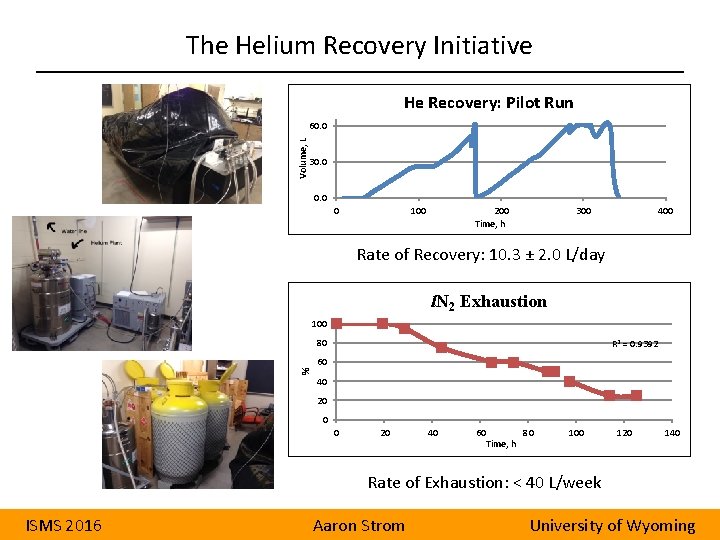
The Helium Recovery Initiative He Recovery: Pilot Run Volume, L 60. 0 30. 0 0 100 200 Time, h 300 400 Rate of Recovery: 10. 3 ± 2. 0 L/day l. N 2 Exhaustion 100 % 80 R 2 = 0. 9392 60 40 20 0 0 20 40 60 Time, h 80 100 120 140 Rate of Exhaustion: < 40 L/week ISMS 2016 Aaron Strom University of Wyoming

Conclusions and Future Plans Deuterated Acetylene Studies It is unlikely that acetylene is able to rotate within solid p. H 2 – Reproduce monomer spectrum – Temperature cycling and [o. H 2] variation – Gearing up for photolysis experiments/kinetic studies The Helium Recovery Initiative – Laser sensor controlled compressor automation – Design of He recovery line from cryostat – Try to conserve your helium! ISMS 2016 Aaron Strom University of Wyoming

Questions? ISMS 2016 Aaron Strom University of Wyoming

Acknowledgements Special thanks to Wes Gates, Morgan Balabanoff, Fred Mutunga, and Dave Anderson. ISMS 2016 Aaron Strom University of Wyoming

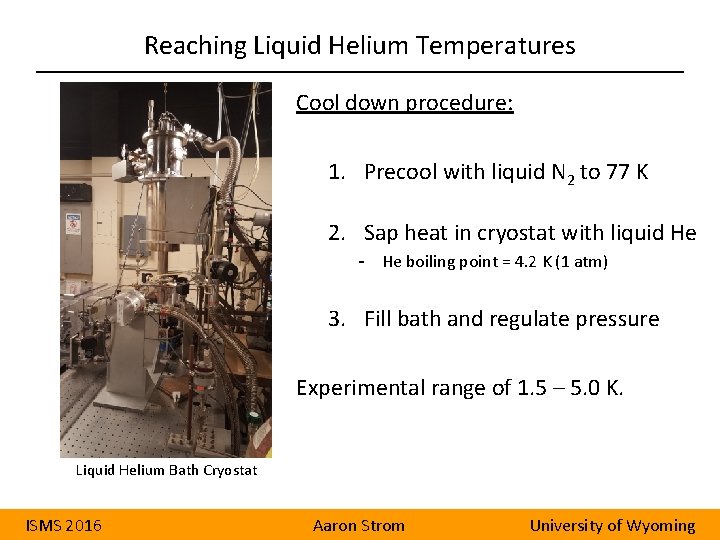
Reaching Liquid Helium Temperatures Cool down procedure: 1. Precool with liquid N 2 to 77 K 2. Sap heat in cryostat with liquid He - He boiling point = 4. 2 K (1 atm) 3. Fill bath and regulate pressure Experimental range of 1. 5 – 5. 0 K. Liquid Helium Bath Cryostat ISMS 2016 Aaron Strom University of Wyoming

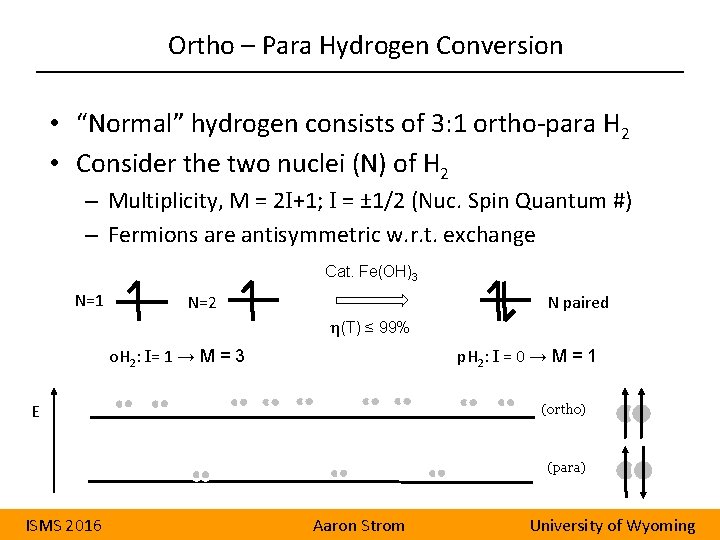
Ortho – Para Hydrogen Conversion • “Normal” hydrogen consists of 3: 1 ortho-para H 2 • Consider the two nuclei (N) of H 2 – Multiplicity, M = 2 I+1; I = ± 1/2 (Nuc. Spin Quantum #) – Fermions are antisymmetric w. r. t. exchange Cat. Fe(OH)3 N=1 N=2 N paired η(T) ≤ 99% o. H 2: I= 1 → M = 3 p. H 2: I = 0 → M = 1 E (ortho) (para) ISMS 2016 Aaron Strom University of Wyoming
 Cryostat procedure
Cryostat procedure Infrared spectroscopy ppt
Infrared spectroscopy ppt Nir spectroscopy instrumentation
Nir spectroscopy instrumentation Ir spectroscopy instrumentation
Ir spectroscopy instrumentation Aromatic compound ir
Aromatic compound ir Infrared spectroscopy theory
Infrared spectroscopy theory Ir spectroscopy sample preparation
Ir spectroscopy sample preparation Crystalline solid
Crystalline solid Evaporation separating mixtures
Evaporation separating mixtures Solve example
Solve example Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Covalent network solid vs molecular solid
Covalent network solid vs molecular solid Anisotropy
Anisotropy Crystallography types
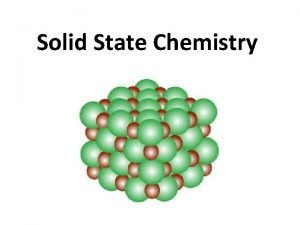
Crystallography types Interpenetration of solids
Interpenetration of solids When a solid completely penetrates another solid
When a solid completely penetrates another solid Crystalline or amorphous
Crystalline or amorphous Acetylene flame temperature
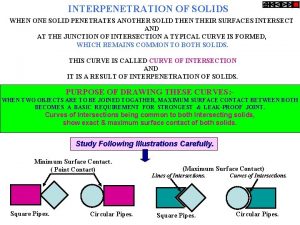
Acetylene flame temperature Oaw welding
Oaw welding Preparation of pyridine from acetylene
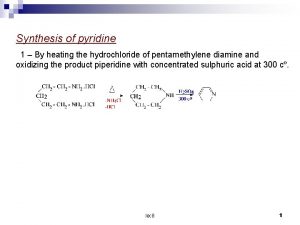
Preparation of pyridine from acetylene Ppe for oxy-acetylene welding
Ppe for oxy-acetylene welding Carburizing flame
Carburizing flame Oxy-acetylene inspection checklist
Oxy-acetylene inspection checklist Oxy acetylene safety rules
Oxy acetylene safety rules Trimerisation de l'acétylène
Trimerisation de l'acétylène Disadvantages of oxy acetylene welding
Disadvantages of oxy acetylene welding Oxy-acetylene welding tip size chart
Oxy-acetylene welding tip size chart 1910 subpart s
1910 subpart s