Diet Coke Warm Up Are the Coke and





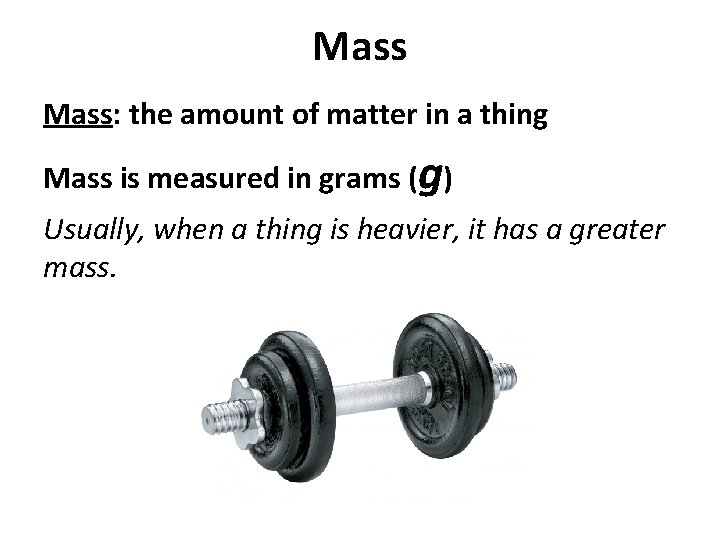

- Slides: 13

Diet Coke Warm Up Are the Coke and Diet Coke different masses? Mass of Coke: Mass of Diet Coke:

Diet Coke Warm Up What will happen when two cans of Diet Coke taped together are dropped in the tank of water?

Diet Coke Warm Up • What happened? Record your observations. • Why do you think that happened?

Properties of Matter Mass, Volume, Density

Matter: anything that has mass and takes up space Different types of matter make up everything around you. Everything is made of matter.
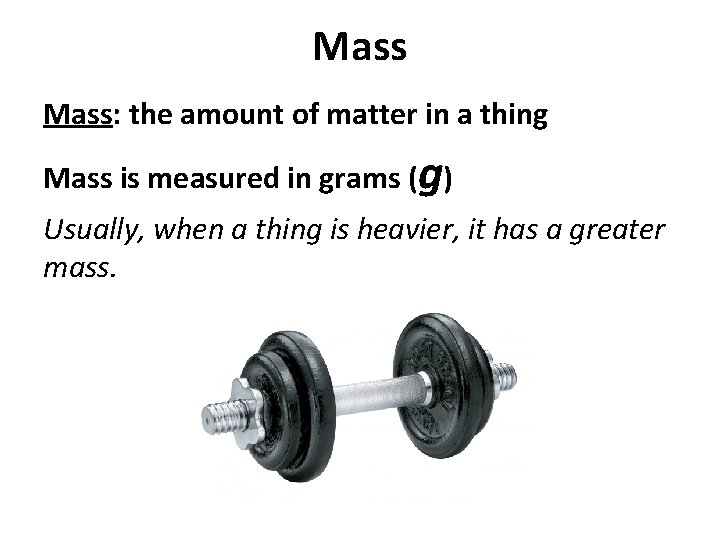
Mass: the amount of matter in a thing Mass is measured in grams (g) Usually, when a thing is heavier, it has a greater mass.

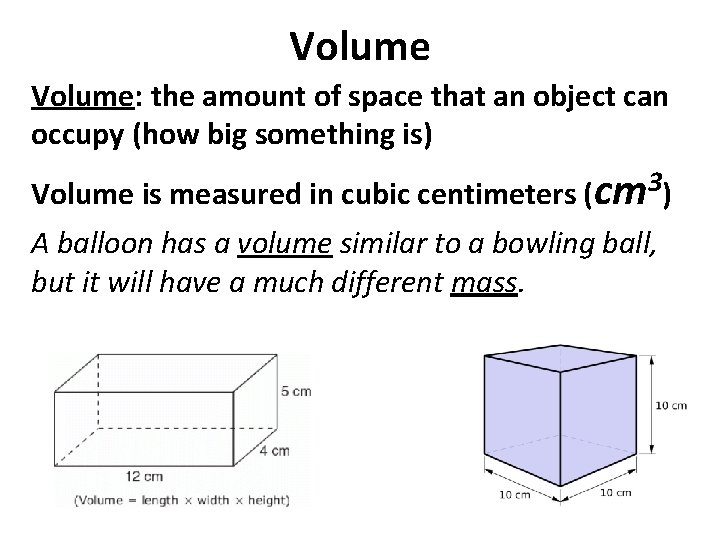
Volume: the amount of space that an object can occupy (how big something is) 3 Volume is measured in cubic centimeters (cm ) A balloon has a volume similar to a bowling ball, but it will have a much different mass.

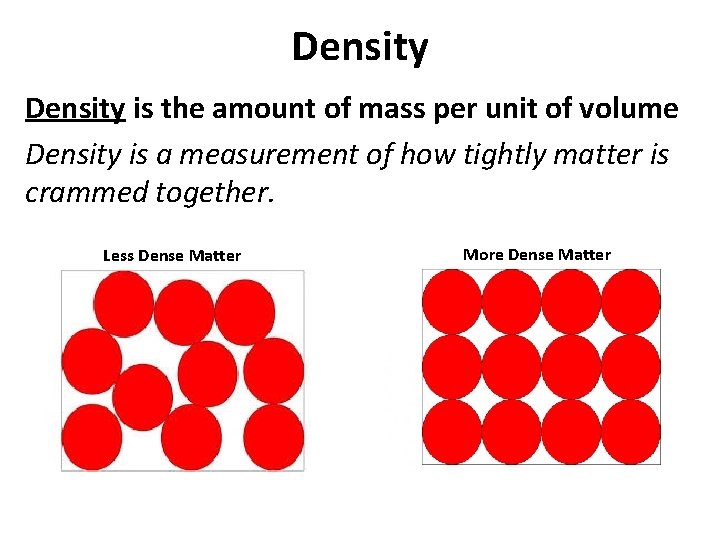
Density is the amount of mass per unit of volume Density is a measurement of how tightly matter is crammed together. Less Dense Matter More Dense Matter

Measuring Matter Activity Purpose • Identify methods and use tools to measure an object’s mass and volume • Calculate density for various objects

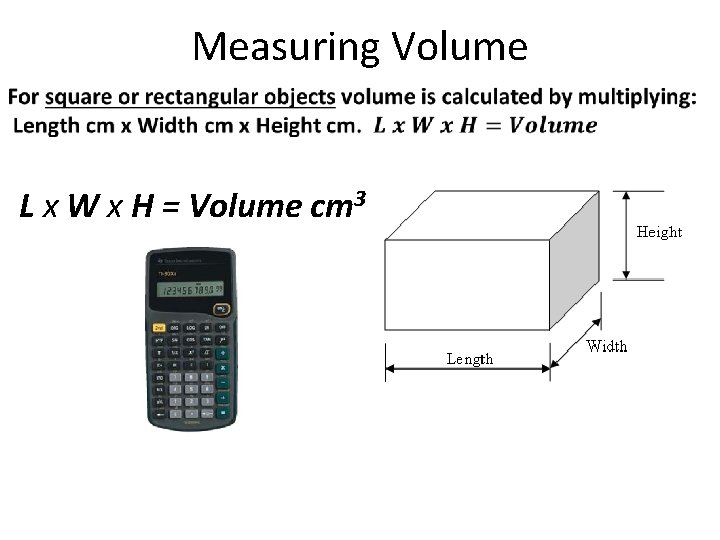
Measuring Volume L x W x H = Volume cm 3

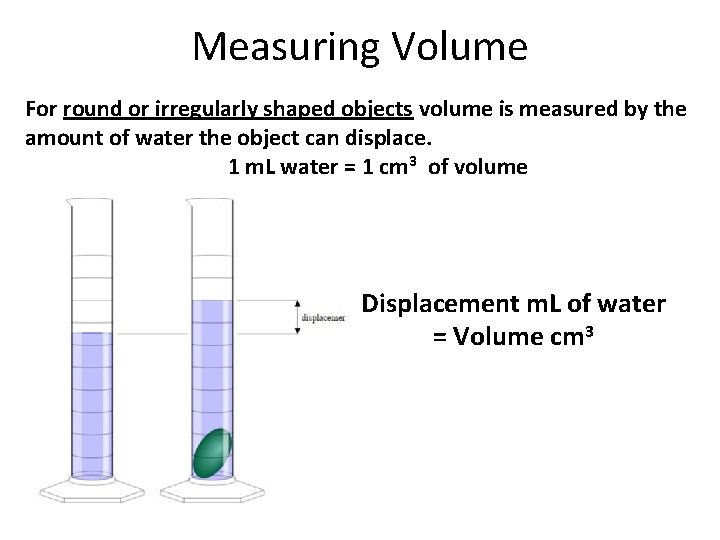
Measuring Volume For round or irregularly shaped objects volume is measured by the amount of water the object can displace. 1 m. L water = 1 cm 3 of volume Displacement m. L of water = Volume cm 3

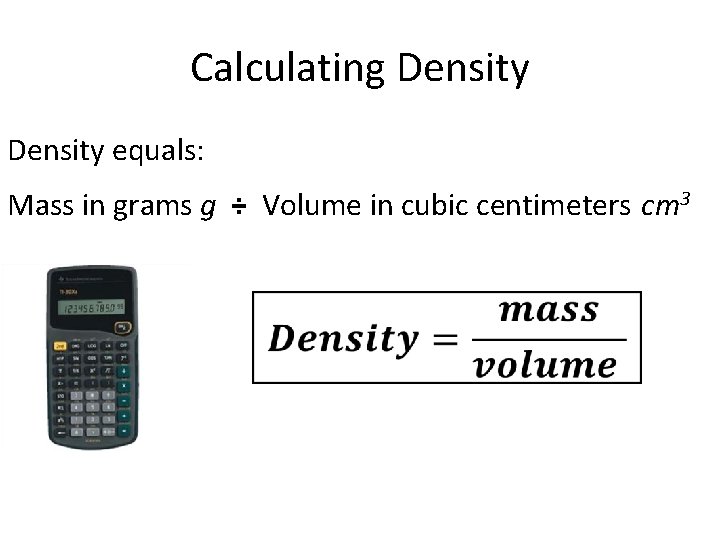
Calculating Density equals: Mass in grams g ÷ Volume in cubic centimeters cm 3

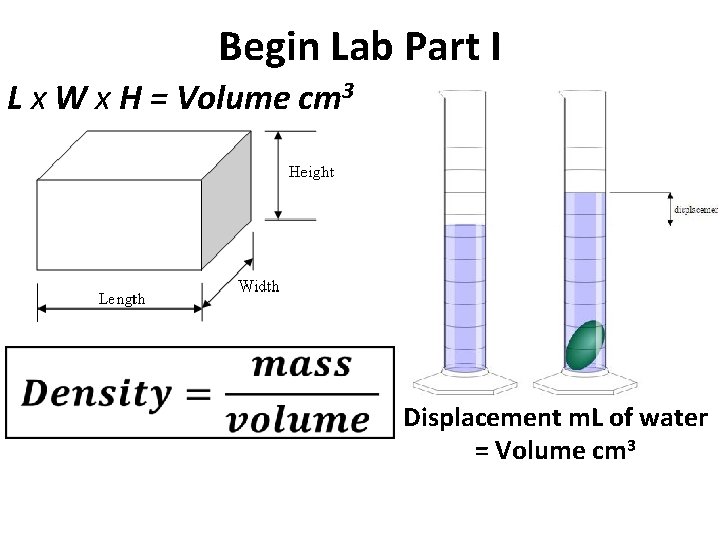
Begin Lab Part I L x W x H = Volume cm 3 Displacement m. L of water = Volume cm 3
 Coke vs diet coke density lab report
Coke vs diet coke density lab report Insidan region jh
Insidan region jh Was diet coke
Was diet coke Dash diet vs mediterranean
Dash diet vs mediterranean Marketing mix de coca cola
Marketing mix de coca cola Thomas coke 8th earl of leicester
Thomas coke 8th earl of leicester Potatoes is countable or uncountable
Potatoes is countable or uncountable Hcv and lcv formula
Hcv and lcv formula Write countable (c) or uncountable (u)
Write countable (c) or uncountable (u) Coke coffee
Coke coffee Killer coke
Killer coke Postcolonial literature
Postcolonial literature Share a coke with lisa
Share a coke with lisa Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay