DENSITY OF SODA AND DIET SODA How to







- Slides: 8

DENSITY OF SODA AND DIET SODA

How to Start your Lab Report Chemistry Name________ Experiment Number #3 Mods_____ Density of Soda and Diet Soda Name(s) of Lab Partner(s) Date_____

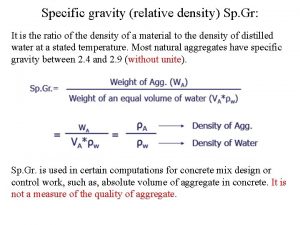
Objective The objective of this lab is to compare the density of soda and diet soda mathematically.

Lab Safety (skip 8 lines)

Procedure 1. Record the mass of a clean, dry graduated cylinder. 2. Measure 5. 0 ml of degassed soda as accurately as possible. 3. Place the graduated cylinder and soda on balance and record mass. 4. Calculate the mass of 5. 0 ml of soda.

5. Add degassed soda to graduated cylinder to a total volume of 10. 0 ml and mass 6. Repeat these steps using 15. 0 ml and 20. 0 ml of soda 7. Repeat steps 2 -6 using degassed diet soda.

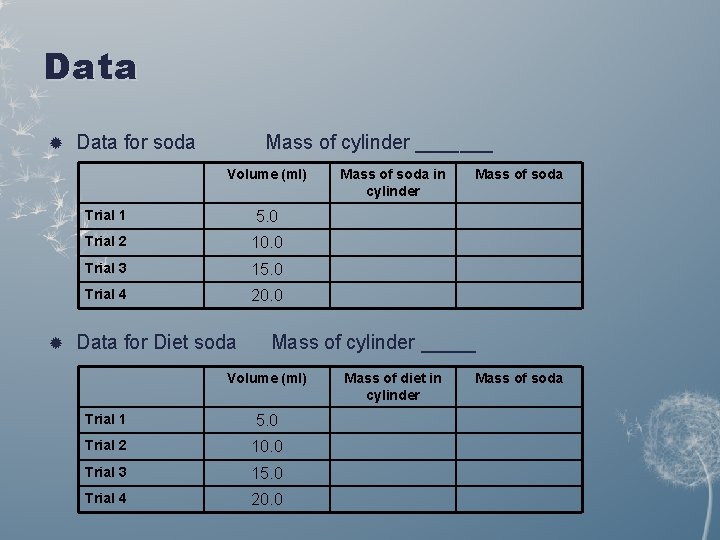
Data for soda Mass of cylinder _______ Volume (ml) Trial 1 5. 0 Trial 2 10. 0 Trial 3 15. 0 Trial 4 20. 0 Data for Diet soda Mass of soda in cylinder Mass of soda Mass of cylinder _____ Volume (ml) Trial 1 5. 0 Trial 2 10. 0 Trial 3 15. 0 Trial 4 20. 0 Mass of diet in cylinder Mass of soda

Conclusion The density of coke is ______. The density of diet coke is ______. The density of water is ______. The reason that the _____ coke floats is because ___________. I was accurate/not accurate in my soda measurements because my percent error was _______; I was accurate/not accurate in my diet soda measurements because my percent error was _______. 1 more sentence- what can you do to be more accurate?