Alkynes C C Synthesis of Acetylene Heat coke
















- Slides: 31

Alkynes C C

Synthesis of Acetylene Heat coke with lime in an electric furnace to form calcium carbide. Then drip water on the calcium carbide. coke lime * *This reaction was used to produce light for miners’ lamps and for the stage.

Acidity of Acetylene and Terminal Alkynes H C C

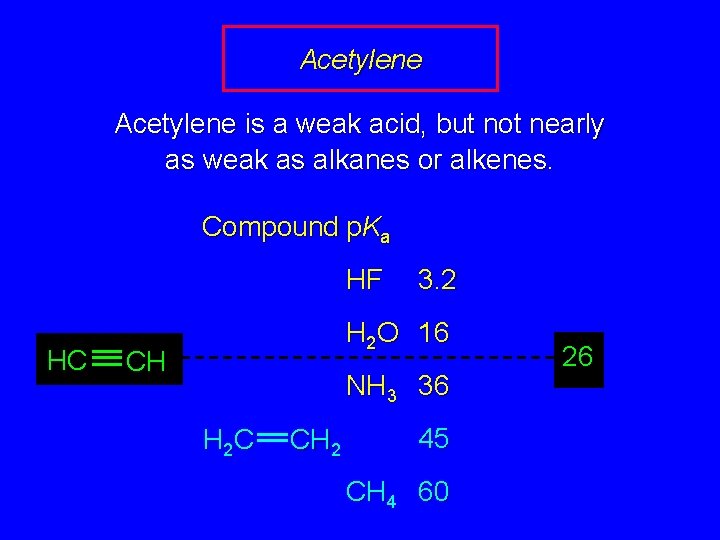
Acidity of Hydrocarbons In general, hydrocarbons are exceedingly weak acids Compound p. Ka HF 3. 2 H 2 O 16 NH 3 36 H 2 C CH 2 45 CH 4 60

Acetylene is a weak acid, but not nearly as weak as alkanes or alkenes. Compound p. Ka HF HC 3. 2 H 2 O 16 CH NH 3 36 H 2 C CH 2 45 CH 4 60 26

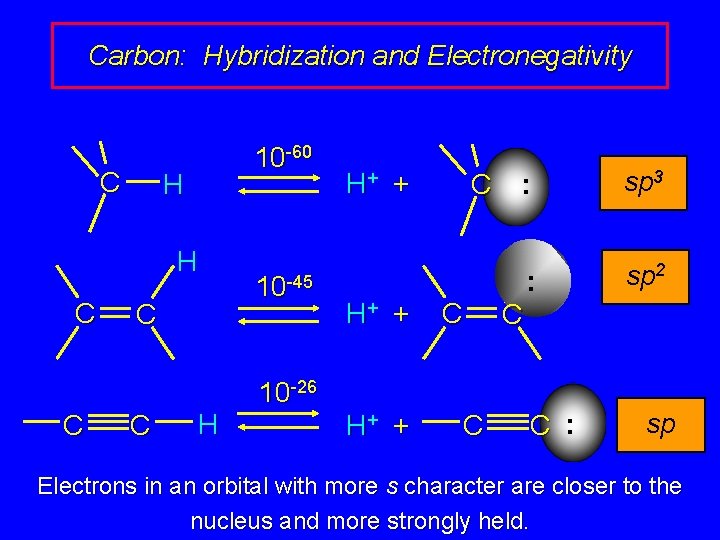
Carbon: Hybridization and Electronegativity C 10 -60 H H C C H 10 -45 H+ + C C 10 -26 H+ + C : sp 3 : sp 2 C C : sp Electrons in an orbital with more s character are closer to the nucleus and more strongly held.

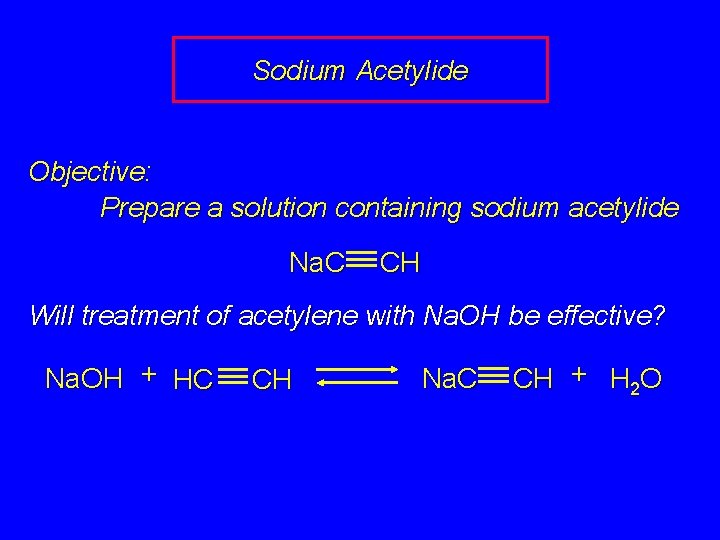
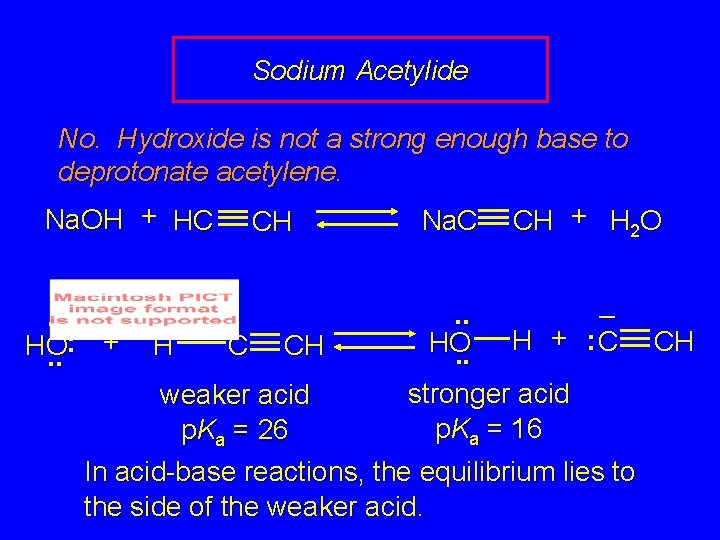
Sodium Acetylide Objective: Prepare a solution containing sodium acetylide Na. C CH Will treatment of acetylene with Na. OH be effective? Na. OH + HC CH Na. C CH + H 2 O

Sodium Acetylide No. Hydroxide is not a strong enough base to deprotonate acetylene. Na. OH + HC. . – : + HO. . H CH C CH Na. C CH + H 2 O . . – + : C H HO. . stronger acid p. Ka = 16 weaker acid p. Ka = 26 In acid-base reactions, the equilibrium lies to the side of the weaker acid. CH

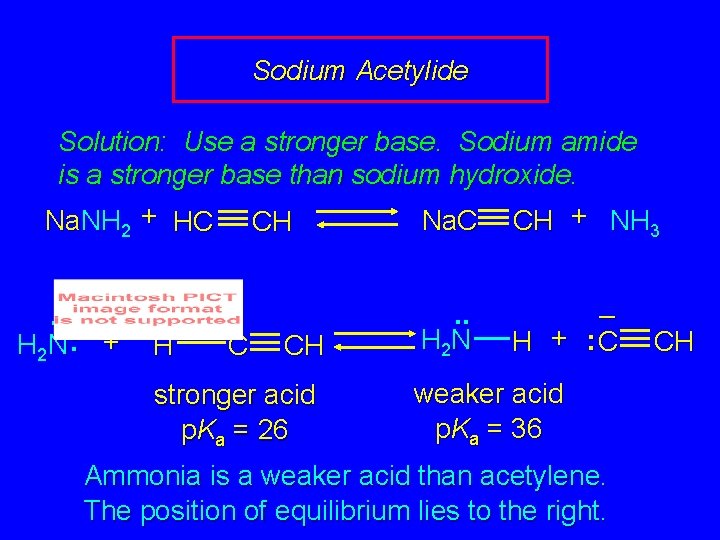
Sodium Acetylide Solution: Use a stronger base. Sodium amide is a stronger base than sodium hydroxide. Na. NH 2 + HC CH Na. C CH + NH 3. . – H 2 N : + H C CH stronger acid p. Ka = 26 . . H 2 N – H + : C weaker acid p. Ka = 36 Ammonia is a weaker acid than acetylene. The position of equilibrium lies to the right. CH

Preparation of Alkynes having other functions by reactions with Acetylide and Terminal Alkynes

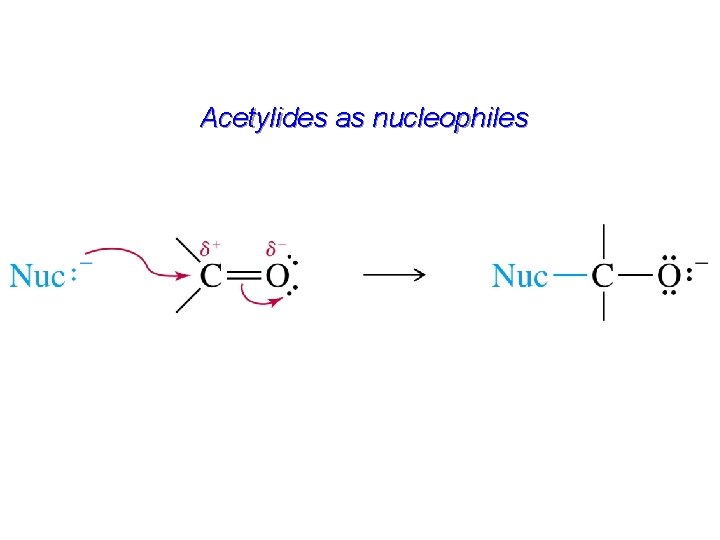
Acetylides as nucleophiles






Preparation of Alkynes by Alkylation of Acetylene and Terminal Alkynes

Preparation of Alkynes There are two main methods for the preparation of alkynes: Carbon-carbon bond formation alkylation of acetylene and terminal alkynes Functional-group transformations elimination

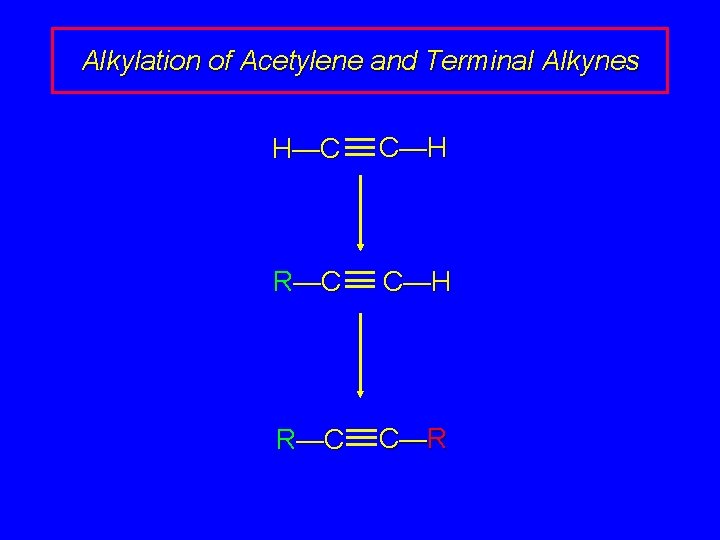
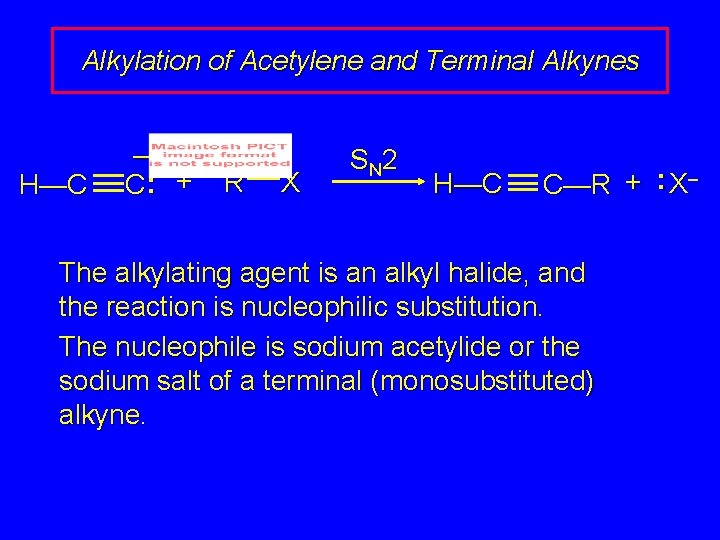
Alkylation of Acetylene and Terminal Alkynes H—C C—H R—C C—R

Alkylation of Acetylene and Terminal Alkynes H—C – C: + R X S N 2 H—C C—R + : X– The alkylating agent is an alkyl halide, and the reaction is nucleophilic substitution. The nucleophile is sodium acetylide or the sodium salt of a terminal (monosubstituted) alkyne.

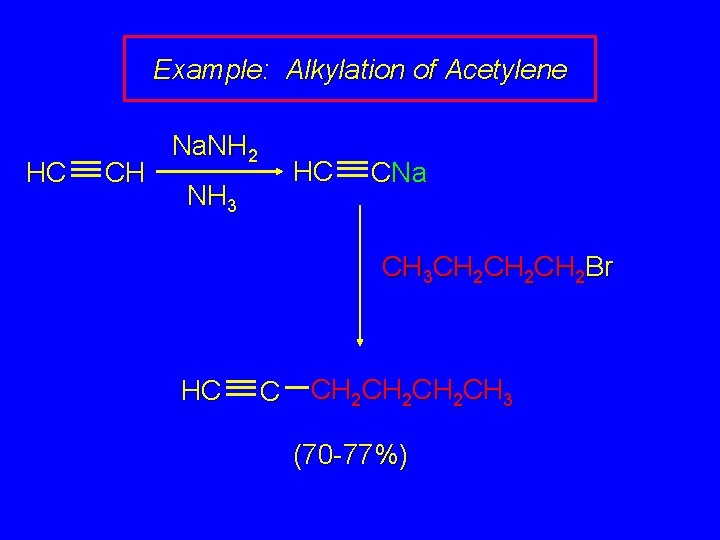
Example: Alkylation of Acetylene HC CH Na. NH 2 HC NH 3 CNa CH 3 CH 2 CH 2 Br HC C CH 2 CH 2 CH 3 (70 -77%)

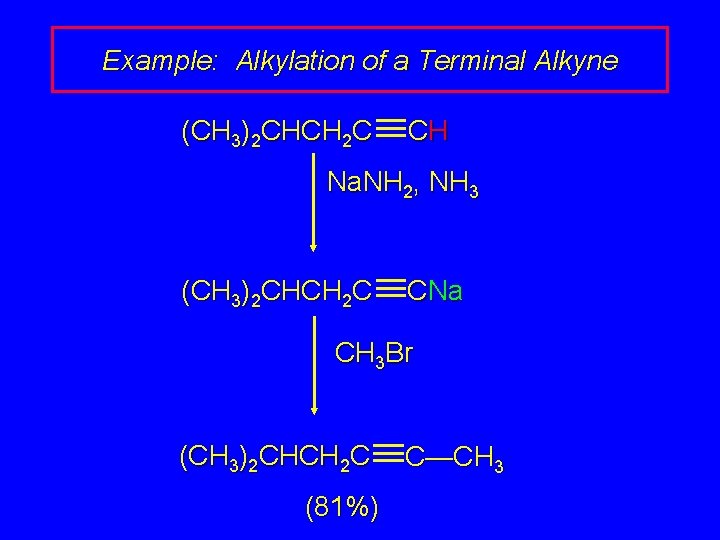
Example: Alkylation of a Terminal Alkyne (CH 3)2 CHCH 2 C CH Na. NH 2, NH 3 (CH 3)2 CHCH 2 C CNa CH 3 Br (CH 3)2 CHCH 2 C (81%) C—CH 3

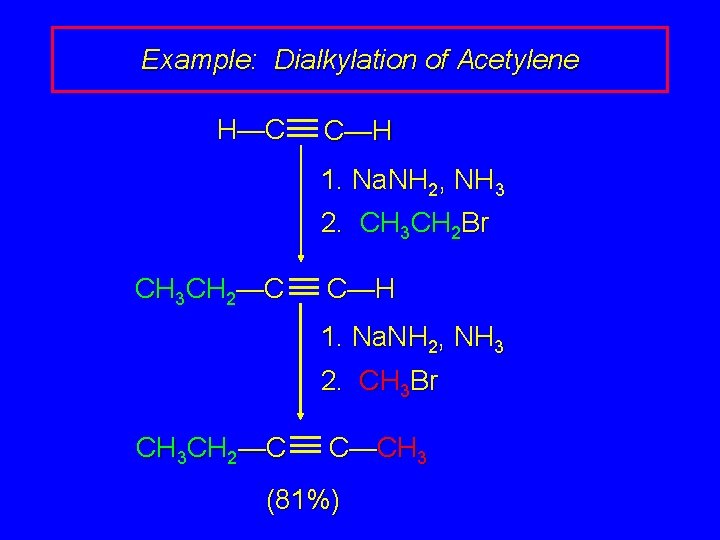
Example: Dialkylation of Acetylene H—C C—H 1. Na. NH 2, NH 3 2. CH 3 CH 2 Br CH 3 CH 2—C C—H 1. Na. NH 2, NH 3 2. CH 3 Br CH 3 CH 2—C C—CH 3 (81%)

Limitation Effective only with primary alkyl halides Secondary and tertiary alkyl halides undergo elimination


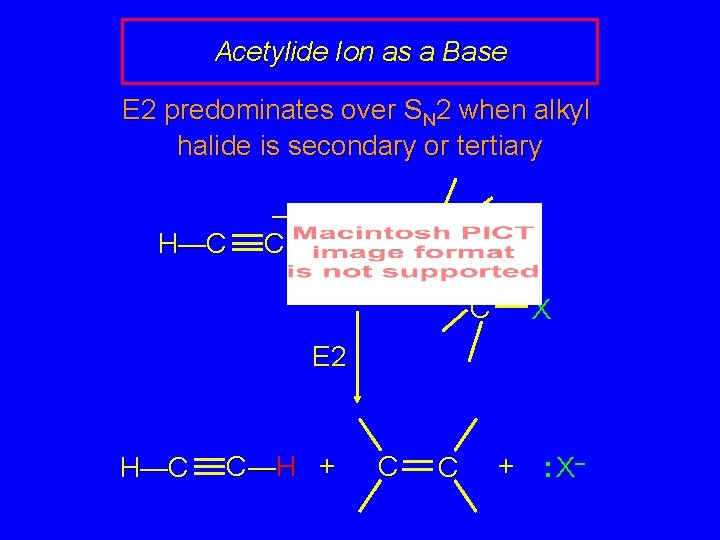
Acetylide Ion as a Base E 2 predominates over SN 2 when alkyl halide is secondary or tertiary H—C – C: H C C X E 2 H—C C —H + C C + : X–

Preparation of Alkynes by Elimination Reactions

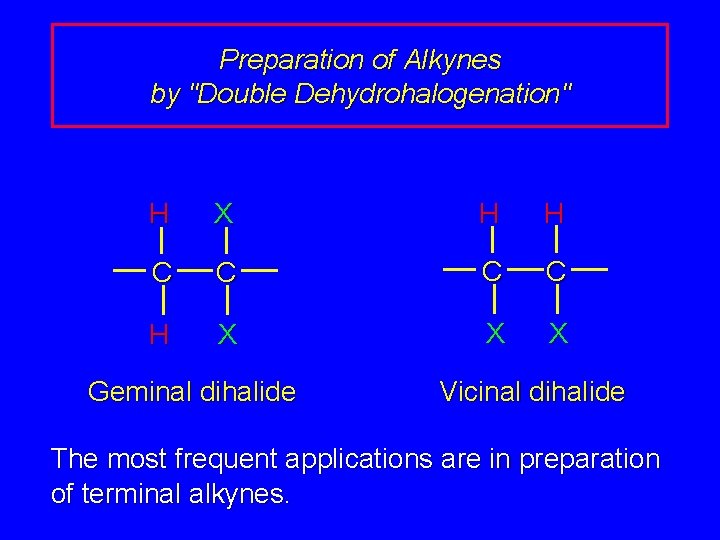
Preparation of Alkynes by "Double Dehydrohalogenation" H X H H C C H X X X Geminal dihalide Vicinal dihalide The most frequent applications are in preparation of terminal alkynes.

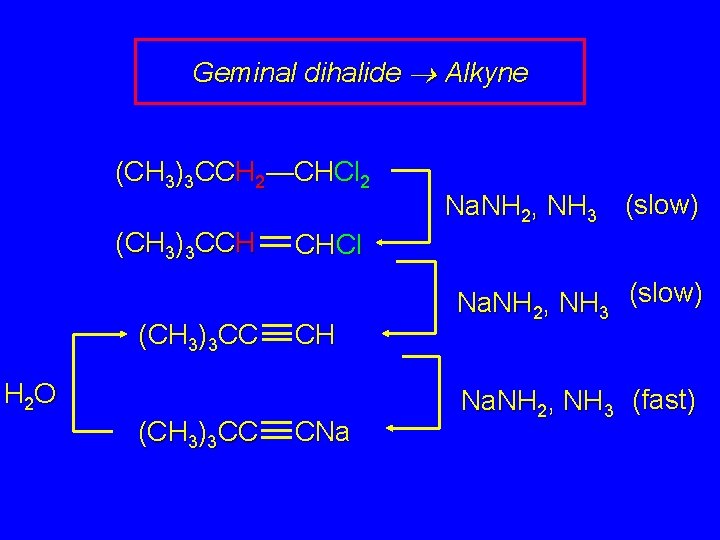
Geminal dihalide Alkyne (CH 3)3 CCH 2—CHCl 2 1. 3 Na. NH 2, NH 3 2. H 2 O (CH 3)3 CC CH (56 -60%)

Geminal dihalide Alkyne (CH 3)3 CCH 2—CHCl 2 (CH 3)3 CCH (CH 3)3 CC CHCl CH H 2 O (CH 3)3 CC Na. NH 2, NH 3 (slow) CNa Na. NH 2, NH 3 (slow) Na. NH 2, NH 3 (fast)

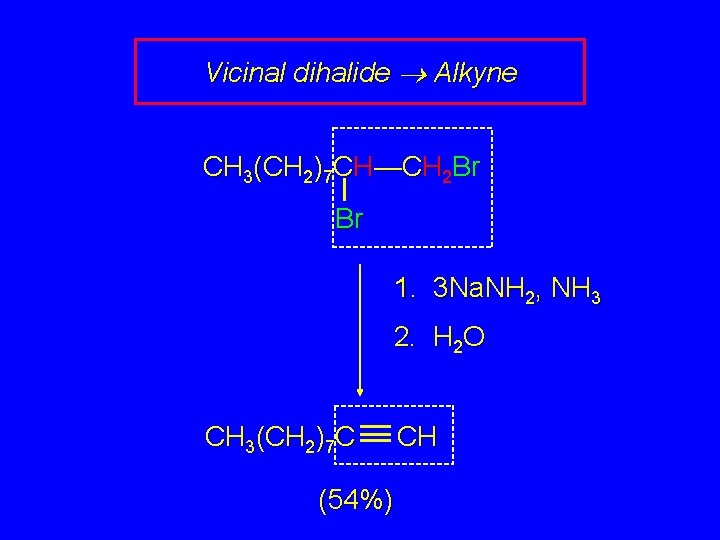
Vicinal dihalide Alkyne CH 3(CH 2)7 CH—CH 2 Br Br 1. 3 Na. NH 2, NH 3 2. H 2 O CH 3(CH 2)7 C (54%) CH
 Coke vs diet coke density lab report
Coke vs diet coke density lab report Killer coke
Killer coke Pepsi vs coke marketing strategy
Pepsi vs coke marketing strategy Can of coke countable or uncountable
Can of coke countable or uncountable Neocolonialism
Neocolonialism Holkham hall family
Holkham hall family Was diet coke
Was diet coke Share a coke with lisa
Share a coke with lisa Potato is countable or uncountable noun
Potato is countable or uncountable noun Coke coffee
Coke coffee Otto hoffman's method
Otto hoffman's method Combustion of alkynes
Combustion of alkynes Alkynes structural formula
Alkynes structural formula Iupac name
Iupac name Alkyne
Alkyne Alkenes general formula
Alkenes general formula Alkynes
Alkynes Anti markovnikov rule
Anti markovnikov rule First 10 members of alkynes
First 10 members of alkynes Alkanes alkenes alkynes
Alkanes alkenes alkynes Alkynes
Alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Syn addition
Syn addition Aldol
Aldol Hybridization of alkynes
Hybridization of alkynes Halogenation of alkynes
Halogenation of alkynes Alkynes
Alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Heat exchanger network design example
Heat exchanger network design example Oxy-acetylene welding tip size chart
Oxy-acetylene welding tip size chart Gas welding safety tips
Gas welding safety tips Gas welding flames
Gas welding flames