Accreditation An institution or a program meets standards










































































- Slides: 108

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Laboratory Method Verification Dr / Aida Ahmed Abd Elhameed Lecturer of Clnical Pathology


Quality Doing the right thing right, from Accreditation • the first time and every time An institution or a program meets • standards of quality set forth by an • accrediting agency •

Accreditation An institution or a program meets standards of quality set forth by an Accreditation • accrediting agency An institution or a program meets • standards of quality set forth by an • accrediting agency •

Validation / Verification? ? Method validation: (Manufacturer concern) Establishing the performance of a new diagnostic tool. Confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been fulfilled’ (doing correct test). . . ISO 9001: 2005

Method verification: (Lab / user concern) A process to determine performance characteristics before a test system is. Accreditation utilized • for patient testing. An institution or a program meets • Confirmation, through the of forth by an • standards ofprovision quality set accrediting agency • objective evidence, that specified requirements have been fulfilled’ (doing test correctly)……ISO 9001: 2005

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

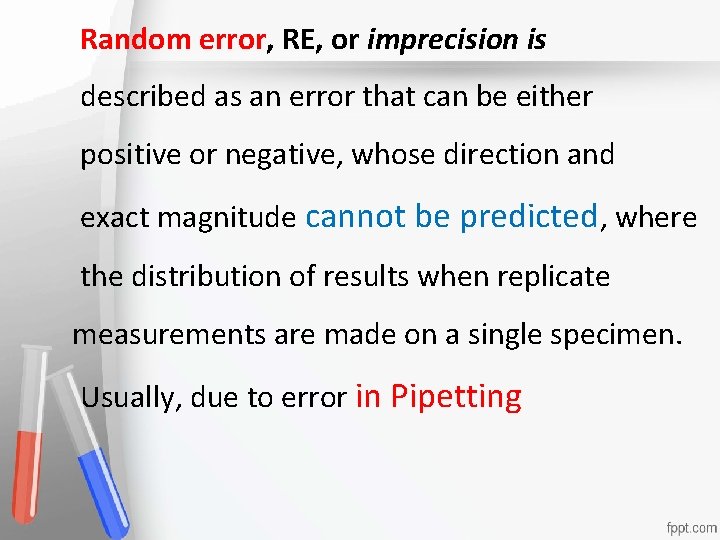
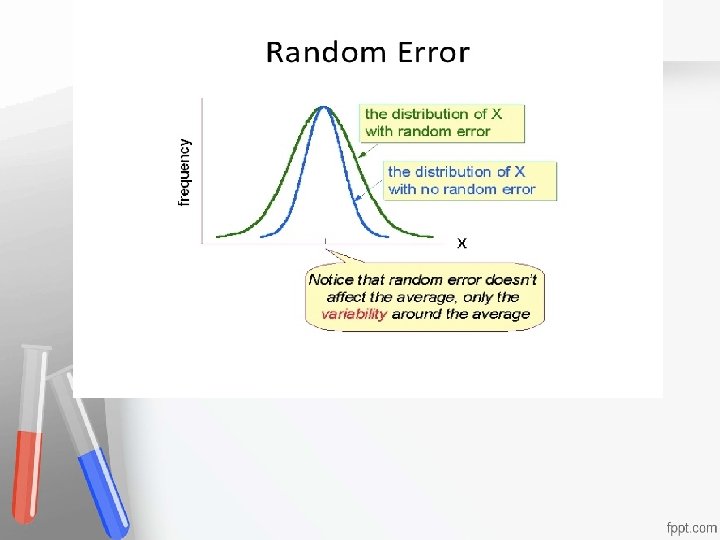
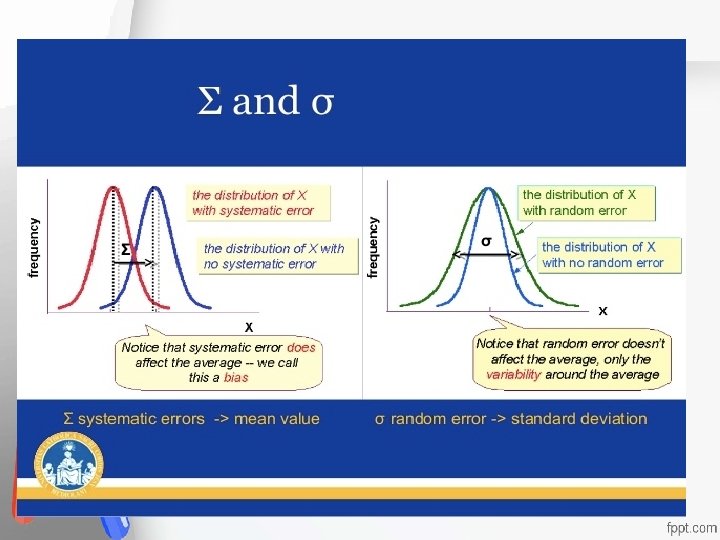
Random error, RE, or imprecision is described as an error that can be either Accreditation • positive or negative, whose direction and An institution or a program meets • exact magnitude cannot be predicted, where standards of quality set forth by an • the distribution of resultsaccrediting when replicate agency • measurements are made on a single specimen. Usually, due to error in Pipetting

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

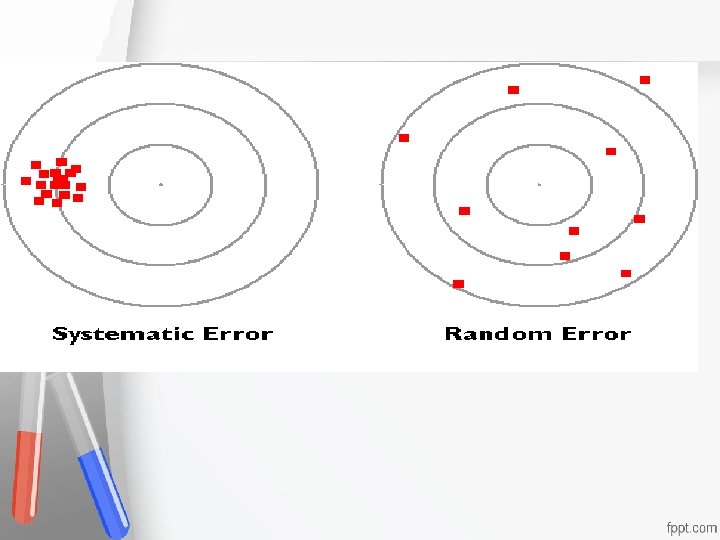
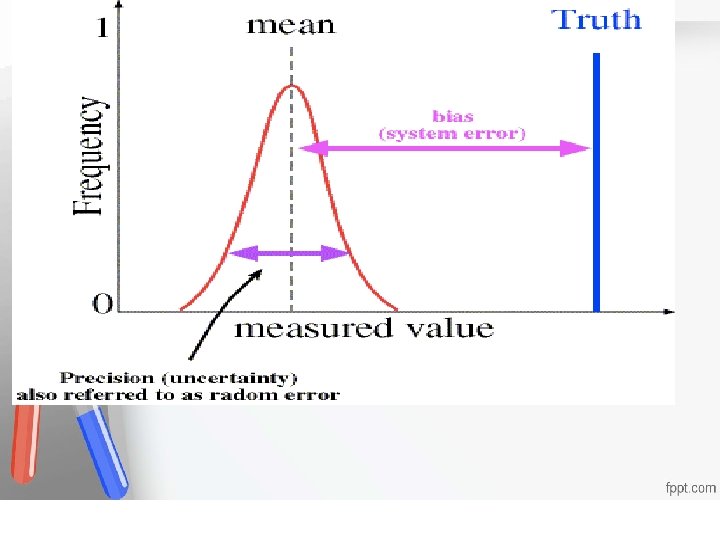
Systematic error, SE, or inaccuracy is an error that is always in one direction, displacing the mean of the distribution Accreditation • from its original value. An institution or a program meets • In contrast to random errors, systematic standards of quality set forth by an • errors are in one direction and cause all the accrediting agency • test results to be either high or low. Either constant or proportionate Usually, due to error in calibration

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

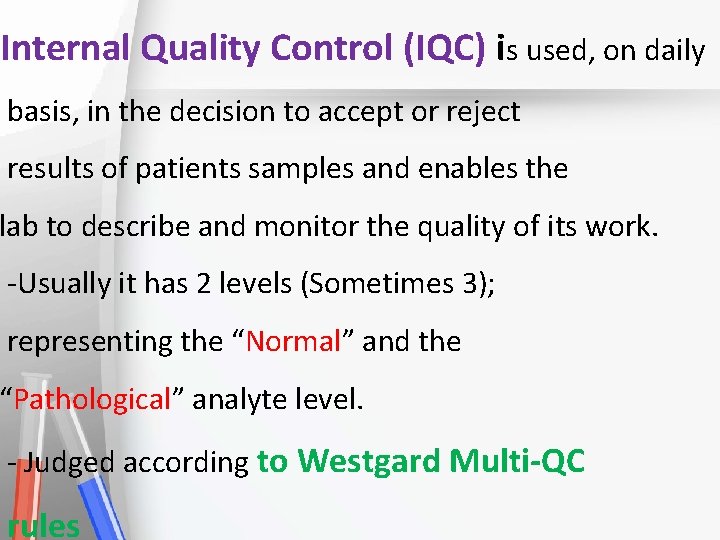
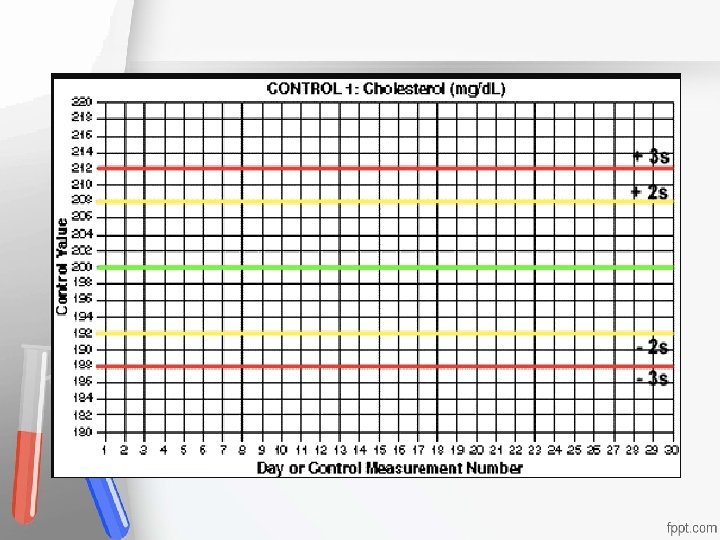
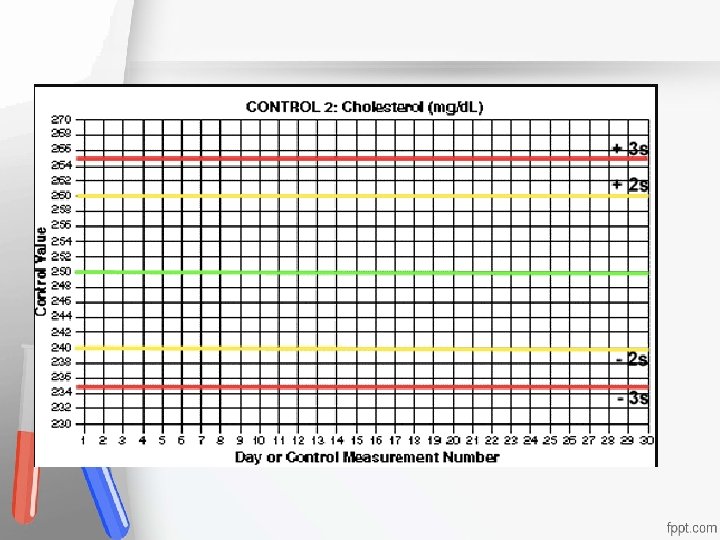
Internal Quality Control (IQC) is used, on daily basis, in the decision to accept or reject Accreditation • results of patients samples and enables the An institution or a program meets • lab to describe standards and monitor the quality of its work. of quality set forth by an • -Usually it has 2 levels (Sometimes 3); accrediting agency • representing the “Normal” and the “Pathological” analyte level. - Judged according to Westgard Multi-QC rules

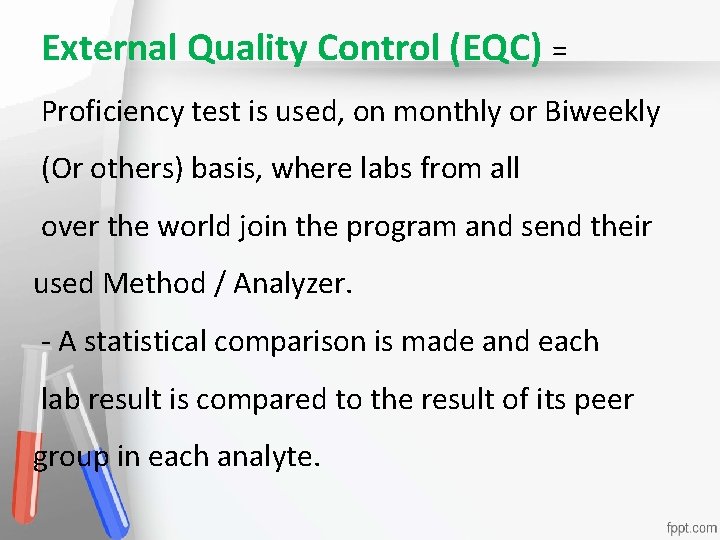
External Quality Control (EQC) = Proficiency test is used, on monthly or Biweekly Accreditation • (Or others) basis, where labs from all An institution or a program meets • over the world join the program and send their standards of quality set forth by an • used Method / Analyzer. accrediting agency • - A statistical comparison is made and each lab result is compared to the result of its peer group in each analyte.

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

The following items need verification Analytical Specificity: Interference studies Accreditation Analytical Sensitivity: Calibration curve An institution or a program meets Detection limit standards of quality set forth by an accrediting agency Reportable range: Linearity experiment Precision: Replication study Accuracy: Bias / Recovery study Reference Intervals • •

Analytical Specificity The ability of an analytical method to detect Accreditation “ONLY” the analyte of interest. or a program meets An institution standards of quality set forth by an accrediting agency Freedom from interference by any element or compound other than the analyte of interest • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

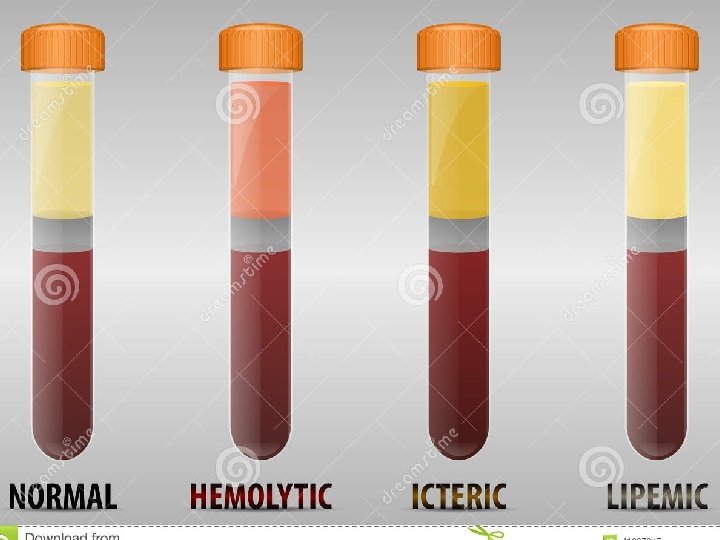
Analytical Specificity - Analytical Specificity is verified using Accreditation interference studies. An institution or a program meets - A validated method, known to be set freeforth of by an standards of quality accrediting the interfering substance is used. A series agency • • of samples containing increased concentrations of the interfering substance are analyzed using that method, and themethod under study, then both results are compared

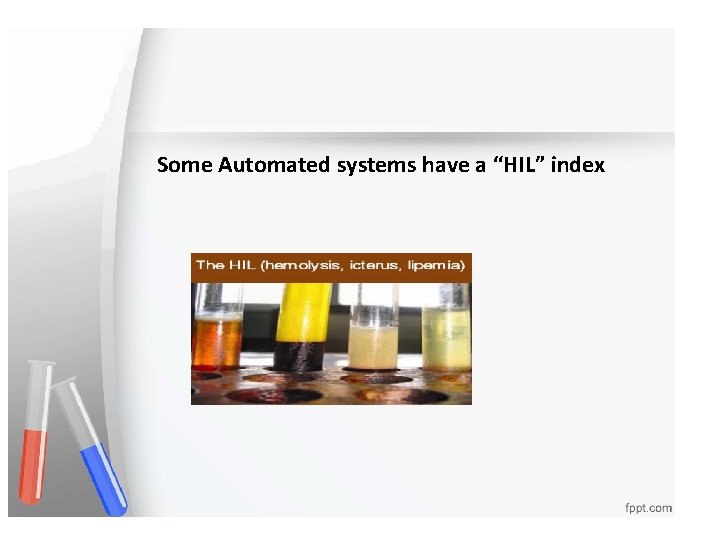
Some Automated systems have a “HIL” index

Analytical Sensitivity - The ability of an analytical method. Accreditation to • An institution a program meets • detect a low concentration of or a given quality. The setlower forth by an • substance in a standards biological of sample. accrediting agency • the detectable concentration, the greater the analytical sensitivity. - Detection limits studies

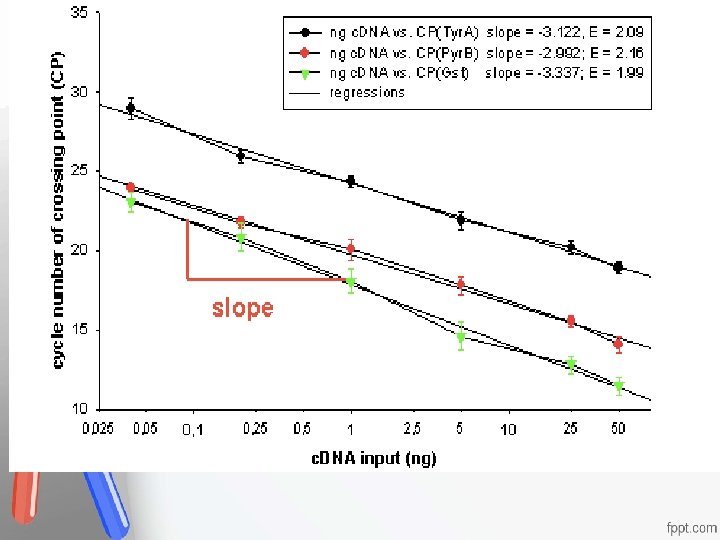
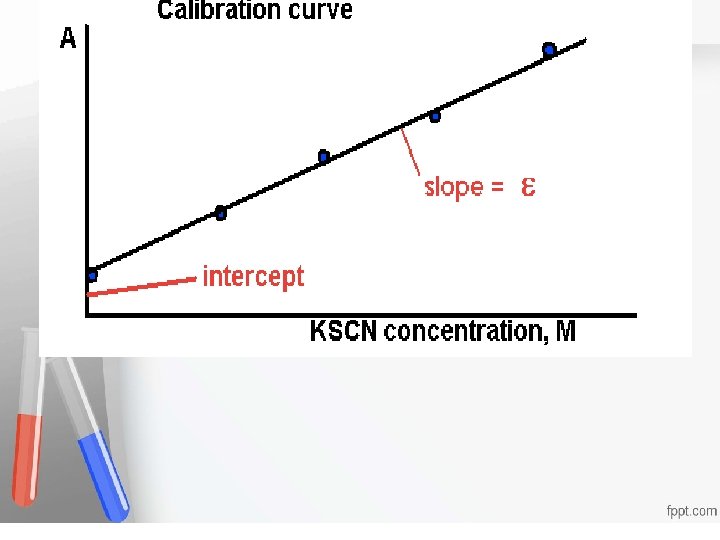
Analytical Sensitivity , -The ability of an analytical method to detect (respond to) a change in Accreditation • An of institution or a. The program meets • concentration the analyte. smaller standards of quality set forth by an • the detectable change (the change in accrediting agency • concentration that can result in a definite change in the reported signal), the greater is the analytical sensitivity. - Slope of the Calibration Curve

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

STANDARD • It is a solution of known concentration. • Formed by dissolving a known amount of Accreditation aqueous solvent. An institution or a program meets of inquality • Ex: Dissolving standards 100 mg glucose 100 ml set forth by an D. W gives a standard of a 100 mg / dl accrediting agency an analyte in a specific volume of an concentration • •

CALIBRATOR • A solution or a device of known. Accreditation • institution or a program meets • quantitative An or qualitative characteristics standards of quality set forth by an • (eg: concentration, activity, intensity) accrediting agency • • Used to calibrate or adjust a measurement procedure. • Matrix is preserved

• The calibrator material is reconstituted & introduced to the analyzer before using a new lot of reagent, and its concentration is Accreditation An isinstitution or a program meets • The calibrator treated like samples and standards of quality the absorbance of the developed colorset is forth by an determined. accrediting agency assigned. • •

If the given concentration is 100 mg / dl, Accreditation the absorbance of this point is determined. An institution or a program meets • By providing the analyzer with the standards of quality set range forth by an agency where this method is linear, accrediting the calibration curve is extended so that any sample concentration lying within that linearity range can be deduced from this curve • •

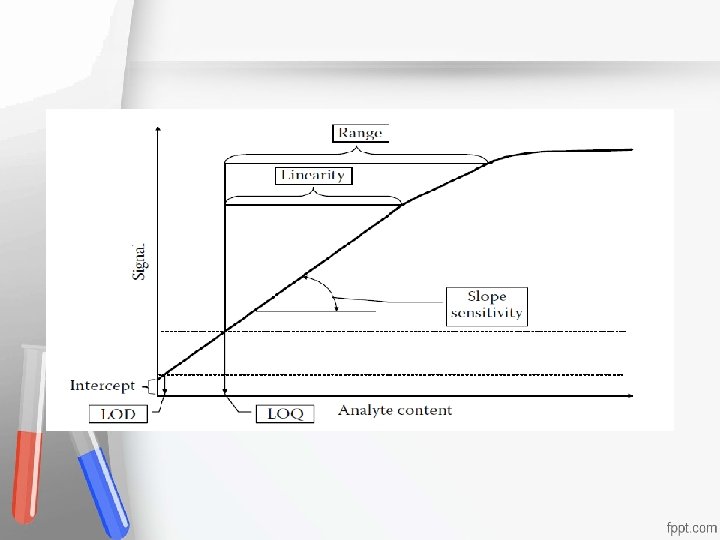
Detection Limits 1. Lower Limit of Detection (LLD)= Limit of the blank (LOB) Accreditation 2. Biologic Limit of Detectionor (BLD)= Limit ofmeets An institution a program Detection (LOD) standards of quality set forth by an 3. Functional Sensitivity (FS)= Limit of accrediting agency Quantification (LOQ) • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

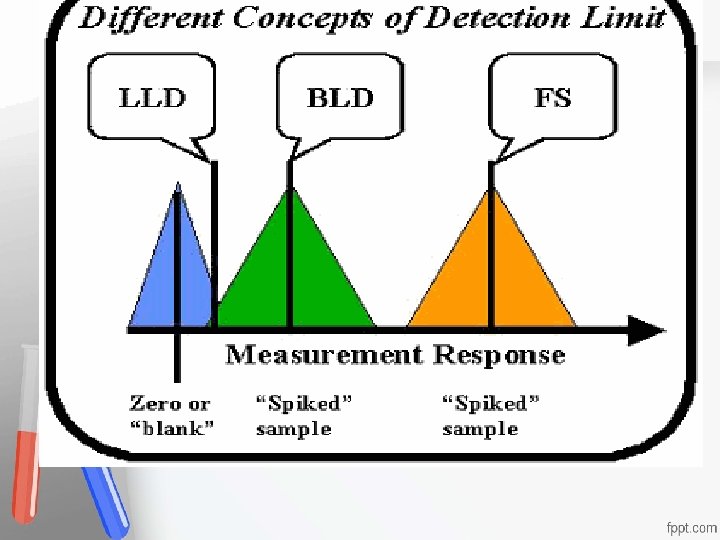
Detection Limits 1. Lower Limit of Detection (LLD)= Limit Accreditation of the blank (LOB) An institution or a program meets Most analytical instruments produce a signal even standards of quality set forth by an when a blank (reagent without analyte) is accrediting agency analyzed. This signal is referred to as the noise • • level. The LLD is the analyte concentration that is required to produce a signal greater than two (or three) times the standard deviation of the noise level

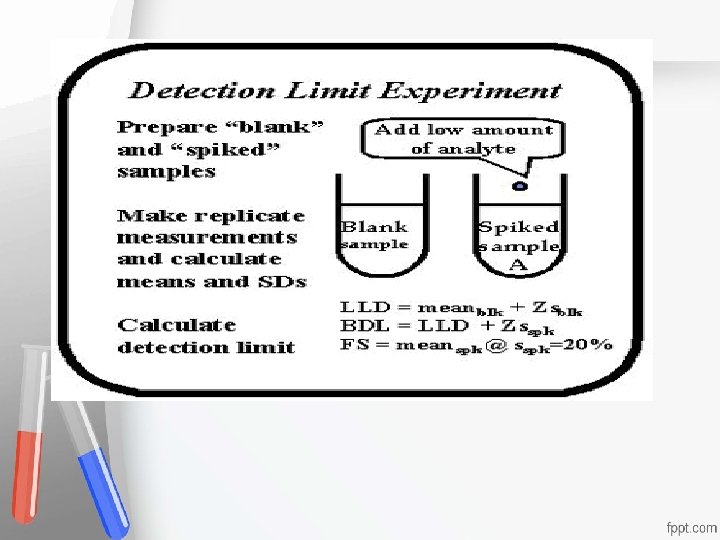
1. Lower Limit of Detection (LLD)= Limit of the blank (LOB) Limit of Detection (LLD) is estimated as, the mean of the blank sample plus 2 or 3 times the SD Accreditation • obtained on the blank sample: An institution or a program meets • LLD = mean(blk) + Zs(blk) standards of quality set forth by an • where the Z-value is usually 2 or 3 accrediting agency •

Detection Limits 2. Biologic Limit of Detection (BLD)= Limit of Detection (LOD) -It is the limit of blank, after the addition of the Accreditation • analyte of interest (Its concentration) Anlowest institution or a program meets • - BLD is estimatedstandards as the LLD plus or 3 timesset theforth by an • of 2 quality standard deviation obtained from the "spiked" accrediting agency • sample BLD = LLD+ Zs(spk) where the Z-value is usually 2 or 3 - Results between BLD & LLD should be reported without quantitation

Detection Limits 3. Functional Sensitivity (FS)= Limit of Quantification (LOQ) -The lowest concentration of target Accreditation compounds • that can be quantified confidently, that meets An institution or a program meets • some pre-specified targets of imprecision, standards of quality set forth by an • commonly accrediting agency • CV=20% - Several spiked concentrations must be studied to determine the precision profile at the low concentration range and to select the lowest concentration at which a 20% CV is obtained

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

To Sum Up! 1. Lo. B is the highest apparent analyte concentration expected to be found when replicates of a blank sample containing no Accreditation • analyte are tested. An institution or a program meets • 2. Lo. D is the lowest analyte concentration likely to standards from of quality set and forthatby an • be reliably distinguished the Lo. B accrediting agency • which detection is feasible. 3. Lo. Q is the lowest concentration at which the analyte can not only be reliably detected, but at which some predefined goals are met. The Lo. Q may be equivalent to the Lo. D or it could be at a much higher concentration

Reportable Range = Analytical Measurement Range It is the range of numeric results. Accreditation a method can produce without any special specimen An institution or a program meets pre-treatment, such as dilution standards of quality set forth by an accrediting agency • •

Accreditation It should be verified, for the manufacturer’s An institution or a program meets claim, using the linearity experiment. standards of quality set forth by an It is performed using either calibrators, accrediting agency proficiency samples, • •

or samples Serial dilutions will be made covering the Accreditation whole analytical measurement range, and An institution or a program meets reaching as close as possible to the claimed standards of quality set forth by an values of the manufacturer. accrediting agency Each dilution is to be processed in duplicate to remove the element of imprecision • •

Accreditation The observed measures (on the X-axis) are An institution or ameasures program meets plotted against the expected (on quality set forth by is an the Y-axis), standards and a lineofpoint to point graph constructed for each analyte. accrediting agency • •

The line is judged visually for its linearity and according to each experiment, the analytical measurement range of each method is verified, where any patient result Accreditation obtained in the future, outside the verified An institution or a program meets range, cannot be released without further standards quality set forth by an processing (Dilution or of concentration accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

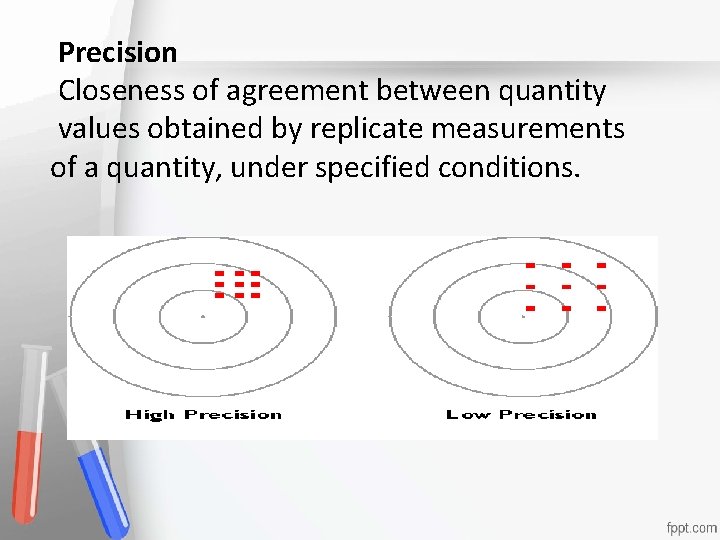
Precision Closeness of agreement between quantity values obtained by replicate measurements Accreditation of a quantity, under specified conditions. An institution or a program meets standards of quality set forth by an accrediting agency • •

Precision should be assessed using quality control material (A minimum. Accreditation of 2 • levels), or pooled (Of minimum two meets • Anserum institution or a program of quality set forth by an • concentration standards levels) accrediting agency • Each level of the QC material / pooled serum is measured 5 times per day (Within run), for 5 days (in between runs

Precision Accreditation • The measures obtained from this An institution or a program meets • precision study are to be collected, standards of quality set forth by an • and the mean, SD, and CV are accrediting agency • calculated for each parameter, for the used QC levels / pooled serum. ﻁ

The obtained CVs, are statistically compared to the manufacturer’s claim using. ANOVA test of significance, to Accreditation determine if there is a significant different • An obtained institution CVs or a program meets • between the (and their standards of quality set forth by an • verification intervals), and the manufacturer’s claim ataccrediting a agency • certain CI; usually 95%

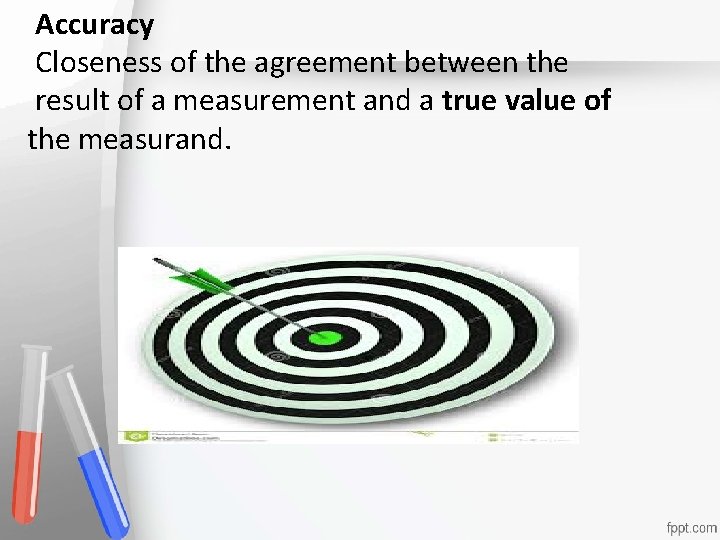
Accuracy Closeness of the agreement between the result of a measurement and a true value of the measurand. Accreditation • An institution or a program meets • standards of quality set forth by an • accrediting agency •

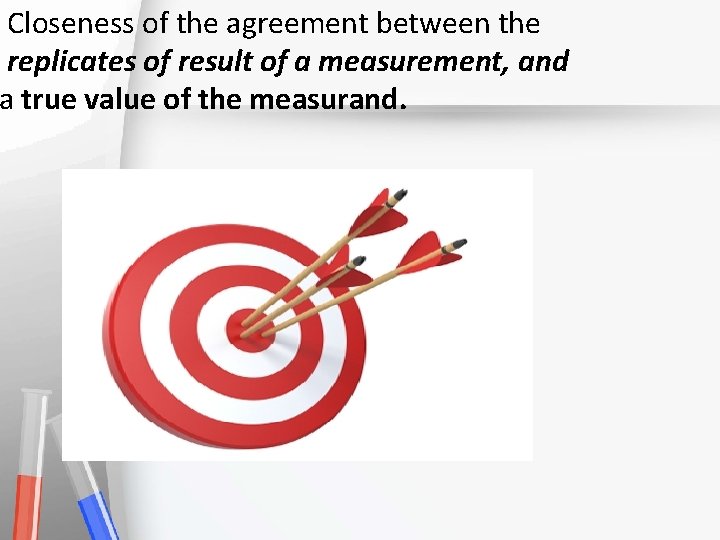
Closeness of the agreement between the replicates of result of a measurement, and a true value of the measurand. Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

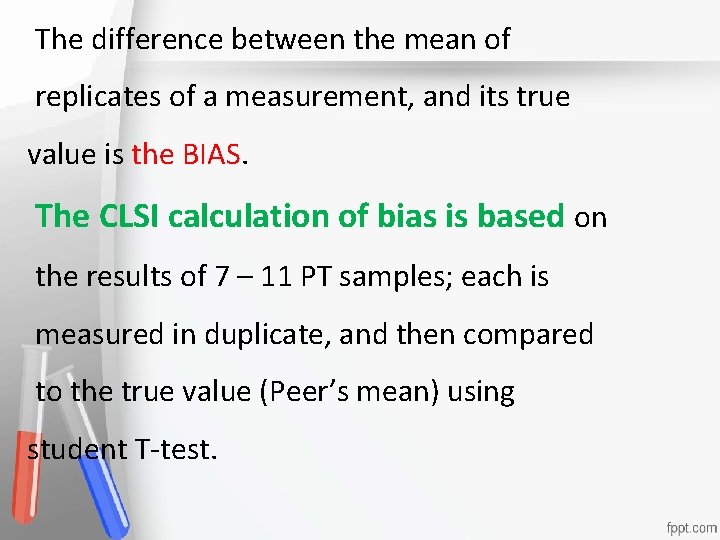
The difference between the mean of replicates of a measurement, and its true Accreditation value is the BIAS. An institution or a program meets The CLSI calculation of bias is based on standards of quality set forth by an the results of 7 – 11 PT samples; each is agency accrediting measured in duplicate, and then compared to the true value (Peer’s mean) using student T-test. • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

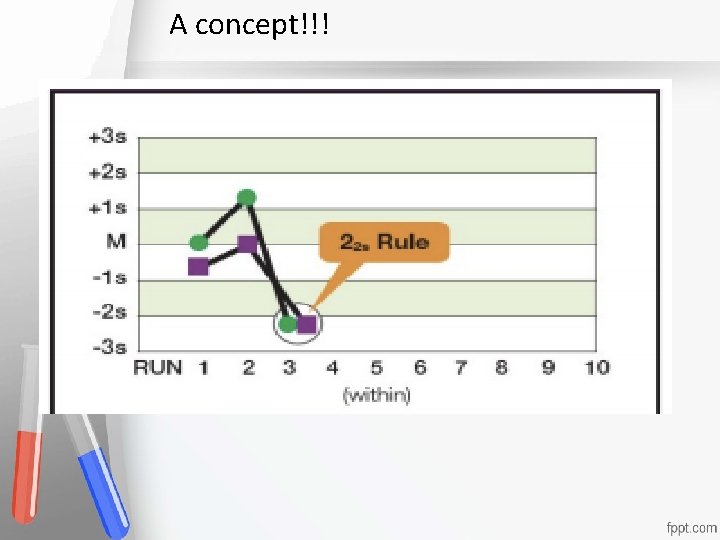
A concept!!! Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

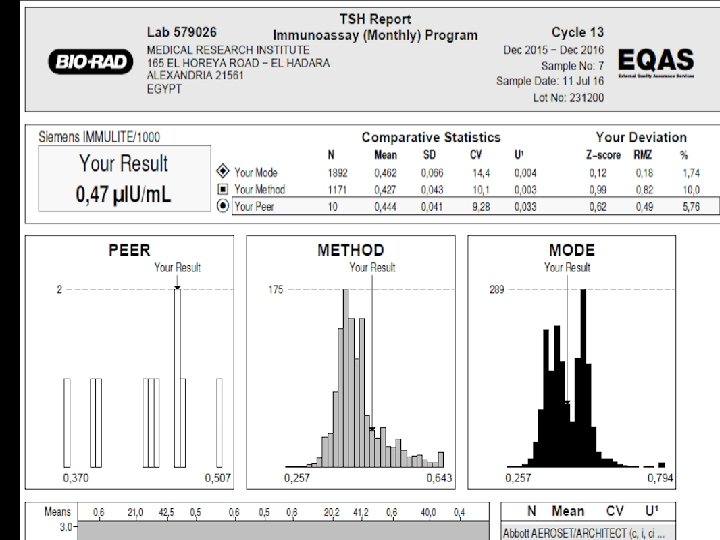
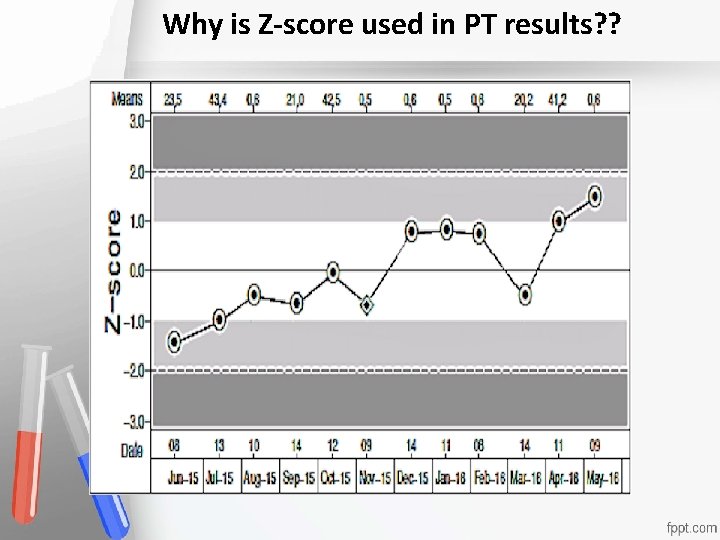
Why is Z-score used in PT results? ? Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Bias is verified for the tested method when there is no significant difference between: • Accreditation An institution or a program meets 1 - Mean Z-score of PT results (7 – 11) is not • standards of quality set forth by an • significantly different from Zero accrediting agency • 2 -Mean Z-score of PT results (7 – 11) is not significantly different from peer’s mean

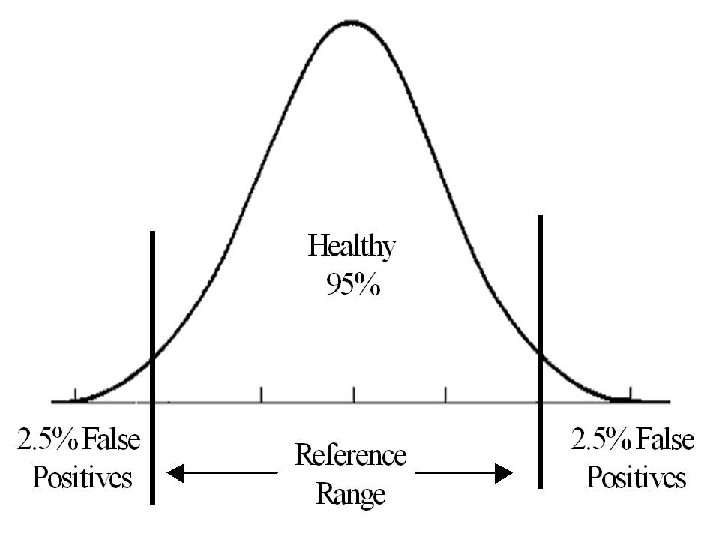
Reference intervals Remember that we are just “Verifying” the Accreditation reference intervals stated by An institution orthe a program meets standards of quality forth by an manufacturer or published in theset literature, accrediting agency and “transferring” them to the lab using the method under study. Establishment” of reference intervals is“ another issue. • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Reference intervals Acceptability of the transfer shall. Accreditation be • assessed by An examining 20 or reference institution a program meets • of quality set forth by an • individuals, standards from our subject population, and accrediting agency • comparing the obtained test results to those of the manufacturer/ Literature. r.

Those 20 individuals should be selected in such a way that will satisfy the exclusion and Accreditation partitioning criteria. An institution or a program meets The test results shouldofbe examined to by an standards quality set forth agency make sure that none of the accrediting results appears to be an outlier • •

Reference intervals The manufacturer's / Literature reference Accreditation • intervals are considered verified if no more An institution or a program meets • than two ofstandards the 20 tested subjects' values of quality set forth by an • accrediting agency • (or 10% of the test results) fall outside those ranges.

Reference intervals Accreditation Exclusion / partitioning criteria include: An institution or a program meets age, sex, fasting status, disease standards of quality sethistory, forth by an accrediting drug history, previous surgeries, andagency time of the cycle / pregnancy for females • •


Method Comparison According to CLSI, at least 40 samples Accreditation should be assayed on both methods under An institution or a program meets examination (two field methods), or between standards of quality set forth by an one tested method and a reference method. accrediting agency Several statistical approaches can be used, one of them is to calculate the correlation coefficient “r” r” should be more than or equal 0. 95“ • •

Total Error It is the summation of both Random and Accreditation Systematic error. An institution or a program meets It is calculated as follow: standards of quality set forth by an TE = Bias (%) + 2 CV accrediting agency It is compared to Biological Variation (or any other specifications) for Total Allowable Error • •

Uncertainty It is an interval around a reported laboratory result that specifies the. Accreditation location of An institution or a program meets the true value with a given probability standards of quality set forth by an It takes into consideration both the accrediting agency imprecision (SD), and the inaccuracy (Bias) It is calculated from the data of 6 month minimum • •

What performance characteristics. Accreditation • An institution or a program meets • are usually validated? standards of quality set forth by an • Reportable range (Linearity) accrediting agency • Precision (or imprecision) Accuracy (or inaccuracy, bias) Reference interval

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

AIDA AHMED •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •

Accreditation An institution or a program meets standards of quality set forth by an accrediting agency • •































