Successful NIH Grant Writing Wafaie Fawzi minahsph harvard
















































- Slides: 53

Successful NIH Grant Writing Wafaie Fawzi mina@hsph. harvard. edu @wafaie_fawzi HBNU Fogarty Global Health Training Program Monthly Mentoring Seminar March 11 th 2020

Today’s Seminar – Part I • Overview of Grants and Grant writing • Searching for Grants • Sections of an NIH Grant • General Grantsmanship Tips

Many thanks for slides sourced from other similar presentations Writing An NIH Research Proposal, by Kelly Edwards, Ph. D, School of Medicine and School of Public Health, University of Washington. Grant Writing: Types of Grants, Identifying Funders, Writing and Selling your Research by Christopher Sudfeld, Sc. D, Harvard T. H. Chan School of Public Health

Sources of Funding • Government(s) • Foundations/philanthropy • Business/private sector • Other

Types of Grants • Research • Career Development/Training • Other (Equipment, etc)

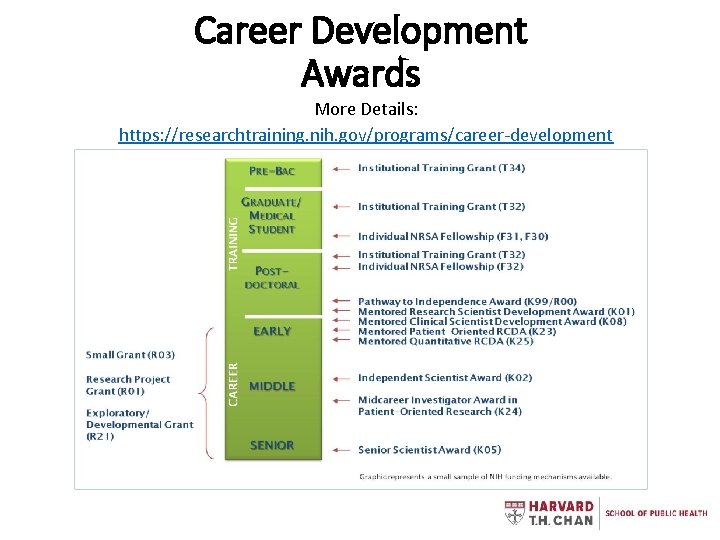
NIH Funding Mechanisms • F = Fellowships (pre- & post-doc) • K = Career Development Awards • T = Training Grants • R = Research Projects • P = Program Project/Center Grants • U = Cooperative Agreements Grants

NIH Research Grants: R 03 • Small grants – Pilot/feasibility studies • Secondary analyses • Development of research methodology or technology • Maximum time = 2 years • Maximum budget = $100, 000 Direct Costs

NIH Research Grants: R 21 • Focus on exploratory/developmental work • Novel/innovative/riskier ideas • Extend previous work in new directions • Maximum time = 2 years • Maximum budget = $275, 000 Direct

NIH Research Grants: R 01 • Research project grant • Broad range of projects • Maximum time = 5 years • Many argue for smaller first project (3 yr) • Maximum budget = $500, 000 / year or more with approval

Career Development Awards More Details: https: //researchtraining. nih. gov/programs/career-development

What does it take to be K-competitive? • Demonstration of commitment to research • At least 1 -2 publications (more is better!) • Evidence of strong mentor-mentee relationship • Clear training plan to show you will develop research skills • Good project

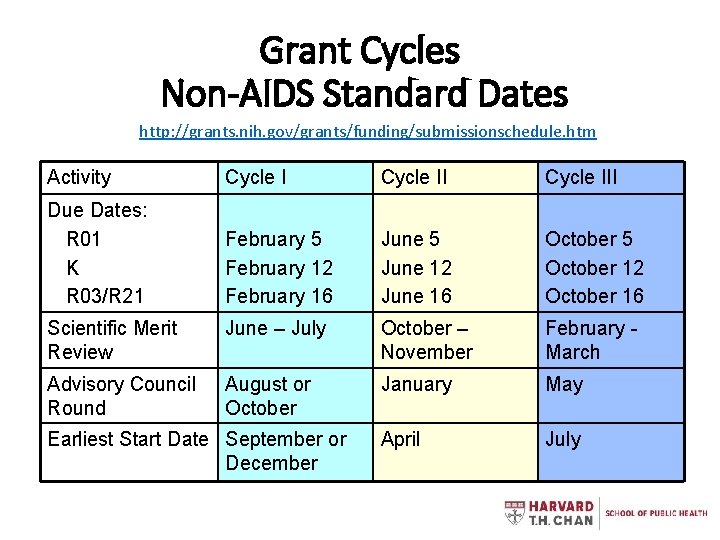
Grant Cycles Non-AIDS Standard Dates http: //grants. nih. gov/grants/funding/submissionschedule. htm Activity Cycle III Due Dates: R 01 K R 03/R 21 February 5 February 12 February 16 June 5 June 12 June 16 October 5 October 12 October 16 Scientific Merit Review June – July October – November February March Advisory Council Round August or October January May April July Earliest Start Date September or December

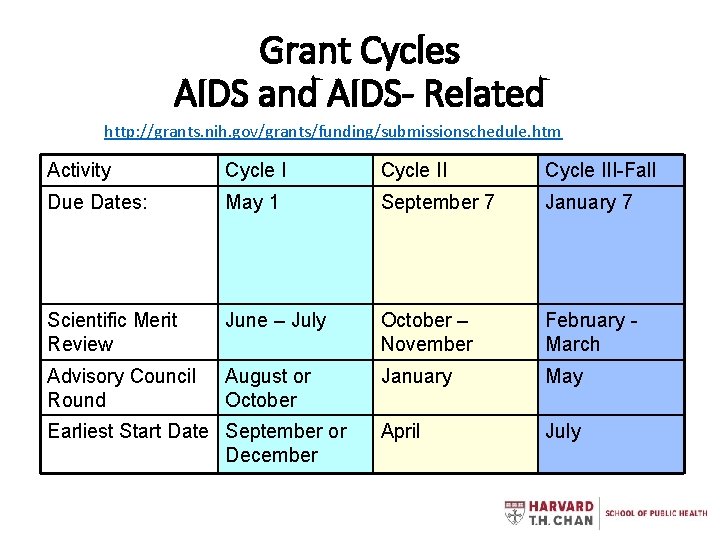
Grant Cycles AIDS and AIDS- Related http: //grants. nih. gov/grants/funding/submissionschedule. htm Activity Cycle III-Fall Due Dates: May 1 September 7 January 7 Scientific Merit Review June – July October – November February March Advisory Council Round August or October January May April July Earliest Start Date September or December

Today’s Seminar – Part I • Overview of Grants and Grant writing • Searching for Grants • Sections of an NIH Grant • General Grantsmanship Tips

Start with a Good Idea! • Does it address an important problem? • Will scientific knowledge be advanced? • Does it build upon or expand current knowledge? • Is it feasible? Who will fund this idea?

Where to search? • University Resources (PIVOT, email announcement) • Online Search Engines (Newton's List, COS, Research, Grants. gov) – Some are pay • Get on mailing lists (Gates, Fogarty Center, etc. ) • Private Foundation Websites • Google Search • Friends, Colleagues, Mentors, Grants Team

Searching All NIH Institutes https: //grants. nih. gov/funding/index. htm

What Grant Type? What Institute? • Step 1: Draft an abstract with Aims (with input from mentors!) • Step 2: Choose an Institute • Read their web pages to learn about THEIR priorities • Decide how your work fits/enhances their research agenda/portfolio • Step 3: Call the Program Officer • Job = advocate for researchers, demystify process • Will help you with “fit” – how your work aligns with Institute mission

27 Institutes/Centers + Director’s Office NCI Cancer NIAMS Arthritis & NIEHS Musculoskeletal/ Skin Environmental Health NCCAM Complementary & Alternative Medicine NEI Eye NIBIB Biomed Imaging & Bioeng. NIGMS General Medical Sciences NCATS Advancing Translational Science NHLBI Heart, Lung, Blood NICHD Child Health & Development NIMH Mental Health CIT Information Technology NHGRI Genome NIDCD Deafness & Comm Disorders NIMHD Minority Health/Disparities CSR Scientific Review NIA Aging NIDCR Dental & Craniofacial Research NINDS Neuro & Stroke FIC Fogarty Int’l Center NIAAA Alcohol NIDDK Diabetes, Digestive & Kidney NINR Nursing Research CC Clinical Center NIAID Allergy/ Infectious Disease NIDA Drug Abuse NLM Library of Medicine OD Office of the Director

Gates Foundation Grand Challenges

Today’s Seminar – Part I • Overview of Grants and Grant writing • Searching for Grants • Sections of an NIH Grant • General Grantsmanship Tips

Grant Applications Each funder has their own template for a proposal and process of submission Some will require 1 -page concept notes or full page proposals with multiple parts RULE #1: Read the application instructions carefully!

Components of an NIH grant Specific Aims- 1 page Research Strategy- R 03/R 21 = 6 pages R 01 = 12 pages • • Background & Significance Innovation Preliminary studies Approach • • • Design Endpoints Data collection Statistical analysis plan Ethical considerations Timeline

Other Components • • Project Summary & Project Narrative Biosketches of Key Personnel Protection of Human Subjects Budget & Budget Justification Resources & Environment Foreign Justification Letters of Support Resource Sharing Plan

Specific Aims (1 page) • • Provides an overview of the details - tells what your proposal is about, and how you will get there Usually starts with 1 - 2 paragraph general overview, then list of specific aims, often ends with a brief statement of importance or what you will learn if successful Keys to success • Important Section! The reader must be convinced that the proposed research is significant and that you have a feasible approach • SMART Objectives (Specific, Measurable, Achievable, Relevant, Time) • Grab the reader attention immediately • Typically 2 - 4 specific aims. Later aims should NOT depend on the success of previous aims

Research Strategy • • • Significance Innovation Approach • • Preliminary studies Design Endpoints Data collection Statistical analysis plan Ethical considerations Timeline

Significance • • • Clearly present the state of knowledge in the area and importance of the proposed research Important to reference all key publications and experiment findings Should be appropriately referenced with an honest and balanced discussion Keys to success • Assume the reviewer is not an expert in this area • Reviewers may not put large focus on this ‘background’ section; however a proposal without a strong significance will generate little enthusiasm.

NIH Reviewers asked to think about…. • • • Does the project address an important problem or a critical barrier to progress in the field? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, or services that drive this field?

Innovation • 1 -2 Paragraphs where succinctly present why your study is exciting, new and important • Often helpful to present multiple aspects of innovation (new intervention, new study population, new statistical methods, new biomarkers…) Keys to success Very Important Section – If the grant does not push science forward it is not likely to be funded If you are not enthusiastic about the innovation… it is unlikely the reviewers will see it different Do not over-hype (or under-hype) your study but present in positive light

NIH Reviewers asked to think about…. • • Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense?

Preliminary Studies • • ~½ to 1 page on your teams previous work has led to the proposed study Demonstrates that you can do what you say you are going to do Keys to success -Use this section to generate excitement for the proposed studies and how you have built your research to this point -Consider figures but be sure they have clear legends and should be large enough for reviewers to easily read

Approach • Approach must directly link to your Specific Aims • Bulk of your Research Strategy where you present how you will complete your primary aims from screening patients to data collection to statistical analysis and interpretation • Need to present how and why you are conducting each of your study procedures and why measurement tools are used

Approach Clinical Studies Should have all the information included in a standard protocol Intervention or system to be studied Target population Inclusion and exclusion criteria Follow-up Schedule Standard of Care Protection Human Subjects All Measures and Instruments Power Calculations Statistical Analysis of All Primary and Secondary Outcomes

NIH Reviewers asked to think about…. • Are the overall strategy, methodology, and analyses wellreasoned and appropriate? • Are potential problems, alternative strategies, and benchmarks for success presented? • If the project is in the early stages of development, will the strategy establish feasibility and how will particularly risky aspects be managed? • If the project involves clinical research, are the plans for protection of human subjects, and inclusion of minorities and members of both sexes/genders, and the inclusion of children, justified in terms of the scientific goals and research strategy proposed?

Today’s Seminar – Part I • Overview of Grants and Grant writing • Searching for Grants • Sections of an NIH Grant • General Grantsmanship Tips

How are NIH applications scored? • Peer Review! Mostly Senior investigators (few Asst. Profs) • 2 reviewers assigned to review in detail; others often only read abstract and aims page • Each assigned reviewer is required to score each of 5 core review criteria • Applications are ranked by the overall score – only the Top 50% are usually discussed for funding

Peer Review 5 Core NIH Review Criteria 1) Significance – Addresses an important problem or critical barrier to progress 2) Investigators – Qualifications of the team 3) Innovation – Novel concepts or approach 4) Approach – Feasibility/strengths/match of strategy to project specific aims. Adequate human subjects protections 5) Environment – Institutional support/resources

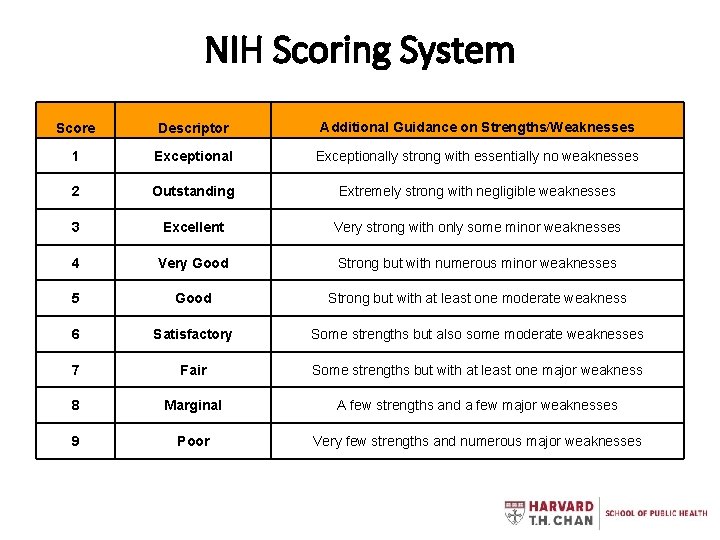
NIH Scoring System Score Descriptor Additional Guidance on Strengths/Weaknesses 1 Exceptionally strong with essentially no weaknesses 2 Outstanding Extremely strong with negligible weaknesses 3 Excellent Very strong with only some minor weaknesses 4 Very Good Strong but with numerous minor weaknesses 5 Good Strong but with at least one moderate weakness 6 Satisfactory Some strengths but also some moderate weaknesses 7 Fair Some strengths but with at least one major weakness 8 Marginal A few strengths and a few major weaknesses 9 Poor Very few strengths and numerous major weaknesses

Today’s Seminar – Part I • Overview of Grants and Grant writing • Searching for Grants • Sections of an NIH Grant • General Grantsmanship Tips

Plan your Time! It may take twice as long as you think call NIH

NIH Grant Writing Tips https: //grants. nih. gov/grants/grant_tips. htm

Writing Tips: Getting ready n. Plan ahead • 6 months pilot work + research question • 6 months writing the grant • Involve mentor / co-investigators with warning n. Write and revise a 1 -2 pg concept paper • Share ahead of every meeting • Revise between meetings • This will become Specific Aims section. . .

Writing Tips: Tricks of the trade • Read successful grants • Sit in on mock reviews • No typos, each page a thing of beauty. . . • K award – write Career Plan in parallel with Research Plan • Involve mentor, co-investigator, biostatistician early (6 -12 months) Inouye SK, Ann Intern Med 2005

Writing Tips • Tell a story. . . • Build your argument • Help reviewers care • Punctuate key points • Write the Aims first…. and Last. • You are writing a prose poem - use subheads/bold key sentences that structure the argument. • Use a conceptual framework and model • Diagram cause-effect or temporal relations • Make the link between aims and products clear

New Investigators: You are the next generation! • NIH website for new investigators: http: //grants. nih. gov/grants/new_investigators/index. htm • New Investigator: has not previously competed successfully as PD/PI for a substantial NIH independent research award. • New and Early Stage Investigator Policies Under this policy, New Investigators within 10 years of completing their terminal research degree or medical residency will be designated Early Stage Investigators (ESIs). Traditional NIH research grant (R 01 s) applications from ESIs will be identified and the career stage of the applicant will be considered at the time of review and award.

Tips for the Junior Investigator • Find a MENTOR • Interdisciplinary collaboration is a MUST! • Know the experts in the “niche area” you are investigating • Begin to develop these relationships today • Share emails, phone numbers, plan a phone conference • Make sure you are getting Funding Opportunity Announcements (FOA) & Program Announcements (PA) • Seek and build a Research Team early in your career • Don’t underestimate the need for a GREAT statistician! • NEVER write a grant alone – you will burn out early on!

Before you submit the grant • Schedule a peer review (internal) • Include persons who sit on study sections • Do early in the process (eg. If June submission – review in early May) • Determine how to include the feedback • External review- experts in the proposed field of inquiry

What makes a Successful Grant Application? • Addresses and ‘important’ problem • High degree of novelty and innovation • Strong track record by a well-qualified team • Clear rationale with supportive preliminary data • Clear and focused approach • Careful attention to details- Spelling, punctuation, grammar, fonts, clarity of figures and tables, etc. • ALL required sections complete

Common Reasons Cited for a Weak Application • Lack of knowledge of published work • Significance not obvious or weak • Lacking focus • Unclear rationale • Lacking appropriate expertise • Approach flawed • Too ambitious • Hard to read - poorly constructed, dense, or filled with typographical/ grammatical errors • Missing Sections • Not a priority of the Funder

Understand Dynamic of Peer Review • Peer reviewers will review many applications. Assume they are overworked, tired, irritable and will not read every word of your application Therefore… • Have a very strong and well-written specific aims • Make your application easy to read and easy to understand • No odd margins, spellings, highlighting, grammar mistakes • The impact and significance should be clear throughout the application • Convince reviewer to be your advocate at the study group or council meeting

Don’t Give Up!!! • Initial failure is very, very common • You can submit one amended application to NIH • Learn from a failed submission • • Discuss with team to decide if problems are repairable Attend diligently to each criticism Keep a positive tone and attitude Revise and submit somewhere else

5 Take-home Tips 1) Start early! 2) Good ideas are foundation to success 3) Seek advice from Mentors and Colleagues 4) Follow grant instructions carefully 5) Don’t give up, Recycle!

Other Components…Next Month • • Project Summary & Project Narrative Biosketches of Key Personnel Protection of Human Subjects Budget & Budget Justification Resources & Environment Foreign Justification Letters of Support Resource Sharing Plan
 Grant writing examples
Grant writing examples Grant writing tutorial
Grant writing tutorial Grant writing usa
Grant writing usa How to reference a case study in harvard style
How to reference a case study in harvard style Harvard academic writing
Harvard academic writing Nih administrative supplement example
Nih administrative supplement example Theresa cruz email
Theresa cruz email Nih transhare
Nih transhare Nih staff scientist
Nih staff scientist Nih all personnel report
Nih all personnel report Jonathan pollock nida
Jonathan pollock nida Building 37 nih
Building 37 nih List of nih institutes
List of nih institutes Dawn corbett nih
Dawn corbett nih Http://ghr.nlm.nih.gov/
Http://ghr.nlm.nih.gov/ Http://ghr.nlm.nih.gov/
Http://ghr.nlm.nih.gov/ Chris baker nih
Chris baker nih Alison lin nih
Alison lin nih Best language nih
Best language nih Nih score
Nih score Qvr nih
Qvr nih Nih cit org chart
Nih cit org chart Web single signon
Web single signon Nih background
Nih background Nih hssip
Nih hssip Diane frasier nih
Diane frasier nih Nih enterprise directory
Nih enterprise directory Expenditure organization
Expenditure organization Nih
Nih Nibib.nih.gov computational
Nibib.nih.gov computational Strides nih
Strides nih K kiosk nih
K kiosk nih Early stage investigator nih
Early stage investigator nih Nist
Nist Nih cit bpa
Nih cit bpa Ncbi blast 2
Ncbi blast 2 Nlm.nih.gov
Nlm.nih.gov Nih assisted referral tool
Nih assisted referral tool Tony beck nih
Tony beck nih Kathryn harris nih
Kathryn harris nih @nengwidiw:nih https://tinyurl.com/mdiafre
@nengwidiw:nih https://tinyurl.com/mdiafre Nih payback agreement t32
Nih payback agreement t32 Nih era comons
Nih era comons Sharon milgram nih
Sharon milgram nih Nih critique template
Nih critique template Guy leschziner
Guy leschziner Michael gottesman nih
Michael gottesman nih Nih supply center
Nih supply center Ghr.nlm.nih.gov
Ghr.nlm.nih.gov Nih
Nih Taxanomical
Taxanomical Nidap nih
Nidap nih Nih oamp
Nih oamp Med pub
Med pub