SNC 1 D Chemistry Particle Theory and Types
































- Slides: 55

SNC 1 D Chemistry

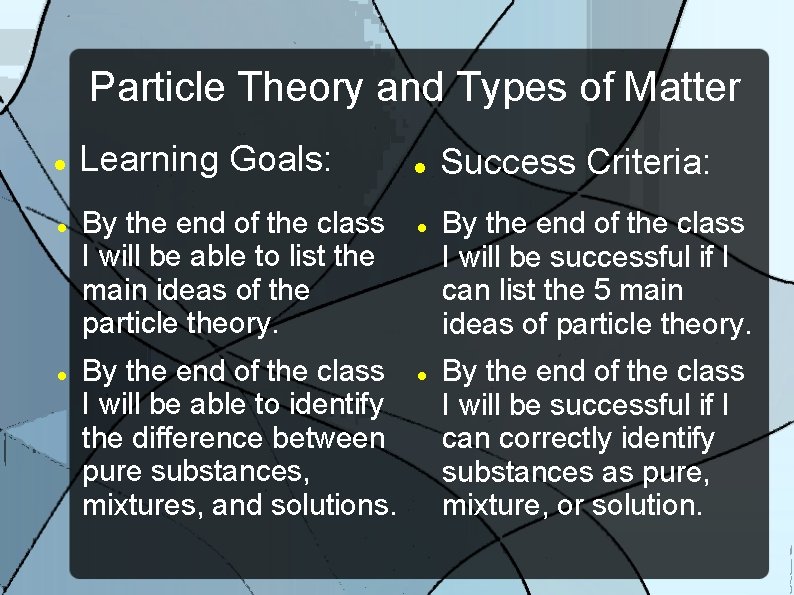
Particle Theory and Types of Matter Learning Goals: By the end of the class I will be able to list the main ideas of the particle theory. By the end of the class I will be able to identify the difference between pure substances, mixtures, and solutions. Success Criteria: By the end of the class I will be successful if I can list the 5 main ideas of particle theory. By the end of the class I will be successful if I can correctly identify substances as pure, mixture, or solution.

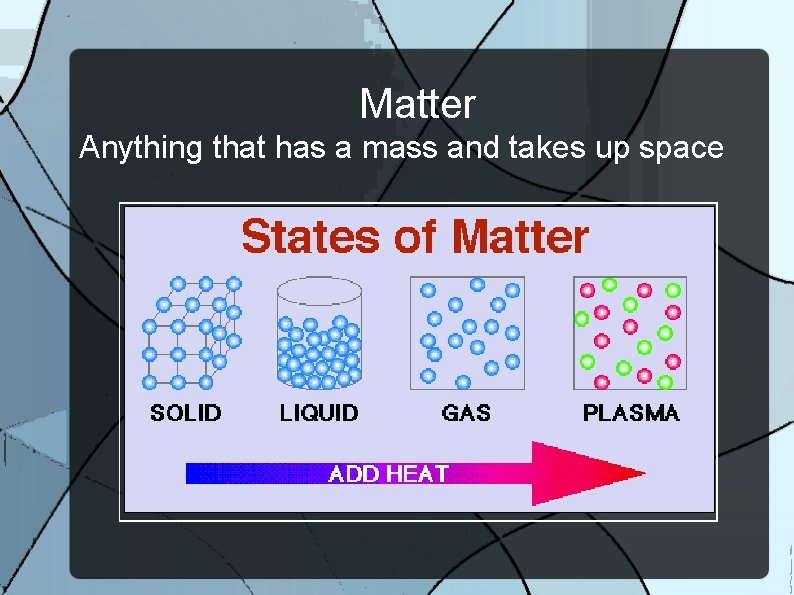
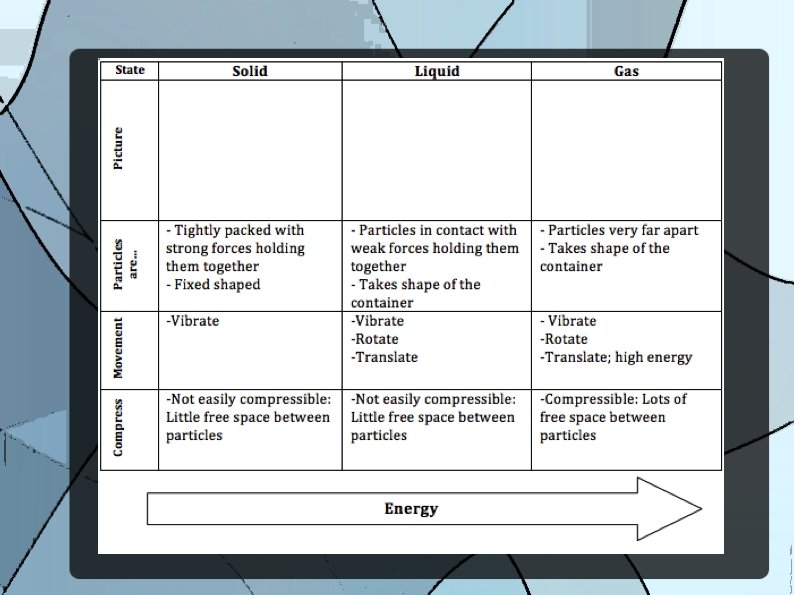
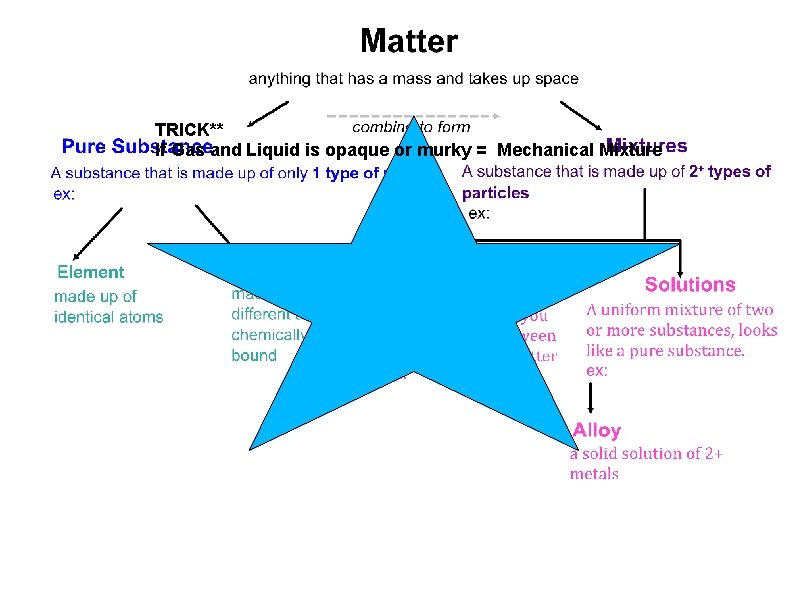
Matter Anything that has a mass and takes up space

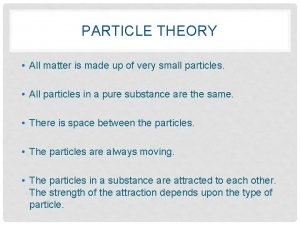
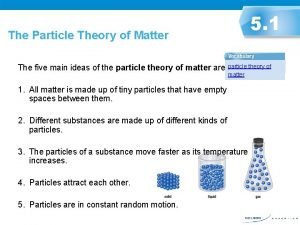
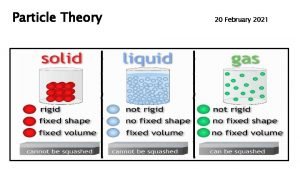
Particle Theory: a theory that describes the composition and behaviour of matter. There are 5 main ideas of the particle theory.

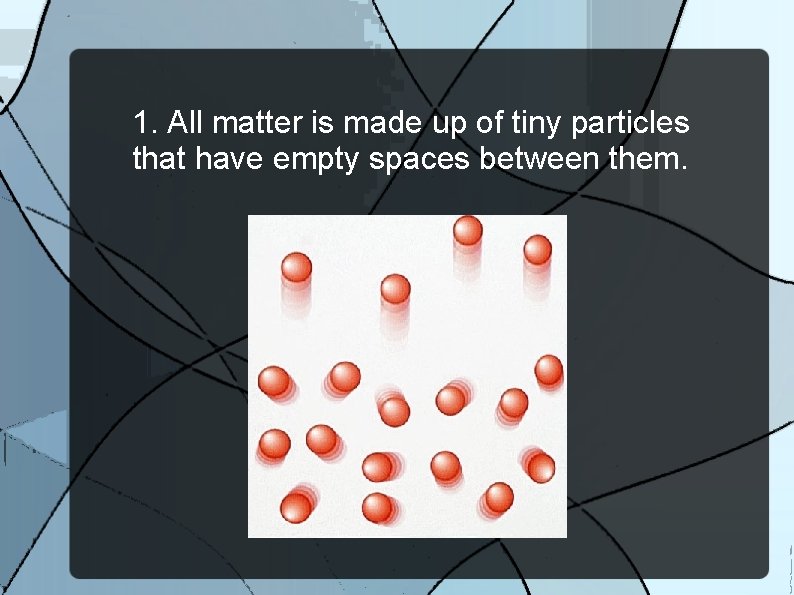
1. All matter is made up of tiny particles that have empty spaces between them.

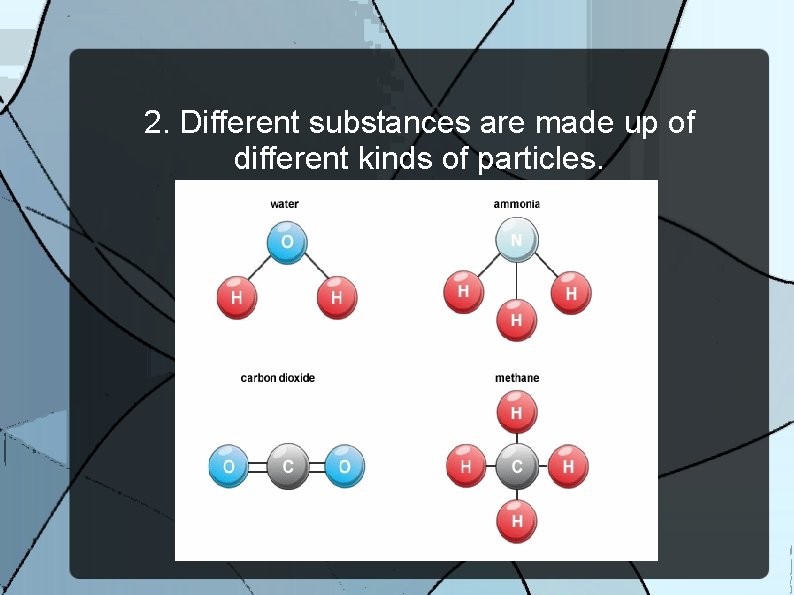
2. Different substances are made up of different kinds of particles.

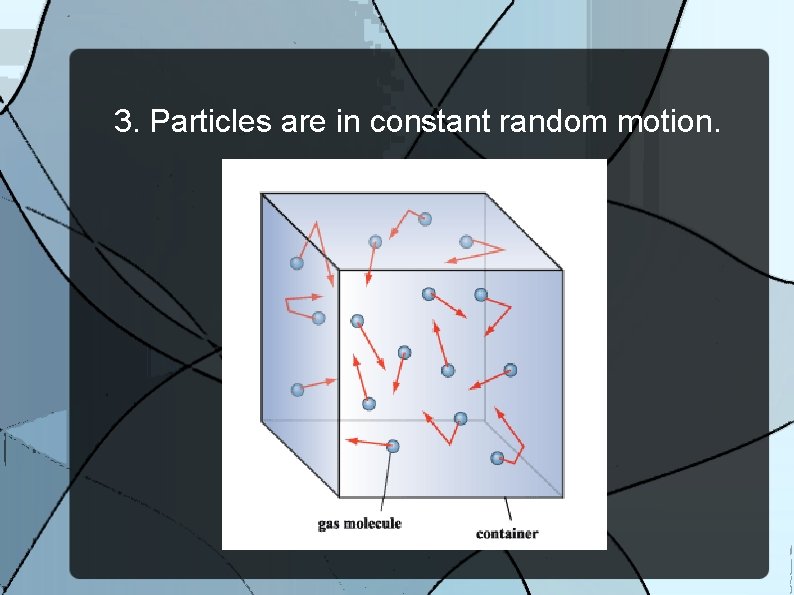
3. Particles are in constant random motion.

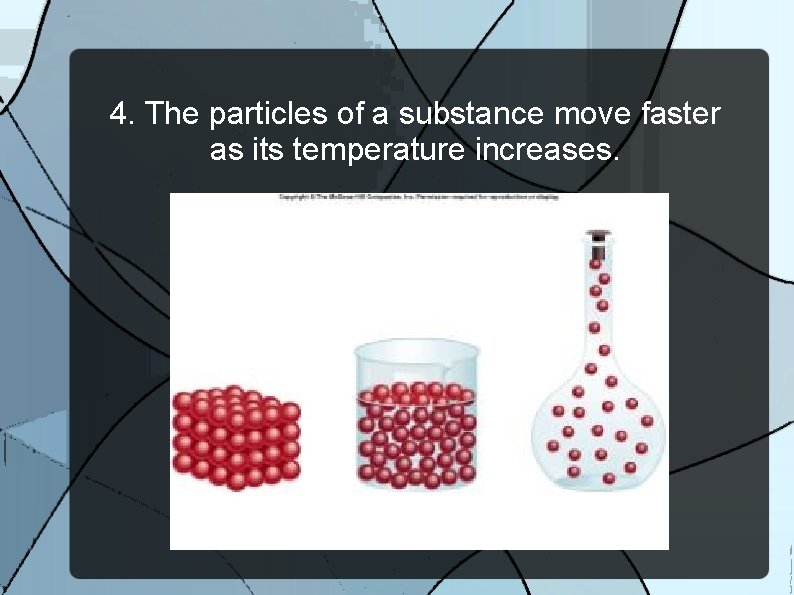
4. The particles of a substance move faster as its temperature increases.

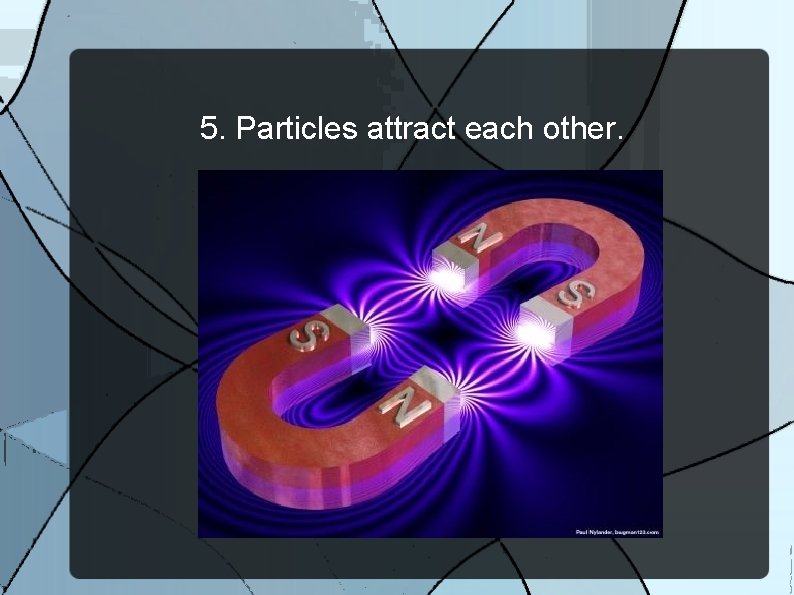
5. Particles attract each other.

Particle Theory Video

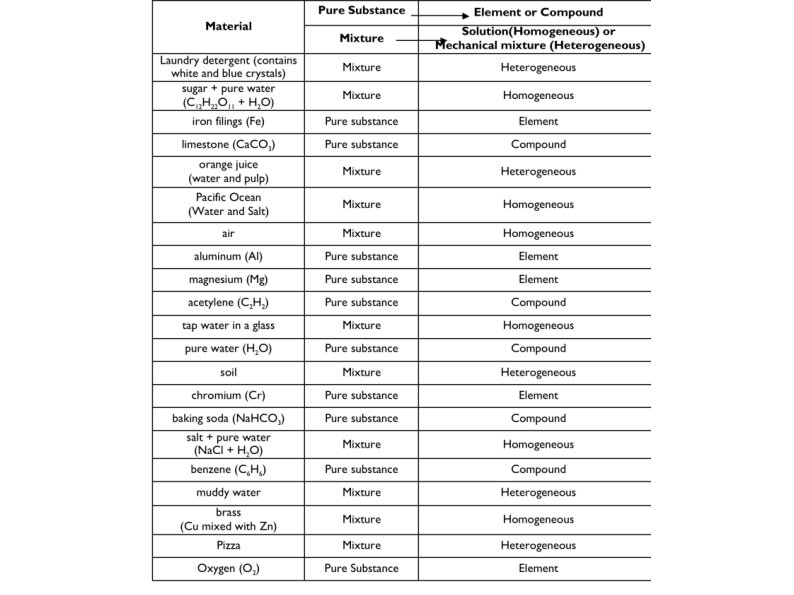
Types of Matter Pure Substances and Mixtures

Pure Substance: a substance that is made up of only one type of particle.

Mixture: a substance that is made up of at least two different types of particles.

Mechanical mixture: a mixture in which you can distinguish between different types of matter.

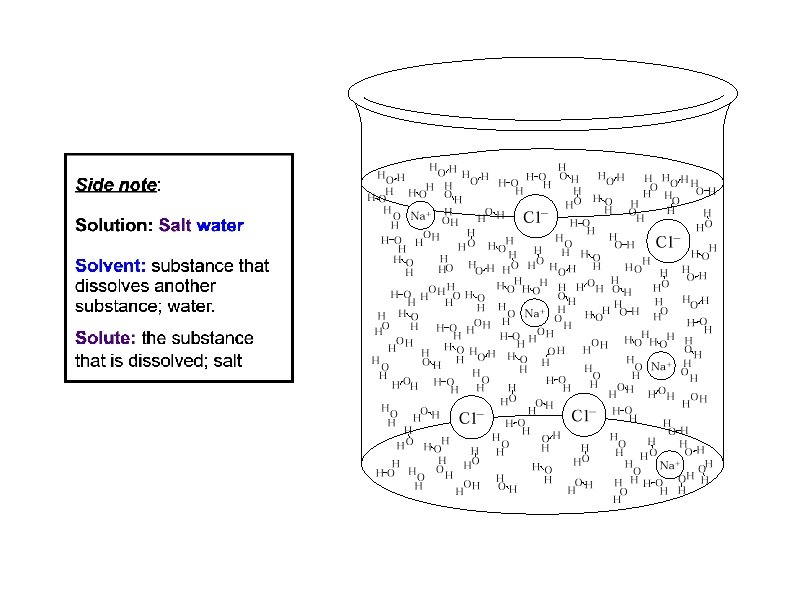
Solution: a uniform mixture of two or more substances.

Alloy: a solid solution of two or more metals.

TRICK** If Gas and Liquid is opaque or murky = Mechanical Mixture




Physical Properties

Physical Properties: A characteristic that can be determined without changing the composition of the substance.

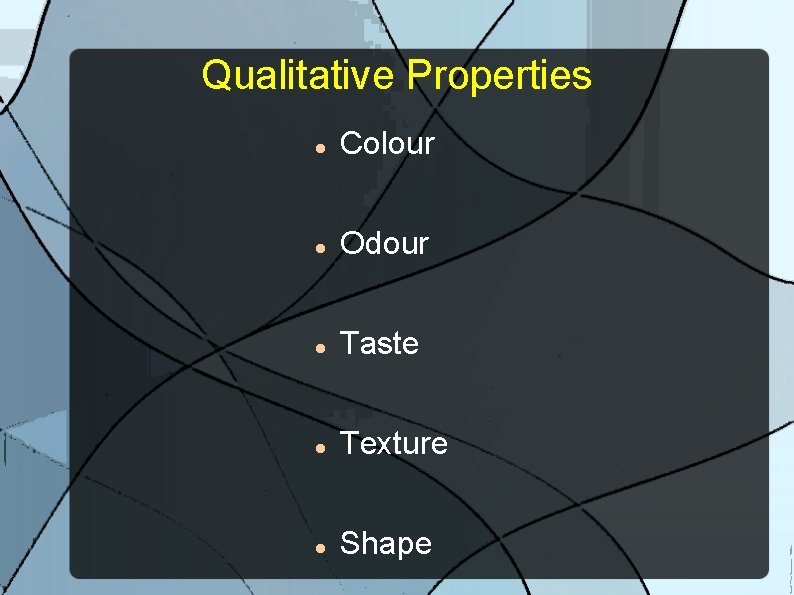
Qualitative Properties of a substance that is not measured and does not have a numerical value.

Qualitative Properties Colour Odour Taste Texture Shape

Qualitative Properties

Quantitative Properties of a substance that is measured and has a numerical value.

Quantitative Properties Lustre Hardness Optical Clarity Malleability Viscosity Ductility Brittleness Electrical Conductivity

Quantitative Properties

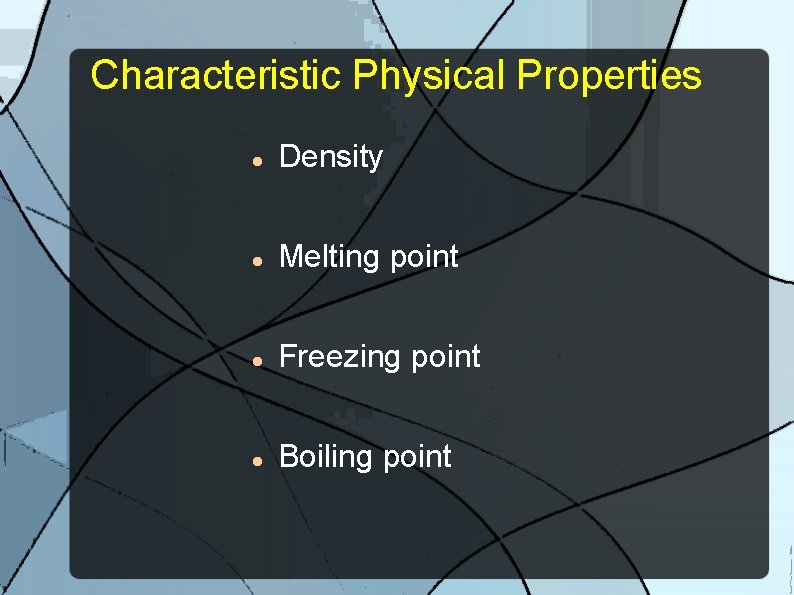
Characteristic Physical Properties A physical property that is unique to a substance and can be used identify the substance.

Characteristic Physical Properties Density Melting point Freezing point Boiling point

Characteristic Physical Properties

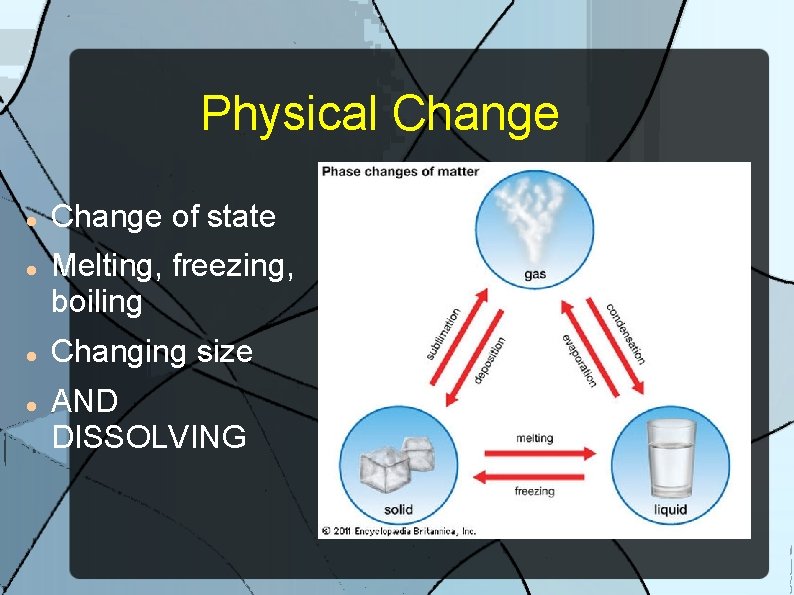
Physical Change A change in which the composition of the substance remains unaltered and no new substances are produced.

Physical Change of state Melting, freezing, boiling Changing size AND DISSOLVING

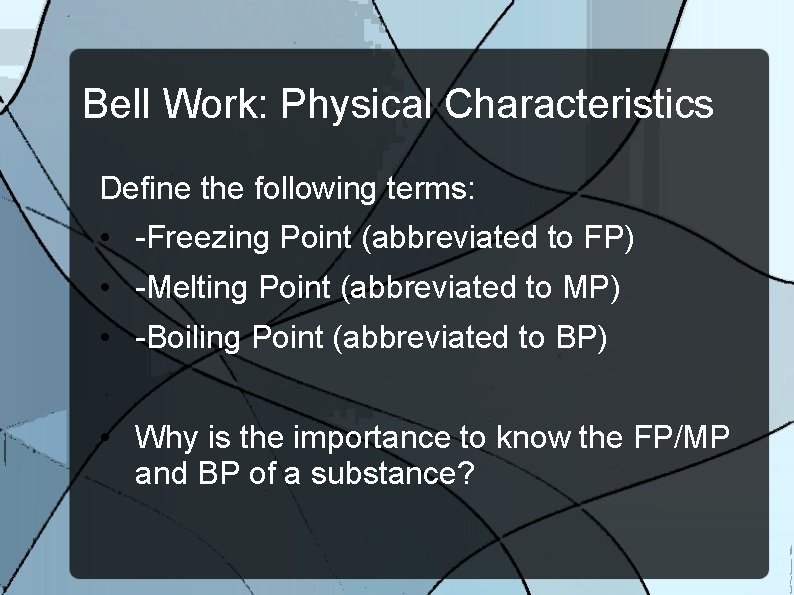
Bell Work: Physical Characteristics Define the following terms: • -Freezing Point (abbreviated to FP) • -Melting Point (abbreviated to MP) • -Boiling Point (abbreviated to BP) • Why is the importance to know the FP/MP and BP of a substance?

Test Tuesday September 29

Density For Full Communication Marks… Sample Problem: Calculate the density of a metal sample that is 18. 00 cm long, 9. 21 cm wide and 4. 45 cm high and that has a mass of 14. 25 kg. What is the identity of the metal? Give: l= 18. 00 cm h= 4. 45 cm w= 9. 21 cm m= 14. 25 kg Required: density of metal (d) Solution: V = l*w*h = 18. 00 cm * 9. 21 cm * 4. 45 cm = 738 cm 3 m = 14. 25 kg =14250 g d = m/v = 14250 g/738 cm 3 = 19. 3 g/cm 3 Statement: The density of the metal is 19. 3 g/cm 3. This metal is gold.

Chemical Properties & Changes

Chemical Properties: A characteristic of a substance that is determined when the composition of the substance is changed and one or more new substances is created

Fireworks Fireworks contain ingredients such as metal flakes, fuel and a bursting charge These substances react together to produce new substances, some of which are visible in the smoke The entire reaction produces a great deal of energy; which appears in the form of light, sound, thermal energy and high-speed motion high into the sky

Advantages of Chemical Properties In our daily lives we mix different substances together to create products that we want Examples: Baking soda causes a cake to rise Bacterial cultures turn milk into cheese Chemicals clean our jewellery

Chemical Changes A change in the starting substance and the production of one or more new substances

What do you think are examples of chemical changes?

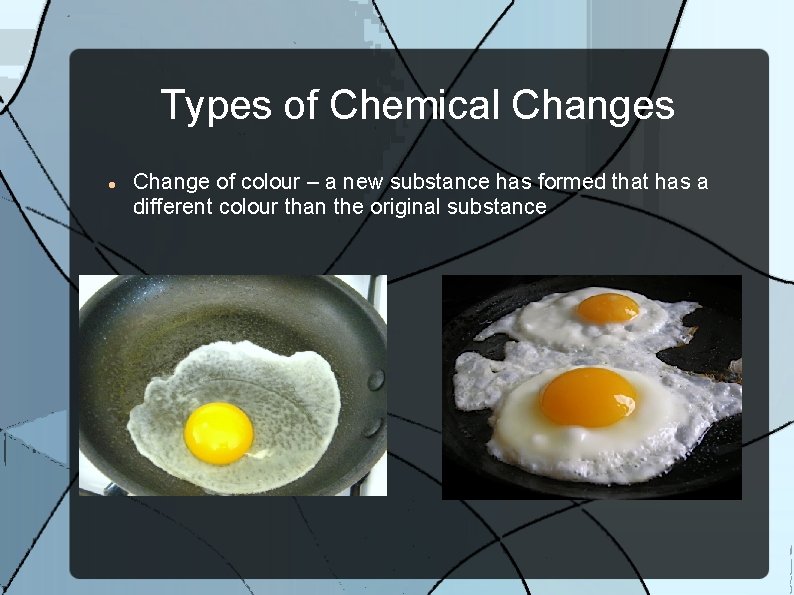
Types of Chemical Changes Change of colour – a new substance has formed that has a different colour than the original substance

A change of odour – a new substance has formed that has a detectable odour

Bubbles are visible that are not caused by heating – a new substance is produced in the form of a gas

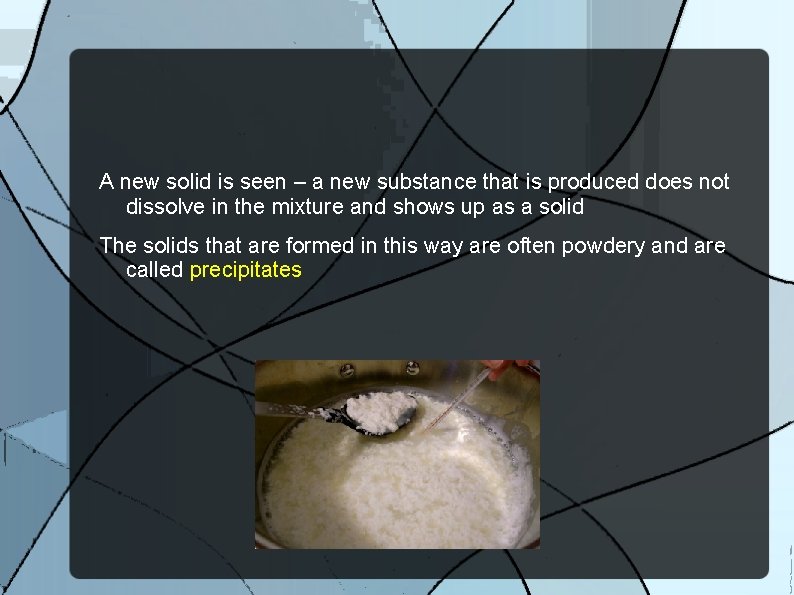
A new solid is seen – a new substance that is produced does not dissolve in the mixture and shows up as a solid The solids that are formed in this way are often powdery and are called precipitates

A change in temperature or light – energy is released or absorbed during the chemical change, and is detected as a change in temperature or light

Endothermic Vs. Exothermic Rxh Exothermic: exo~ “exit” thermic ~“hot” - - Heat Releasing Endothermic: endo~ “within” thermic ~“hot” • - Heat Absorbing

Demonstration Before: - Describe the physical properties of the materials. Separate each property in a chart as either qualitative or quantitative. After: • Describe the physical properties after the change. • Is the a physical or chemical change? • Is this an example of an endothermic or exothermic reaction

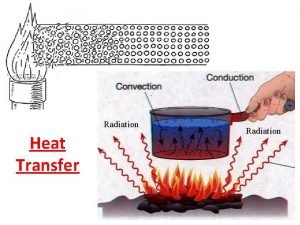
Changing States -Melting ice: endothermic or exothermic? -Freezing ice: endothermic or exothermic?

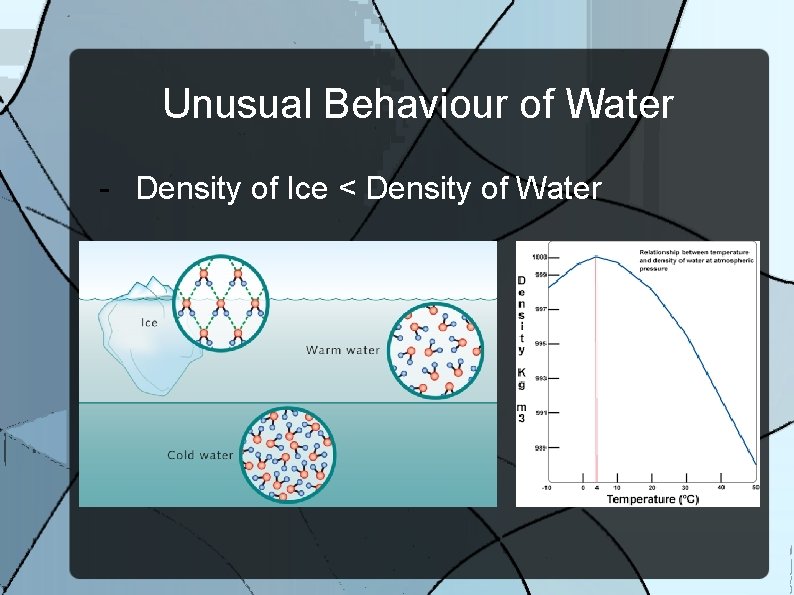
Unusual Behaviour of Water - Density of Ice < Density of Water

Unusual Behaviour of Water - Density of Ice < Density of Water

Pros and Cons of Water’s Unusual Characteristic Physical Properties Pros: Cons:

Pros and Cons of Water’s Unusual Characteristic Physical Properties Pros: - Allows aquatic life to survive Cons: - Potential for pipes to burst - Cannot freeze water in a glass

Salt and Ice - Adding salt to water alters the characteristic physical properties. - When do we take advantage of this? Brainstorm possible alternatives
 Hpps symbols
Hpps symbols Rombencefalo
Rombencefalo Snc1d chemistry
Snc1d chemistry Snc lajaa venissieux
Snc lajaa venissieux Snc
Snc Craneorraquisis
Craneorraquisis Snc database
Snc database Particle theory dissolving
Particle theory dissolving Closely packed in an orderly manner
Closely packed in an orderly manner What is the particle theory of matter
What is the particle theory of matter Particle theory of matter
Particle theory of matter Radiation
Radiation 5 particle theory of matter
5 particle theory of matter Particle theory of matter
Particle theory of matter Www.youtube.com
Www.youtube.com Particle theory of light
Particle theory of light Particle theory of matter
Particle theory of matter Pure substance vs element
Pure substance vs element Kinetic particle theory of matter
Kinetic particle theory of matter Sublimation deposition condensation evaporation
Sublimation deposition condensation evaporation He proposed the particle theory of light
He proposed the particle theory of light Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Synthesis reaction cartoon example
Synthesis reaction cartoon example Vsepr theory project
Vsepr theory project Chemistry collision theory
Chemistry collision theory Particle vs rigid body
Particle vs rigid body Particle equilibrium in 2d and 3d engineering mechanics
Particle equilibrium in 2d and 3d engineering mechanics Kinetics of a particle force and acceleration
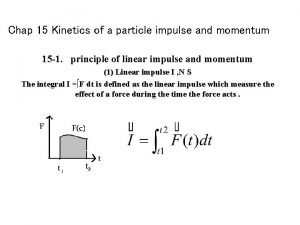
Kinetics of a particle force and acceleration Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Youtube https //www.youtube.com/watch v=vnp84pn0mjq
Youtube https //www.youtube.com/watch v=vnp84pn0mjq Red liquid element
Red liquid element 4 types of chemical reactions
4 types of chemical reactions 5 types of reactions chemistry
5 types of reactions chemistry All types of reaction in chemistry
All types of reaction in chemistry Synthesis and combustion reaction
Synthesis and combustion reaction Trait approaches to leadership
Trait approaches to leadership Continental drift vs plate tectonics theory
Continental drift vs plate tectonics theory Continental drift theory and plate tectonics theory
Continental drift theory and plate tectonics theory Neo classical organizational theory
Neo classical organizational theory Theory x and theory y of motivation
Theory x and theory y of motivation Semantic satiation
Semantic satiation Theory of chromatography
Theory of chromatography Title theory and lien theory
Title theory and lien theory X and y theory
X and y theory Game theory and graph theory
Game theory and graph theory Comparison between mot and vbt
Comparison between mot and vbt Basic principle of valence bond theory
Basic principle of valence bond theory Theory x and theory y
Theory x and theory y Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Sof4 lewis structure
Sof4 lewis structure Theory x and y douglas mcgregor
Theory x and y douglas mcgregor Solid heat
Solid heat Wet etch clean filters
Wet etch clean filters Wave particle duality questions
Wave particle duality questions