Types of Reactions and Predicting Products SNC 2












- Slides: 22

Types of Reactions and Predicting Products SNC 2 D

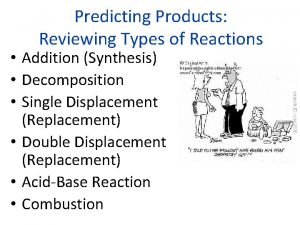
Types of Reactions can be divided into 4 main types: n Synthesis n Decomposition n Single Displacement/Replacement n Double Displacement/Replacement

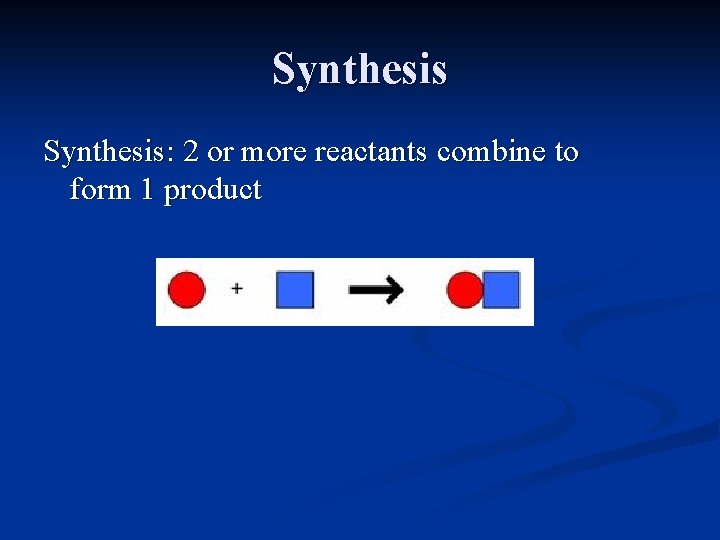
Synthesis: 2 or more reactants combine to form 1 product

Synthesis: 2 or more reactants combine to form 1 product

Synthesis: 2 or more reactants combine to form 1 product e. g. Mg + O 2 -> Mg. O

Synthesis: 2 or more reactants combine to form 1 product e. g. 2 Mg + O 2 -> 2 Mg. O

Synthesis: 2 or more reactants combine to form 1 product e. g. 2 Mg + O 2 2 Mg. O S + Zn Zn. S

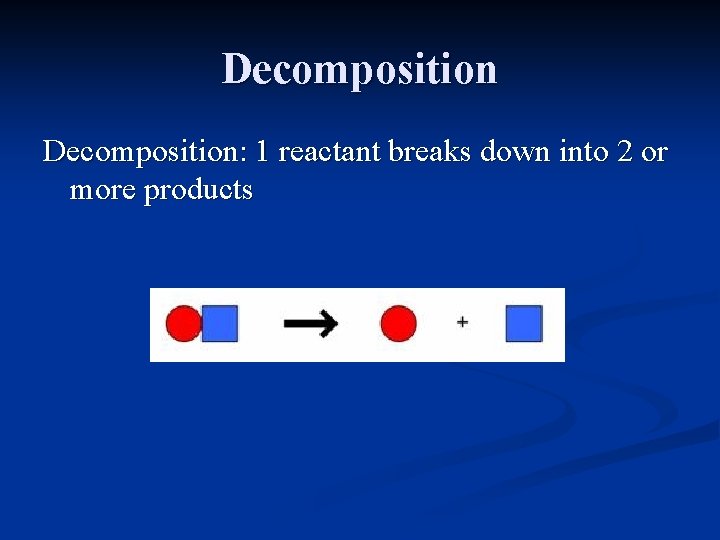
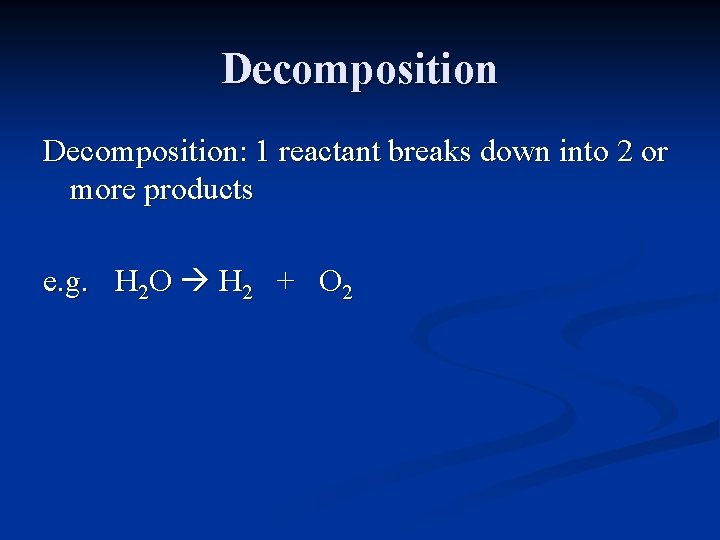
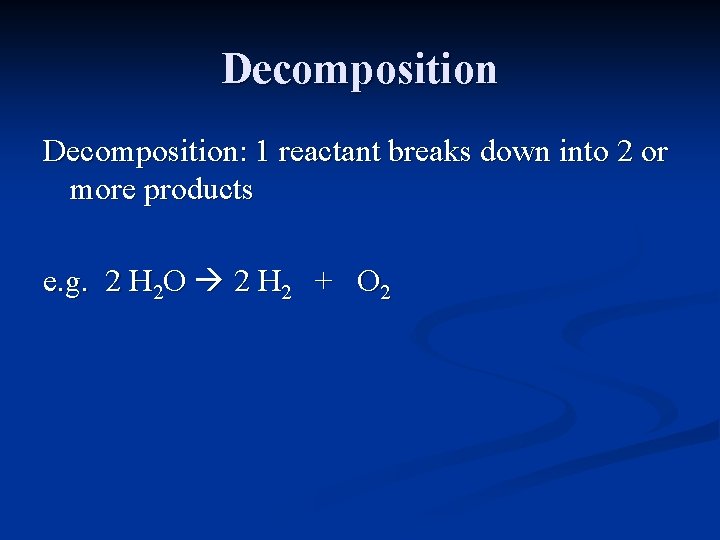
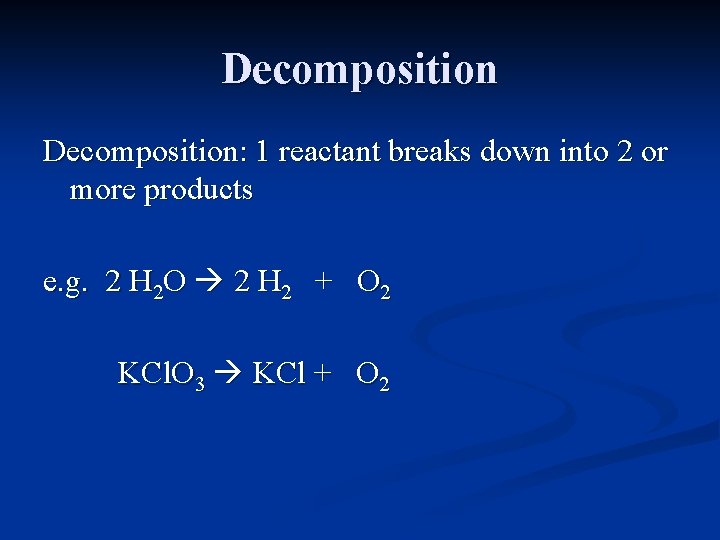
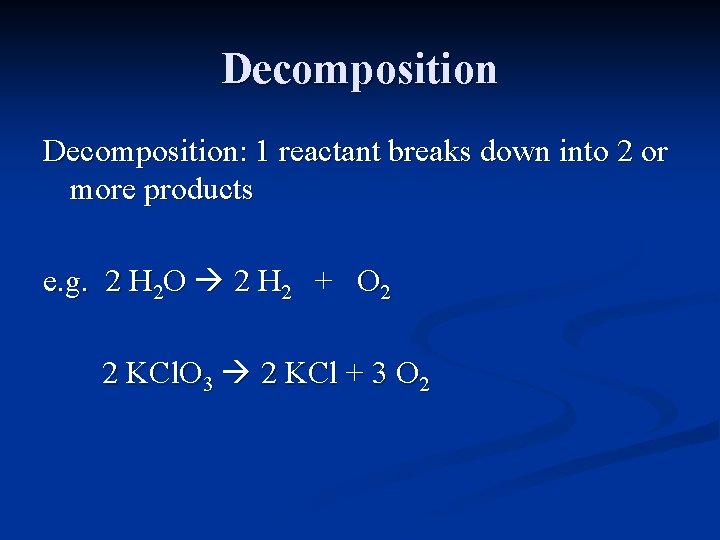
Decomposition: 1 reactant breaks down into 2 or more products

Decomposition: 1 reactant breaks down into 2 or more products e. g. H 2 O H 2 + O 2

Decomposition: 1 reactant breaks down into 2 or more products e. g. 2 H 2 O 2 H 2 + O 2

Decomposition: 1 reactant breaks down into 2 or more products e. g. 2 H 2 O 2 H 2 + O 2 KCl. O 3 KCl + O 2

Decomposition: 1 reactant breaks down into 2 or more products e. g. 2 H 2 O 2 H 2 + O 2 2 KCl. O 3 2 KCl + 3 O 2

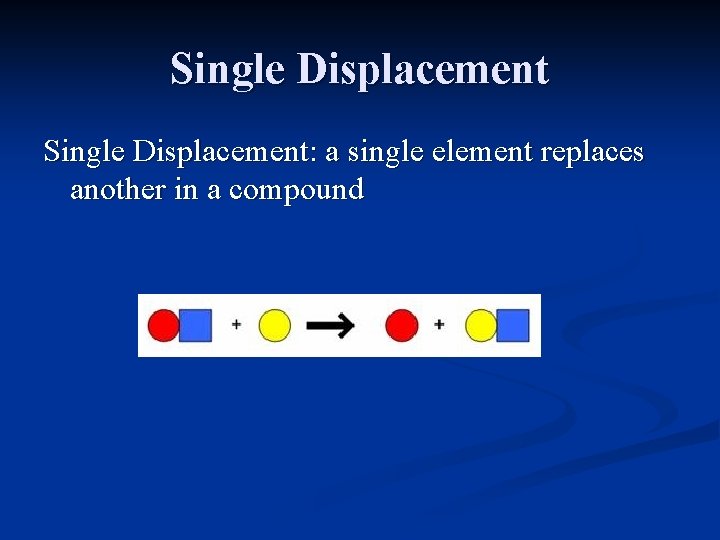
Single Displacement: a single element replaces another in a compound

Single Displacement: a single element replaces another in a compound e. g. Mg. Br 2 + Cl 2 Mg. Cl 2 + Br 2 Negative ions replace negative ions!

Single Displacement: a single element replaces another in a compound e. g. Fe + Cu. SO 4

Single Displacement: a single element replaces another in a compound e. g. Fe + Cu. SO 4 Cu + Fe. SO 4 And positive ions replace positive ions!

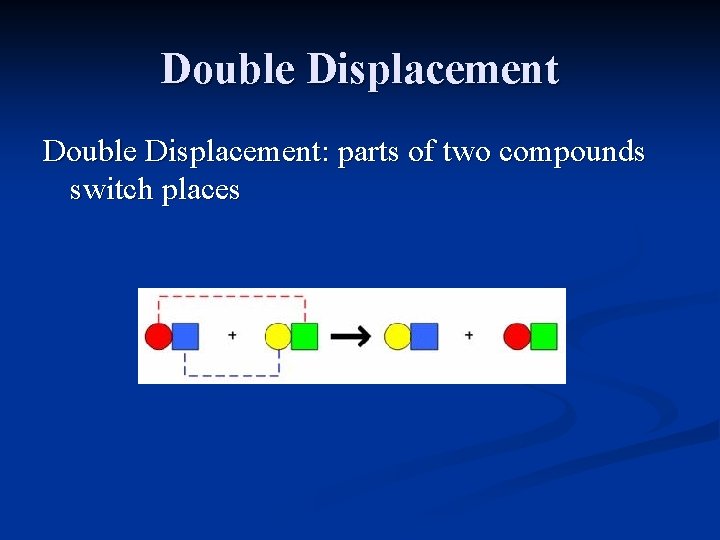
Double Displacement: parts of two compounds switch places

Double Displacement: parts of two compounds switch places e. g. Na. OH + Fe. Cl 3

Double Displacement: parts of two compounds switch places e. g. Na. OH + Fe. Cl 3 Fe(OH)3 + Na. Cl

Double Displacement: parts of two compounds switch places e. g. 3 Na. OH + Fe. Cl 3 Fe(OH)3 + 3 Na. Cl

Double Displacement: parts of two compounds switch places e. g. 3 Na. OH + Fe. Cl 3 Fe(OH)3 + 3 Na. Cl Remember that positive ions pair with negative ions.

Combustion And remember that there also exist combustion reactions: e. g. the combustion of hydrocarbons like methane CH 4 + O 2 CO 2 + H 2 O
 Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions More predicting products of chemical reactions
More predicting products of chemical reactions Combination reaction example
Combination reaction example Predicting single replacement reactions
Predicting single replacement reactions Potassium chloride precipitate
Potassium chloride precipitate Stoichiometry predicting amounts in reactions
Stoichiometry predicting amounts in reactions Single replacement activity series
Single replacement activity series Predicting redox reactions
Predicting redox reactions Predicting products
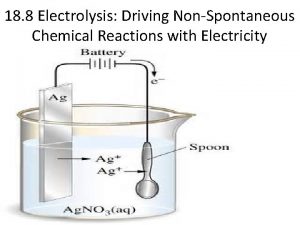
Predicting products Predicting products of electrolysis
Predicting products of electrolysis Poisonous and infectious material symbol
Poisonous and infectious material symbol Snc y snp diferencias
Snc y snp diferencias Snc1d chemistry
Snc1d chemistry Snc lajaa venissieux
Snc lajaa venissieux Snc società
Snc società Síndrome de kleeblattschädel
Síndrome de kleeblattschädel Snc database
Snc database Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions 20 examples of redox reaction
20 examples of redox reaction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers