Predicting Redox Reactions 9 3 Predicting Spontaneous Reactions


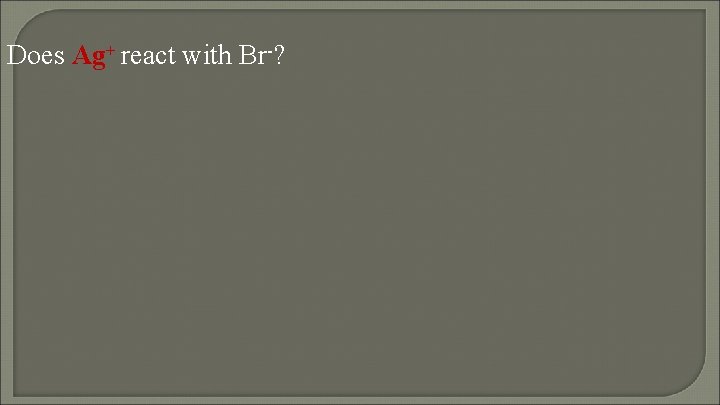
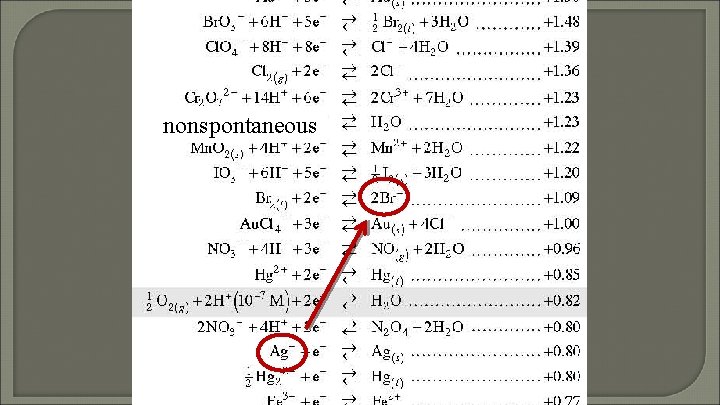


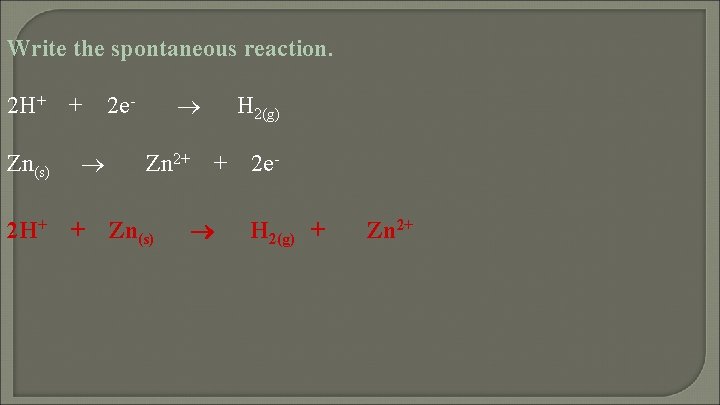


- Slides: 29

Predicting Redox Reactions 9. 3

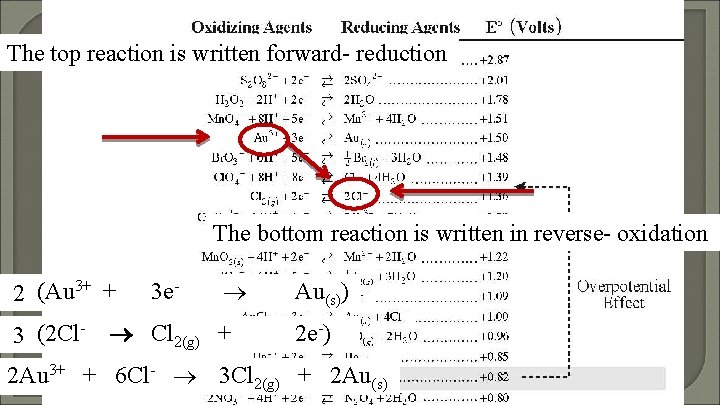
Predicting Spontaneous Reactions Using the Standard Reduction Chart Does Au 3+ react with Cl-? If it does write the spontaneous reaction.

The top reaction is written forward- reduction The bottom reaction is written in reverse- oxidation 2 (Au 3+ + 3 (2 Cl- 3 e- Cl 2(g) + Au(s)) 2 e-) 2 Au 3+ + 6 Cl- 3 Cl 2(g) + 2 Au(s)
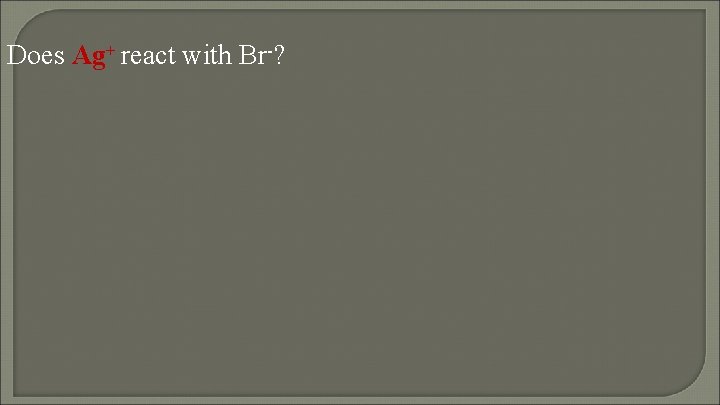
Does Ag+ react with Br-?
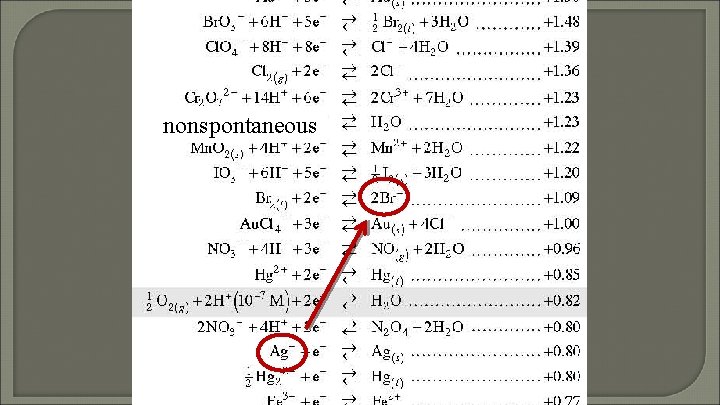
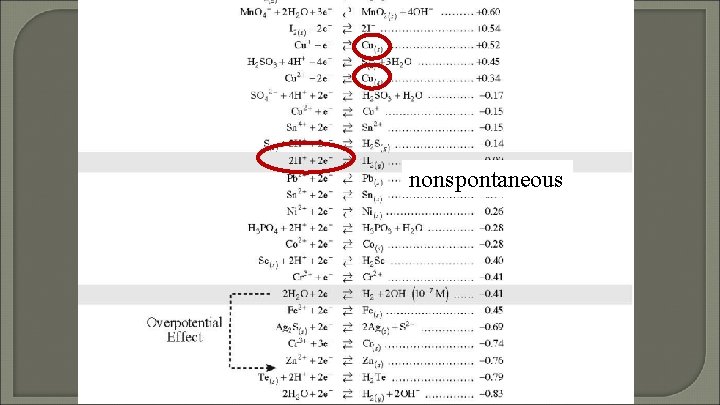
nonspontaneous

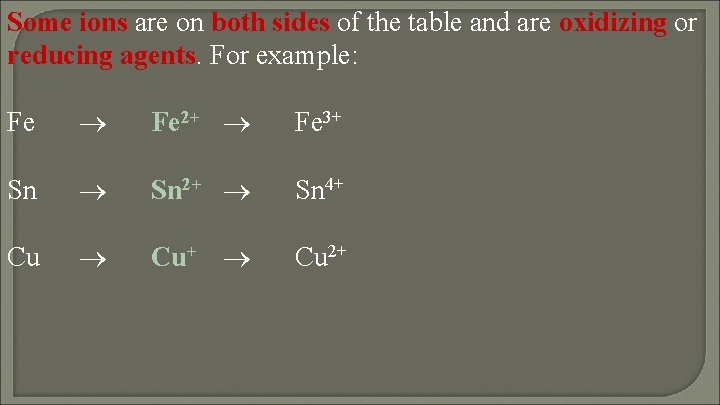
Some ions are on both sides of the table and are oxidizing or reducing agents. For example: Fe Fe 2+ Fe 3+ Sn Sn 2+ Sn 4+ Cu Cu+ Cu 2+
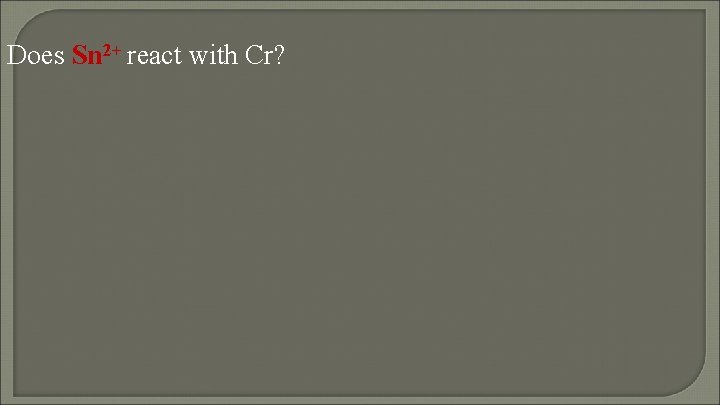
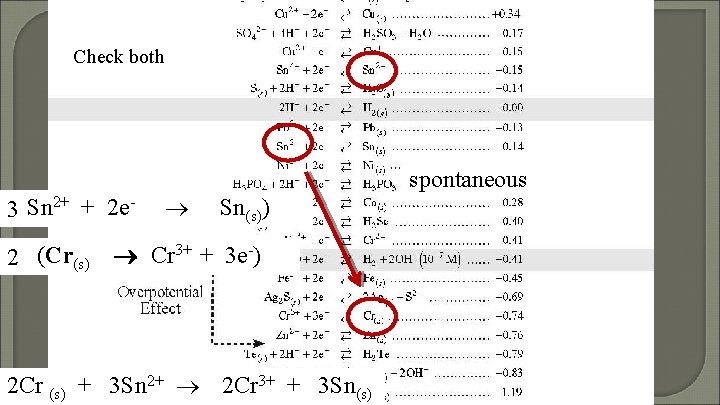
Does Sn 2+ react with Cr?

Check both 3(Sn 2+ + 2 e 2 (Cr(s) spontaneous Sn(s)) Cr 3+ + 3 e-) 2 Cr (s) + 3 Sn 2+ 2 Cr 3+ + 3 Sn(s)

Can you keep HCl in a Cu container? Explain!

nonspontaneous

Can you keep HCl in a Cu container? Explain! Yes, nonspontaneous. H+ is a weaker oxidizing agent than Cu 2+ and Cu+.

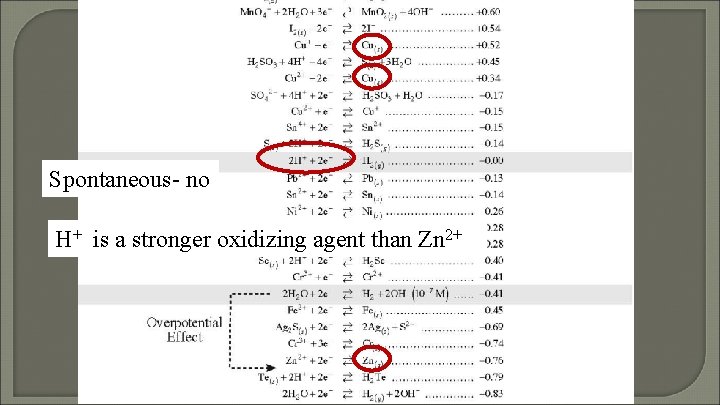
Can you keep HCl in a Zn container? Explain!

Spontaneous- no H+ is a stronger oxidizing agent than Zn 2+
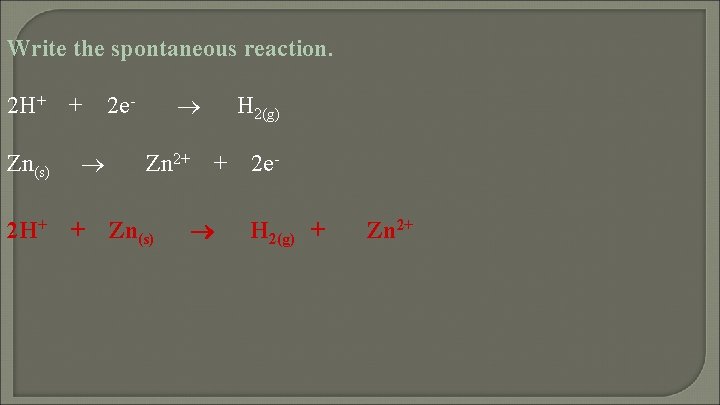
Write the spontaneous reaction. 2 H+ Zn(s) 2 H+ + + 2 e- Zn 2+ Zn(s) H 2(g) + 2 e. H 2(g) + Zn 2+

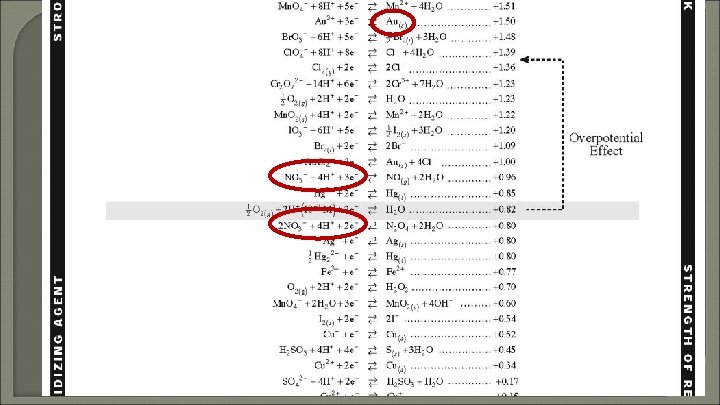
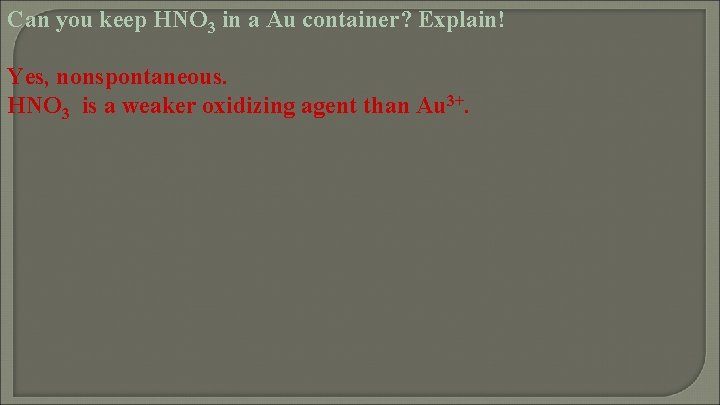
Can you keep HNO 3 in a Au container? Explain!


Can you keep HNO 3 in a Au container? Explain! Yes, nonspontaneous. HNO 3 is a weaker oxidizing agent than Au 3+.

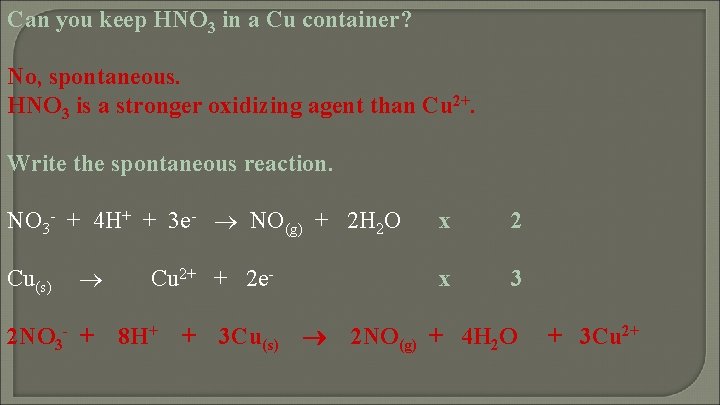
Can you keep HNO 3 in a Cu container? Explain!

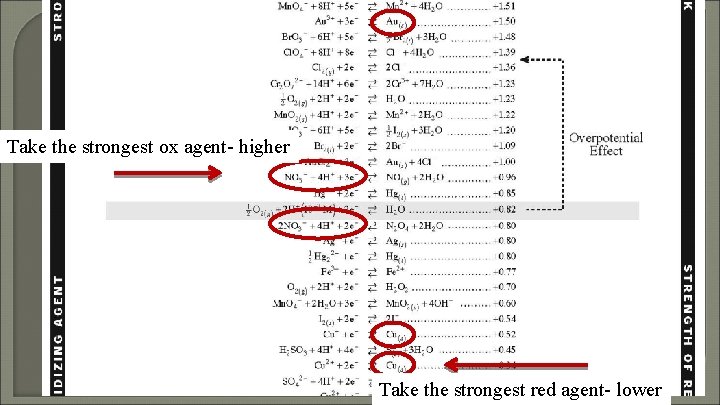
Take the strongest ox agent- higher Take the strongest red agent- lower

Can you keep HNO 3 in a Cu container? No, spontaneous. HNO 3 is a stronger oxidizing agent than Cu 2+. Write the spontaneous reaction. NO 3 - + 4 H+ + 3 e- NO(g) + 2 H 2 O Cu(s) 2 NO 3 - + Cu 2+ + 2 e 8 H+ + 3 Cu(s) x 2 x 3 2 NO(g) + 4 H 2 O + 3 Cu 2+

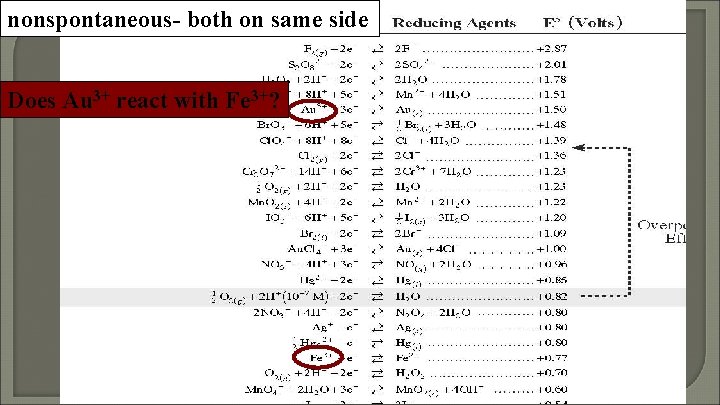
nonspontaneous- both on same side Does Au 3+ react with Fe 3+?

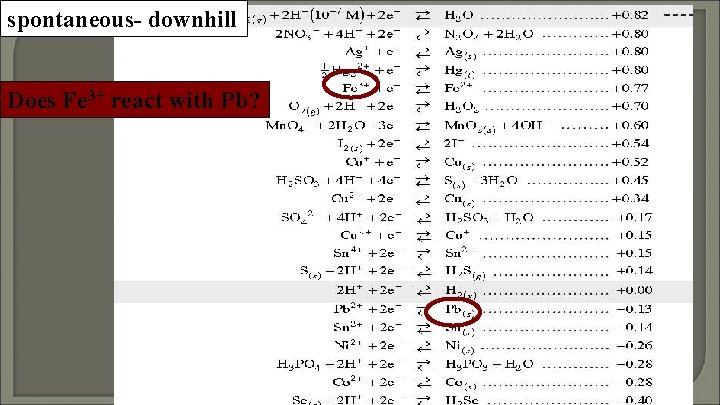
spontaneous- downhill Does Fe 3+ react with Pb?

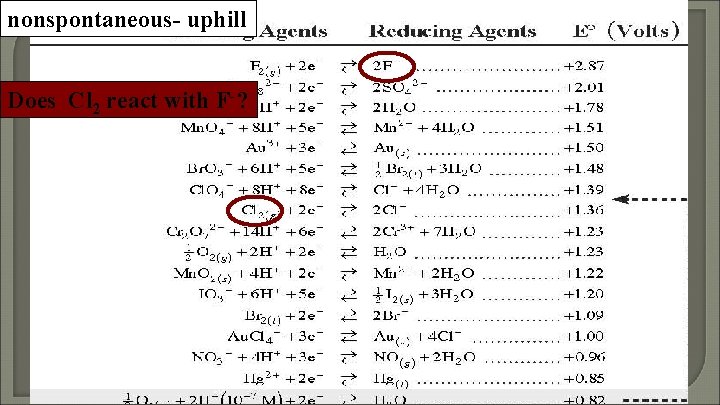
nonspontaneous- uphill Does Cl 2 react with F-?

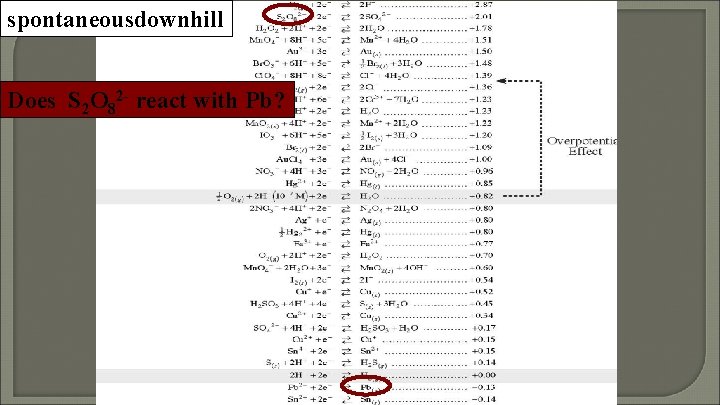
spontaneousdownhill Does S 2 O 82 - react with Pb?

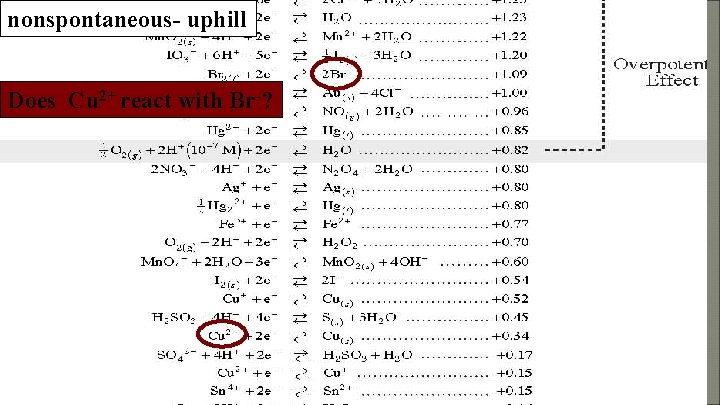
nonspontaneous- uphill Does Cu 2+ react with Br-?

spontaneous- downhill Does Br 2 react with Sn 2+?

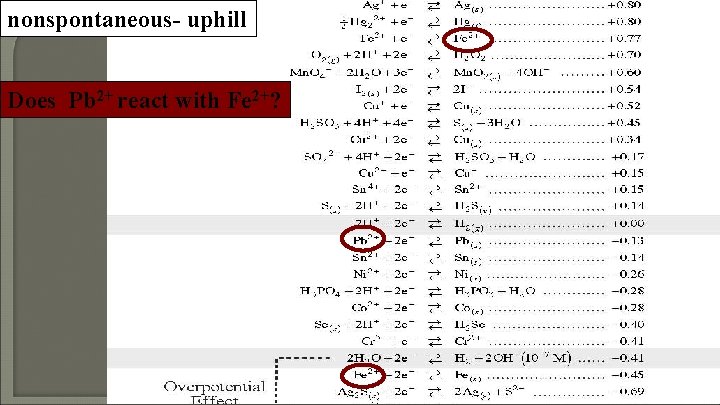
nonspontaneous- uphill Does Pb 2+ react with Fe 2+?

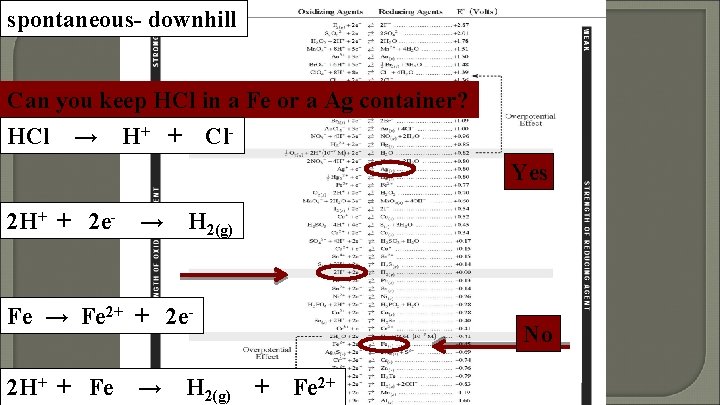
spontaneous- downhill Can you keep HCl in a Fe or a Ag container? HCl → H+ + Cl. Yes 2 H+ + 2 e- → H 2(g) Fe → Fe 2+ + 2 e 2 H+ + Fe → H 2(g) No + Fe 2+

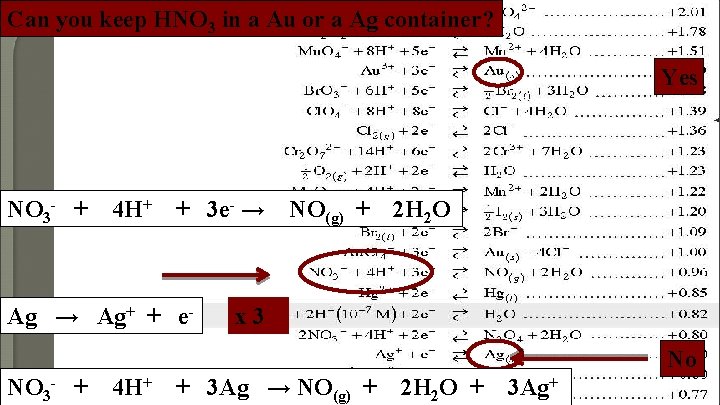
Can you keep HNO 3 in a Au or a Ag container? Yes NO 3 - + 4 H+ + 3 e- → Ag+ + e- NO(g) + 2 H 2 O x 3 No NO 3 - + 4 H+ + 3 Ag → NO(g) + 2 H 2 O + 3 Ag+