Types of Chemical Reactions Predicting Products Notes Review








- Slides: 22

Types of Chemical Reactions Predicting Products Notes


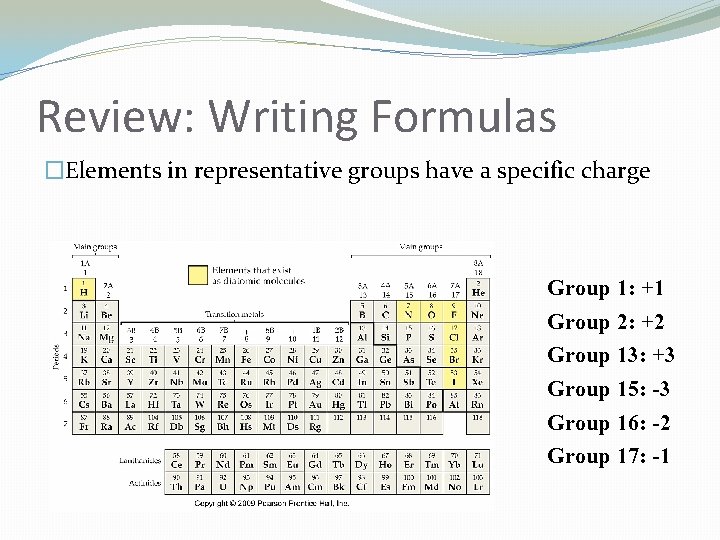
Review: Writing Formulas �Elements in representative groups have a specific charge Group 1: +1 Group 2: +2 Group 13: +3 Group 15: -3 Group 16: -2 Group 17: -1

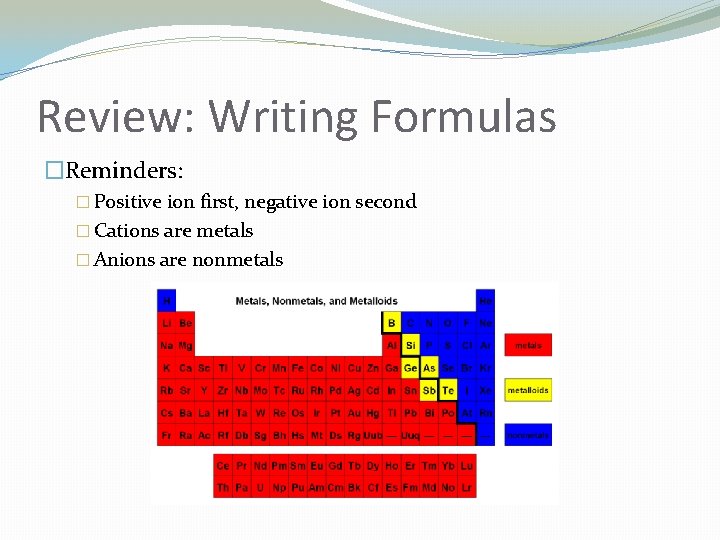
Review: Writing Formulas �Reminders: � Positive ion first, negative ion second � Cations are metals � Anions are nonmetals

Review: Writing Formulas �Write out the ions (atomic symbol with charge) �Charge is memorized (representative groups) �Or look on polyatomic ions sheet �Transition metals should be given to you in the problem or work backwards from the given formulas �Criss cross the charges. �The charge on one ion becomes the subscript of the other ion. �ADD THIS TO NOTES: If more than one polyatomic ion, need to use parentheses! �Let’s practice #1 -10


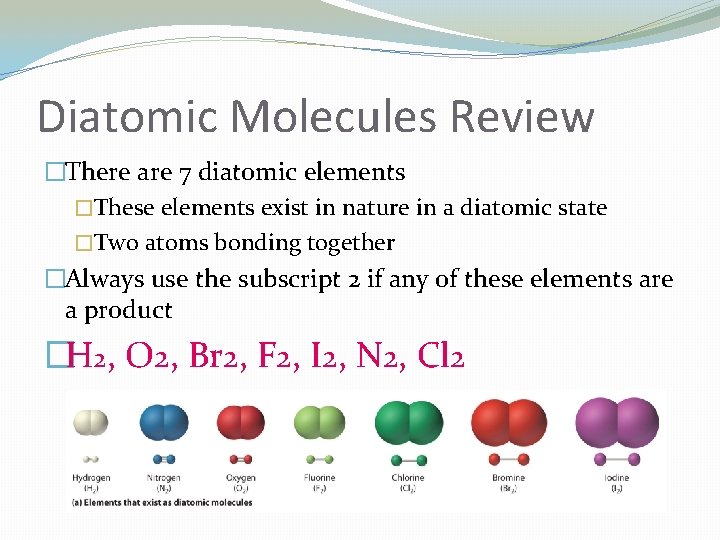
Diatomic Molecules Review �There are 7 diatomic elements �These elements exist in nature in a diatomic state �Two atoms bonding together �Always use the subscript 2 if any of these elements are a product �H 2, O 2, Br 2, F 2, I 2, N 2, Cl 2

Diatomic Molecules Review �How do you remember them? �HOBr. FINCl

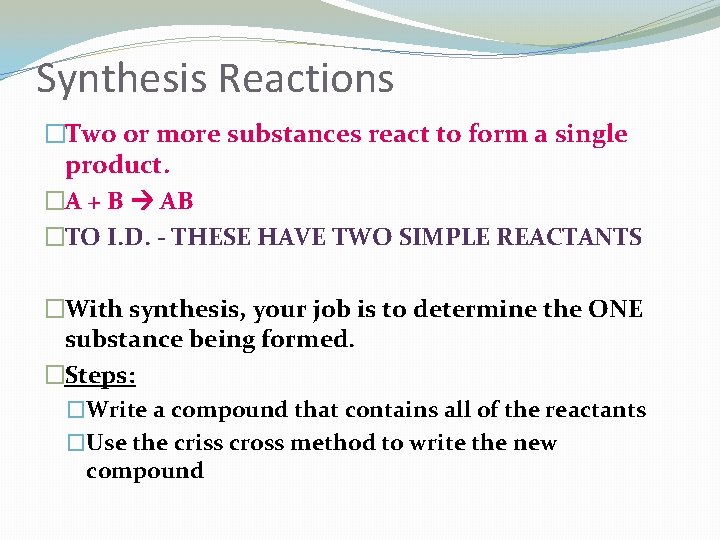
Synthesis Reactions �Two or more substances react to form a single product. �A + B AB �TO I. D. - THESE HAVE TWO SIMPLE REACTANTS �With synthesis, your job is to determine the ONE substance being formed. �Steps: �Write a compound that contains all of the reactants �Use the criss cross method to write the new compound

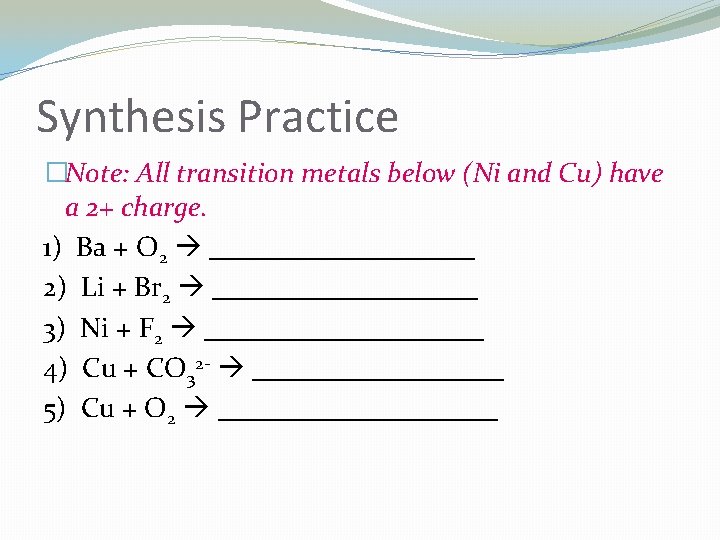
Synthesis Practice �Note: All transition metals below (Ni and Cu) have a 2+ charge. 1) Ba + O 2 __________ 2) Li + Br 2 __________ 3) Ni + F 2 __________ 4) Cu + CO 32 - _________ 5) Cu + O 2 __________

Decomposition Reactions � A single compound breaks down into two or more substances � AB A + B � TO I. D. - THESE HAVE ONE REACTANT �With decomposition, your job is to determine what the compound is breaking down into �Steps: � Attempt to break the compound into two elements or two compounds � Split the single substance into two parts � Don’t forget diatomic molecules

Decomposition Practice 1) Ca. CO 3 _______________ 2) Al 2(CO 3)3 _______________ 3) HCl. O 3 ________________ 4) Mg(OH)2 _______________ Don’t forget: HOBr. FINCl

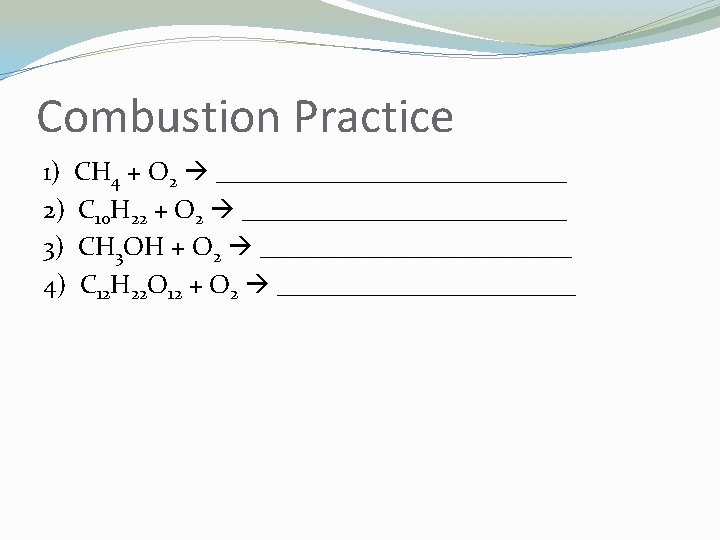
Combustion Reactions �Hydrocarbon reacts with O 2 to produce CO 2 and H 2 O �Cx. Hy +O 2 CO 2 + H 2 O �TO I. D. – CH COMPOUND REACTING WITH OXYGEN �Steps: �Products are always CO 2 and H 2 O

Combustion Practice 1) CH 4 + O 2 ______________ 2) C 10 H 22 + O 2 _____________ 3) CH 3 OH + O 2 ____________ 4) C 12 H 22 O 12 + O 2 ____________

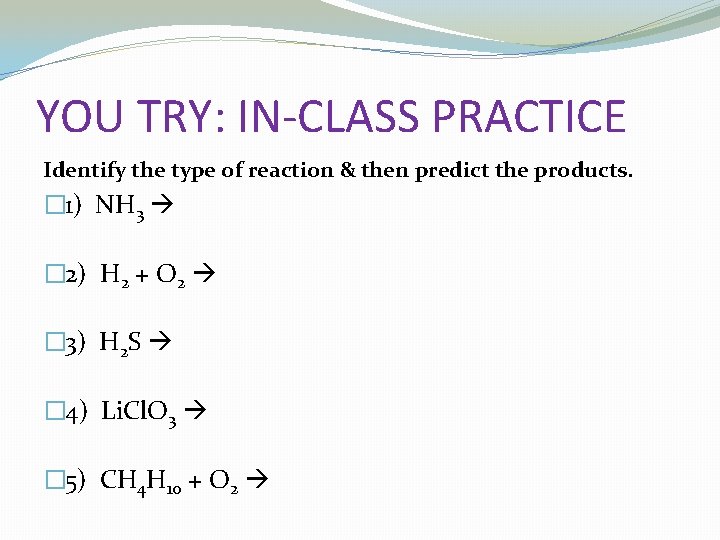
YOU TRY: IN-CLASS PRACTICE Identify the type of reaction & then predict the products. � 1) NH 3 � 2) H 2 + O 2 � 3) H 2 S � 4) Li. Cl. O 3 � 5) CH 4 H 10 + O 2

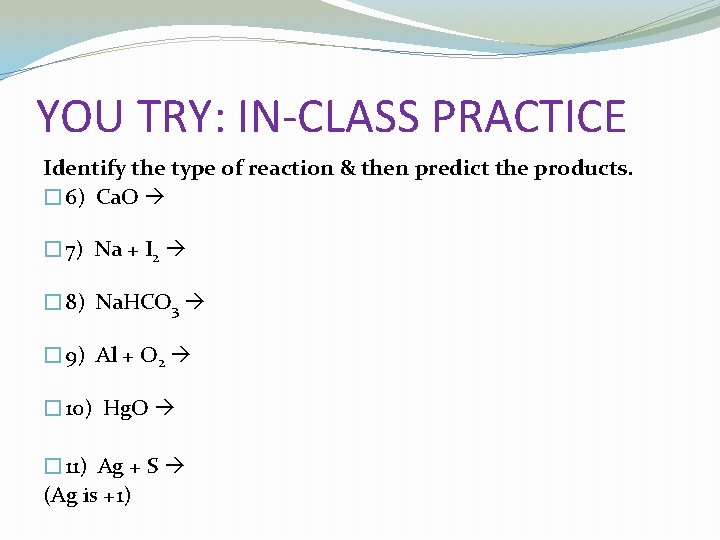
YOU TRY: IN-CLASS PRACTICE Identify the type of reaction & then predict the products. � 6) Ca. O � 7) Na + I 2 � 8) Na. HCO 3 � 9) Al + O 2 � 10) Hg. O � 11) Ag + S (Ag is +1)

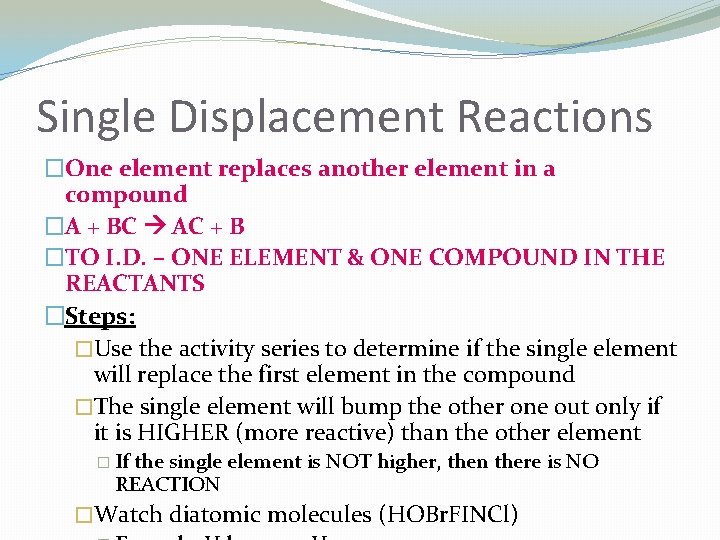
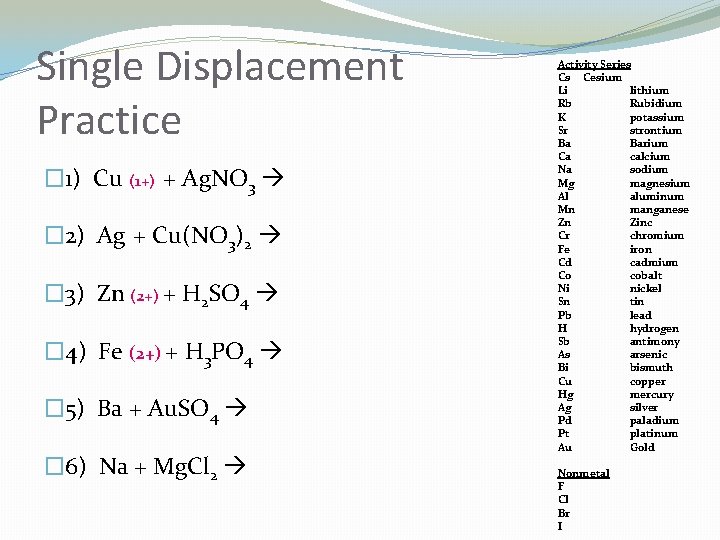
Single Displacement Reactions �One element replaces another element in a compound �A + BC AC + B �TO I. D. – ONE ELEMENT & ONE COMPOUND IN THE REACTANTS �Steps: �Use the activity series to determine if the single element will replace the first element in the compound �The single element will bump the other one out only if it is HIGHER (more reactive) than the other element � If the single element is NOT higher, then there is NO REACTION �Watch diatomic molecules (HOBr. FINCl)

Single Displacement Practice � 1) Cu (1+) + Ag. NO 3 � 2) Ag + Cu(NO 3)2 � 3) Zn (2+) + H 2 SO 4 � 4) Fe (2+) + H 3 PO 4 � 5) Ba + Au. SO 4 � 6) Na + Mg. Cl 2 Activity Series Cs Cesium Li lithium Rb Rubidium K potassium Sr strontium Ba Barium Ca calcium Na sodium Mg magnesium Al aluminum Mn manganese Zn Zinc Cr chromium Fe iron Cd cadmium Co cobalt Ni nickel Sn tin Pb lead H hydrogen Sb antimony As arsenic Bi bismuth Cu copper Hg mercury Ag silver Pd paladium Pt platinum Au Gold Nonmetal F Cl Br I

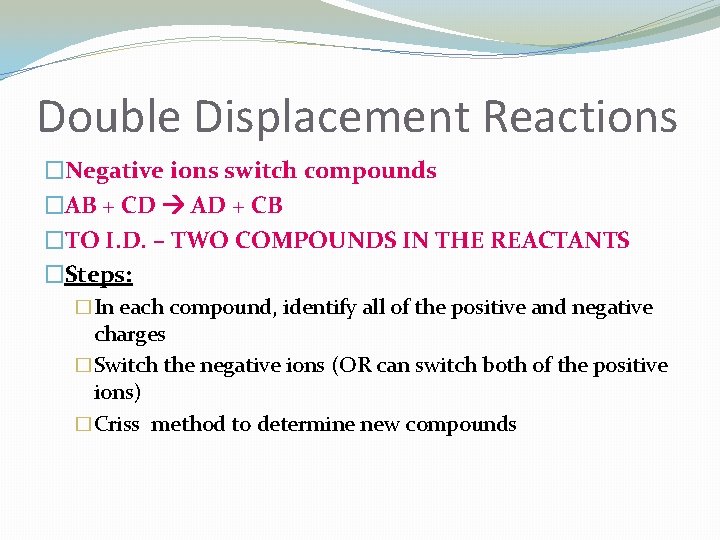
Double Displacement Reactions �Negative ions switch compounds �AB + CD AD + CB �TO I. D. – TWO COMPOUNDS IN THE REACTANTS �Steps: �In each compound, identify all of the positive and negative charges �Switch the negative ions (OR can switch both of the positive ions) �Criss method to determine new compounds

Silver (Ag) is always +1 and zinc (Zn) is +2 To chemical reactions reference sheet: Add NH 4+1 Double Displacement Practice � 1) Ba. Cl 2 + Na 2 CO 3 � 2) Al 2 (SO 4)3 + H 3 PO 4 � 3) HNO 3 + Na. Cl � 4) NH 4 Cl + Ba(OH)2 � 5) Ag. Cl. O 3 + Ni(NO 3)2 � 6) Pb. Cl 4 + H 3 PO 4

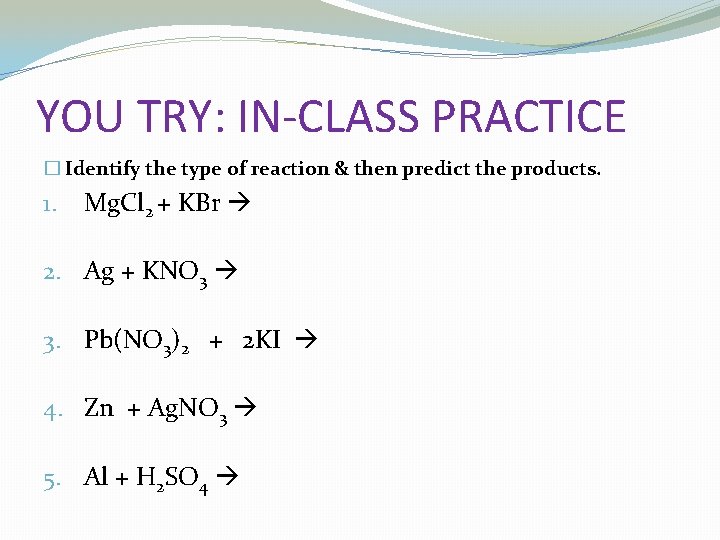
YOU TRY: IN-CLASS PRACTICE � Identify the type of reaction & then predict the products. 1. Mg. Cl 2 + KBr 2. Ag + KNO 3 3. Pb(NO 3)2 + 2 KI 4. Zn + Ag. NO 3 5. Al + H 2 SO 4

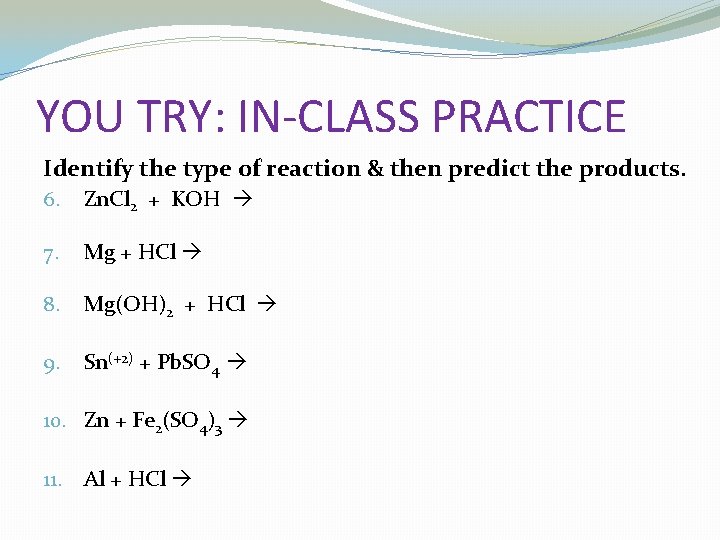
YOU TRY: IN-CLASS PRACTICE Identify the type of reaction & then predict the products. 6. Zn. Cl 2 + KOH 7. Mg + HCl 8. Mg(OH)2 + HCl 9. Sn(+2) + Pb. SO 4 10. Zn + Fe 2(SO 4)3 11. Al + HCl
 Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Combination reaction equation
Combination reaction equation Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Reactants and products
Reactants and products Predicting single replacement reactions
Predicting single replacement reactions Predicting precipitation reactions
Predicting precipitation reactions How to determine if a single replacement reaction occurs
How to determine if a single replacement reaction occurs Predicting single replacement reactions
Predicting single replacement reactions Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Predicting products
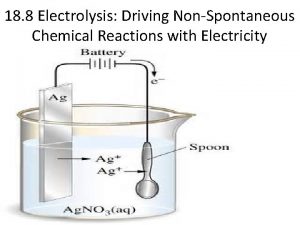
Predicting products Predicting products of electrolysis
Predicting products of electrolysis Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Balancing equations chapter 8
Balancing equations chapter 8 Balancing redox reactions
Balancing redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type