Particle Theory of Matter SNC 1 D Chemistry



- Slides: 19

Particle Theory of Matter SNC 1 D - Chemistry Unit

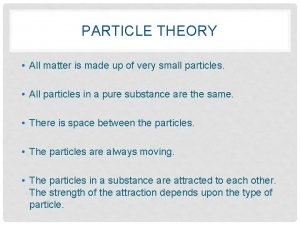
Particle Theory • A theory is a series of statements which are developed to explain several related observations • A model is used as a guide for the imagination when using a theory

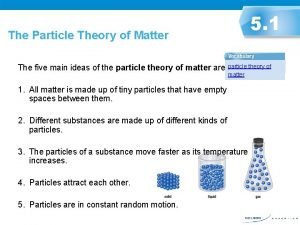
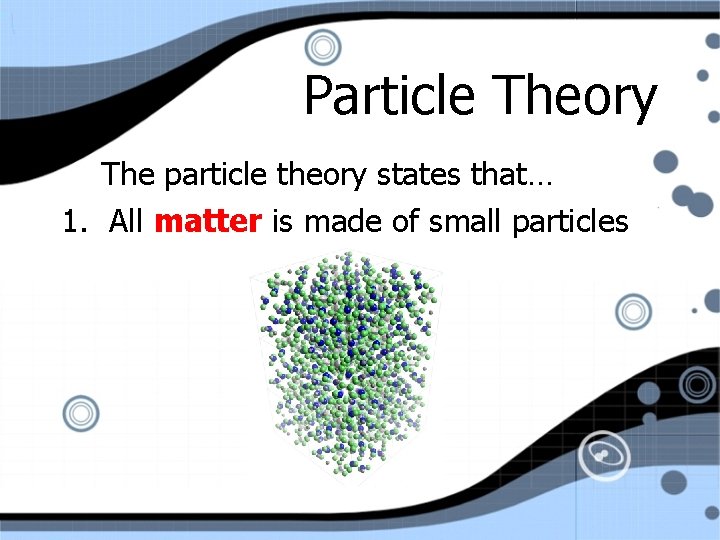
Particle Theory The particle theory states that… 1. All matter is made of small particles

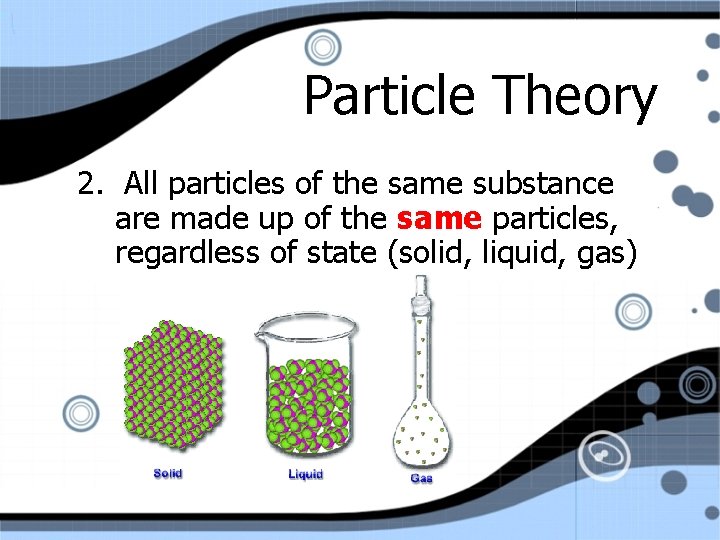
Particle Theory 2. All particles of the same substance are made up of the same particles, regardless of state (solid, liquid, gas)

Particle Theory 3. The particles attract one another. These forces get stronger as the particles get closer

Particle Theory 4. The space between particles are larger compared to the sizes of the particles themselves

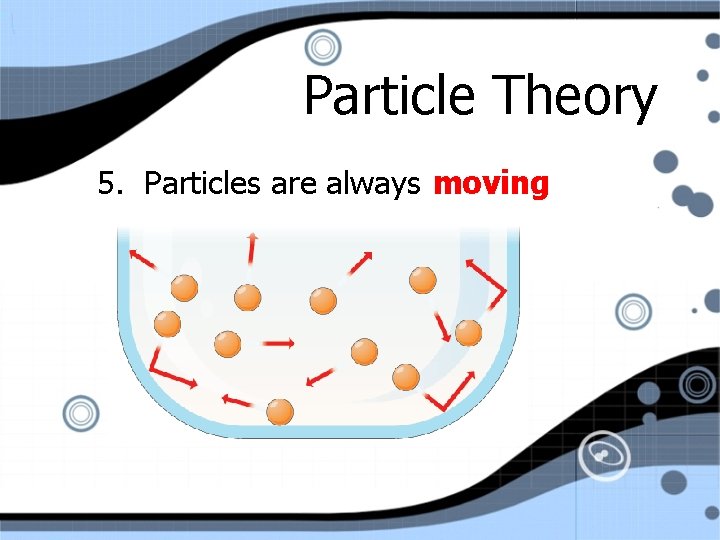
Particle Theory 5. Particles are always moving

States of Matter

States of Matter • Some substances exist only in one state of matter

States of Matter • Some substances exist in more than one state of matter. Water is a common example

Solids • attraction between particles is strong • space between particles is small (particles are close together) • there is little motion of particles - movement is mostly vibrational

Liquids • attraction between particles is medium • space between particles is medium • movement is vibrational and rotational

Gases • attraction between particles is weak • space between particles is large • movement is vibrational, rotational and translational

Gas vs. Vapour gas = gas at room temperature vapour = the gaseous state of a substance that is liquid at room temperature

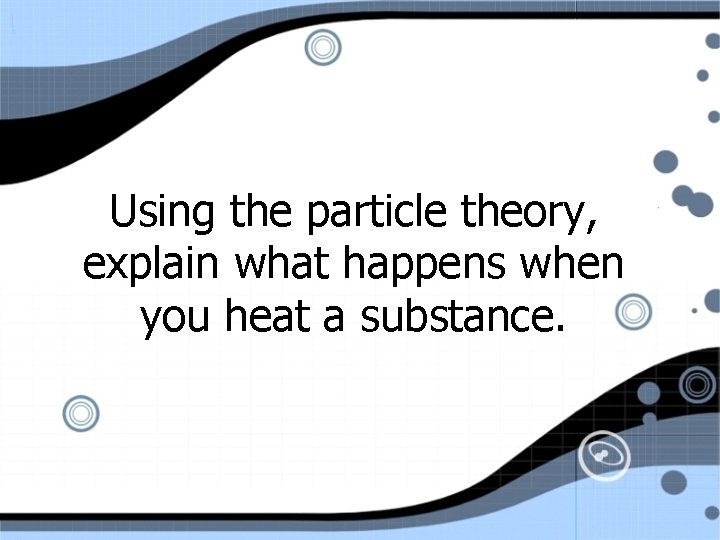
Using the particle theory, explain what happens when you heat a substance.

Heat • heat = energy • added energy breaks the attractive forces between the particles • particles spread apart and movement increases

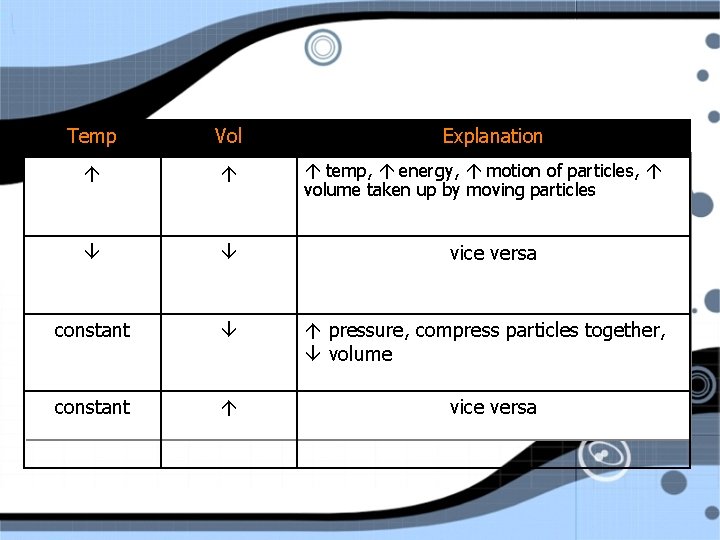
Temp Vol constant Explanation temp, energy, motion of particles, volume taken up by moving particles vice versa pressure, compress particles together, volume vice versa

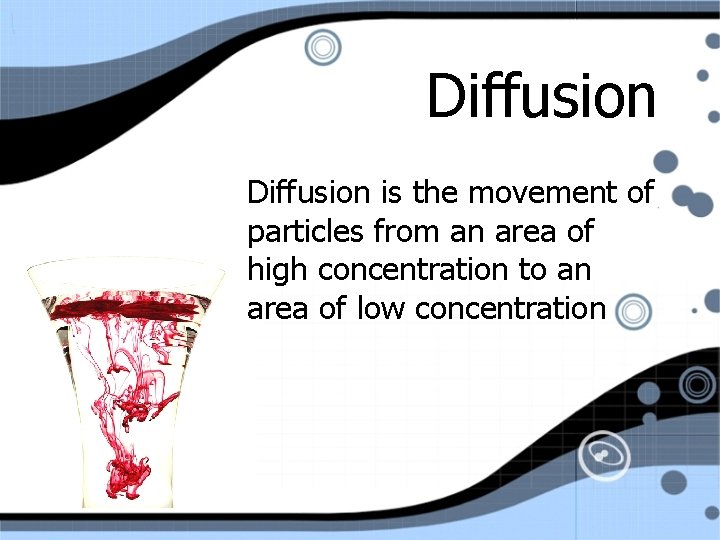
Diffusion is the movement of particles from an area of high concentration to an area of low concentration

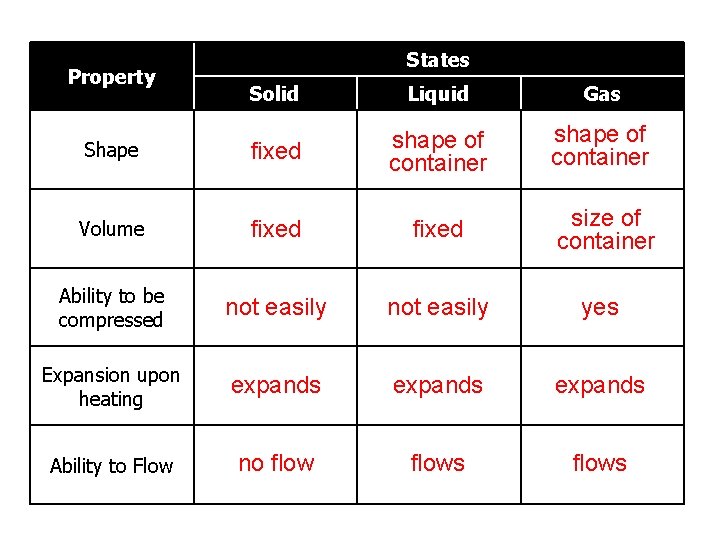
Property States Solid Liquid Gas Shape fixed shape of container Volume fixed size of container Ability to be compressed not easily yes Expansion upon heating expands Ability to Flow no flows
 Particle theory
Particle theory What is the particle theory of matter
What is the particle theory of matter Define particle theory of matter
Define particle theory of matter Particle theory of matter
Particle theory of matter Particle theory
Particle theory What is matter
What is matter Particle theory grade 9
Particle theory grade 9 Particle theory of matter grade 7
Particle theory of matter grade 7 Particle theory evaporation
Particle theory evaporation Hpps symbol
Hpps symbol Rombencefalo
Rombencefalo Water density
Water density Snc lajaa venissieux
Snc lajaa venissieux Snc
Snc Raquisis
Raquisis Snc database
Snc database Particle model of matter exam questions
Particle model of matter exam questions Whats the smallest particle of matter
Whats the smallest particle of matter Whats the smallest particle of matter
Whats the smallest particle of matter Particle diagram of a solid
Particle diagram of a solid