Particle Theory and Dissolving Particle Theory and Dissolving









- Slides: 11

Particle Theory and Dissolving

Particle Theory and Dissolving Ø The particle theory states that: • • All matter is made up of particles. Particles are constantly in motion. The particles of one substance are all the same. There are spaces between particles and those spaces are larger than the particles themselves. • Particles are attracted to one another- the closer the particles the stronger the attraction. • Particles move faster when heated up and slower when cooled down.

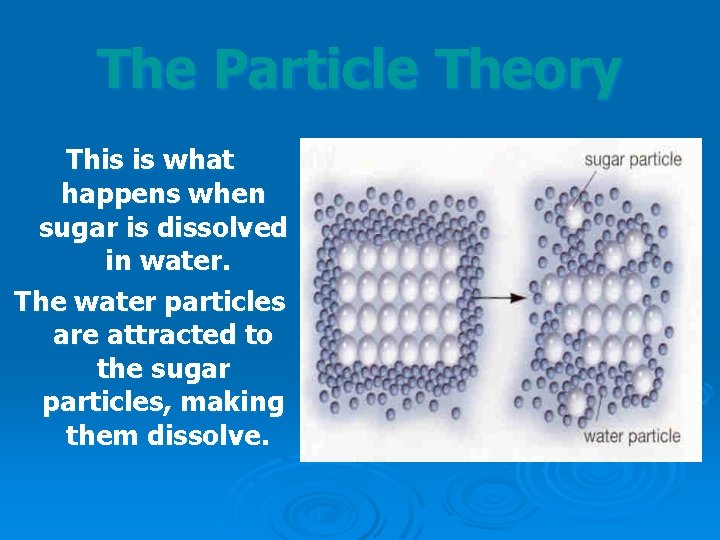
The Particle Theory This is what happens when sugar is dissolved in water. The water particles are attracted to the sugar particles, making them dissolve.

Particle Theory and Dissolving Ø https: //www. youtube. com/watch? v=0 c. PFx 0 w. Fu. V s Ø https: //www. youtube. com/watch? v=sg. Vr 0 zq 6 qio Ø So water molecules are attracted to the sugar molecules and will break down the sugar cube and stick to the sugar spreading evenly in the solution.

Rate of Dissolving When a crystal dissolves, the molecules at the surface of the crystal enter the solution first because they are the ones that are in contact with the moving water molecules. Water molecules can “kick” the sugar molecules from the surface of a sugar crystal.

Dissolution Rate- Heat is added https: //www. youtube. com/watch? v=sg. Vr 0 zq 6 qio Ø When heat is added the particles of water move faster and can break up the molecules of sugar faster therefore dissolving the sugar quicker Ø In addition, more space is created between the molecules to allow for more sugar to be dissolved Ø

Dissolution Rate- Agitation/Stirring Ø Add more energy by agitating the solution. This increases the number of particle collisions.

Dissolution Rate- Increase Surface Area • Another way to increase the number of particle collisions is to break or crush the solute into very small particles. • This increases the surface area of the solute. More surface area means more collisions between solvent and solute particles.

Insolubility

The Particle Theory and Dissolving Ø If a solute dissolves in a particular solvent, we say that it is soluble in that solvent. Ø If a solute does not dissolve, it is insoluble. Ø Sugar, for example, is soluble in water but insoluble in vegetable oil Ø Think of one solute that is insoluble in water. What does this tell you about the particles of this solute in water?

Insolubility