Properties of Matter I What is Matter A
































- Slides: 36

Properties of Matter

I. What is Matter? A. Matter is anything that has mass and takes up space. B. Matter has different properties or characteristics. { Chemistry is the study of the properties of matter and how matter changes. }

II. Composition of Matter Substance – matter with a fixed composition or make up. It also has definite properties. It is pure. a. Sodium Chloride, Na. Cl (table salt) is always exactly the same no matter what brand it is or where it comes from. It is a pure substance.

b. Blueberry muffin batter is made up of different ingredients. Some of the ingredients are substances. Each recipe is different. Each batch is different no matter how hard you try to make it the same. Blueberry muffin batter is not a substance.

Group as “substances” or “not a substance” 1. table salt 2. pizza 3. distilled water 4. silver 5. air 6. mud 7. oxygen 8. Sugar 9. rock 10. tap water

Substance: table salt, sugar, distilled water, silver, oxygen. Not substances: pizza, air, mud, rock, tap water. • How did you do? • Make a list of your own and exchange with a friend. See if you both can sort things out. Discuss your answers.

All matter has two kinds of properties: 1. Physical: can be observed (using your senses. ) Without changing it into another different substance. How hard is it? Is it rough? What color is it? Will it dissolve in water? Is it a solid, a liquid or a gas? Can it bend? The state of matter is a physical property.

These physical properties can be used to classify matter. a. for example, metals have luster (they are shiny) and they conduct electricity. Can you think of other properties metals have in common?

Four States of Matter (review) A. Solid – definite volume and definite shape -particles are tightly packed together - very little motion, vibrate only - strong attraction between particles

B. Liquid - definite volume but no definite shape – particles are tightly packed together – particles have the ability to move (flow) – particle attraction not as strong as that of a solid – viscosity – the resistance of a fluid to flow (strong attraction = high viscosity = slow moving) Ex: gel

D. Gas – no definite volume or shape - particles are far apart - particles move in random motions; very fast - little or no particle attraction - pressure is exerted by the atoms of the gas hitting the sides of the container

E. Plasma – no definite volume or shape - consists of electrically charged particles. - has large amounts of energy - stars!!

2. Chemical property: the property of a pure substance that describes (identifies) its ability to change into a different substance. . If you have a piece of paper and burn it, it is no longer paper. It has let off a gas, the solid has become ash. Heat is released.

Burning, giving off heat, is a chemical property. Chemical properties are also used to classify substances. Some substances change, like iron combines with oxygen to form rust. Some substances like gold do not change or react.

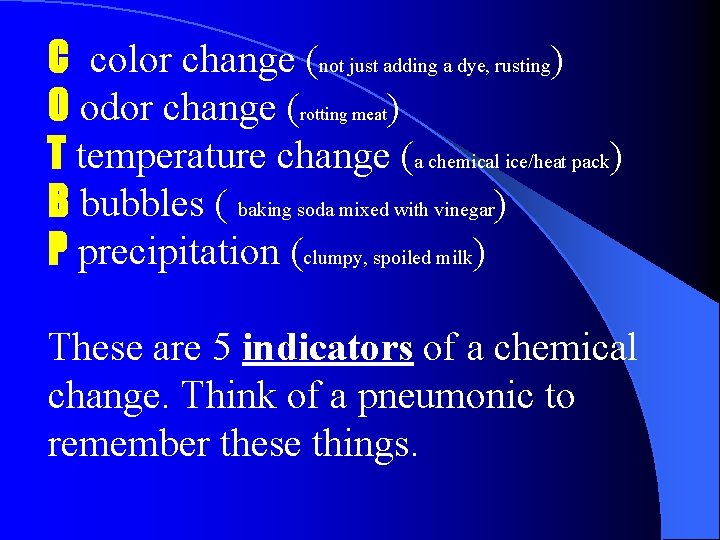
C color change (not just adding a dye, rusting) O odor change (rotting meat) T temperature change (a chemical ice/heat pack) B bubbles ( baking soda mixed with vinegar) P precipitation (clumpy, spoiled milk) These are 5 indicators of a chemical change. Think of a pneumonic to remember these things.

If two or more of these indicators are present then it may be a chemical change! • Adding crystal light to water may change the color and it may have a sweet smell but it is not a chemical change. The crystal light is just dissolved in the water, the water is still water. Evaporate the water and crystal light is at the bottom of the glass. H 2 O has gone into the air. Each thing is still the same substance.

Leaving milk on the counter so it goes bad IS a chemical change. • It smells (odor) • It becomes chunky (precipitate) • The milk may change color also It is no longer milk. Can you come up with another chemical change?

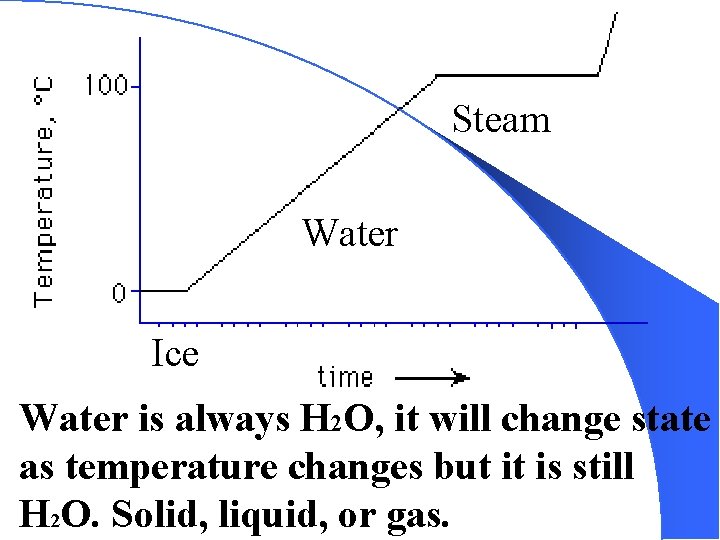
You have a container of water, H 2 O, at room temperature, it is liquid. You put it in a freezer, it becomes a solid as the temperature drops. Is this a physical or chemical change? Support or defend your answer.

Steam Water Ice Water is always H 2 O, it will change state as temperature changes but it is still H 2 O. Solid, liquid, or gas.

- Two types substances: Elements & Compounds

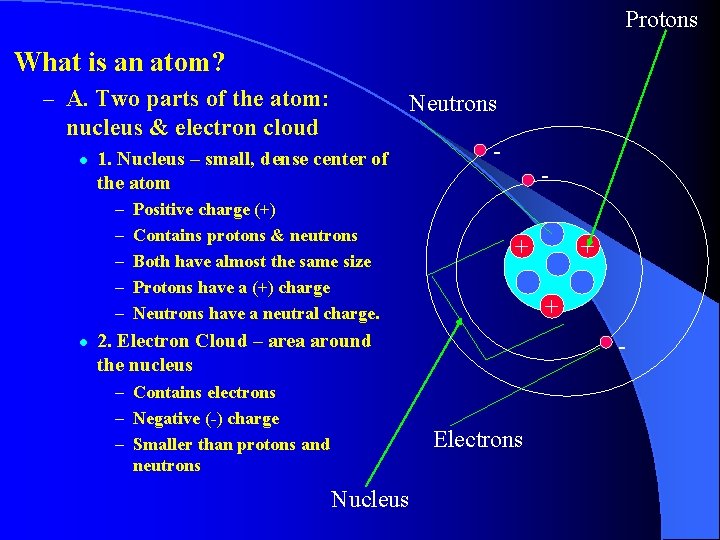
I. Atoms are the tiny units that determine the property of all materials. They are the building blocks of molecules. Atoms are the smallest part of an element that still has the element’s properties. An atom of gold is different from an atom of Oxygen.

Protons What is an atom? – A. Two parts of the atom: Neutrons nucleus & electron cloud l 1. Nucleus – small, dense center of the atom – – – l Positive charge (+) Contains protons & neutrons Both have almost the same size Protons have a (+) charge Neutrons have a neutral charge. - + + 2. Electron Cloud – area around the nucleus – Contains electrons – Negative (-) charge – Smaller than protons and neutrons - Electrons Nucleus +

B. Atoms have no overall charge. Equal number of protons and electrons Equal number of (+) and (-); they cancel each other out.

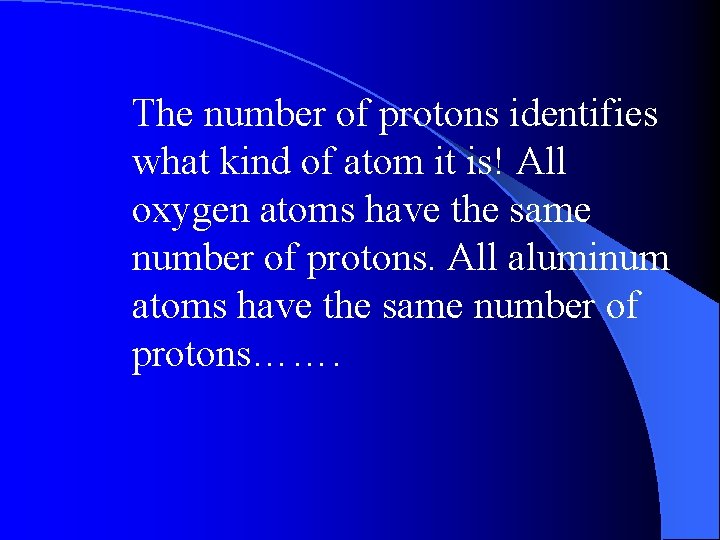
The number of protons identifies what kind of atom it is! All oxygen atoms have the same number of protons. All aluminum atoms have the same number of protons…….

a. Element – a substance that cannot be broken down into simpler substances. An element is the simplest substance. - Symbols are used to represent names. O oxygen, He helium - 1 st letter capital; 2 nd letter lower case if there is one - Identified by its specific physical and chemical properties

• When atoms combine they form a chemical bond. The bond is a “force of attraction”. • Atoms combine to form molecules.

b. Compound – substance made of atoms of more than one element bound together in a specific ratio. (2 or more elements combine chemically to make a compound) H 2 O, water, is always 2 Hydrogen atoms and one Oxygen atom.

l Combine chemically – New compound has totally different properties than the original elements. 1. Oxygen and Hydrogen are gasses but water H 2 O is a liquid – Always combine in a specific ratio 1. Ex: H 2 O = 2 H’s (hydrogen) 1 O (oxygen) Water is always H 2 O

2. Mixture – combination of more than one pure substance (element, compound, or both) which are not chemically combined but are together in the same place. . - combines physically - each part keeps its own identity - combines in any ratio

Two types of mixtures: 1. heterogeneous hetero = different 2. homogeneous homo = the same

l a. Heterogeneous mixture – can see the different parts that make it up; nonuniform; Ex: vegetable soup, salads l b. Homogeneous mixture- cannot see the different parts that make it up; uniform; also called a solution (solid, liquid or gas) l Air, brass, plastic, crystal light Solute – substance being dissolved (usually in the smallest amount) l Solvent – substance doing the dissolving (usually in the largest amt) l Solution – all of it together l

l E. Properties of Matter – 4 types 1. Chemical Property – the way a substance reacts with others to form new substances with different properties. a. describe how a substance reacts b. involve the reactivity of elements or compounds c. flammability, combustibility

l 2. Physical Propertycharacteristic of a substance that can be observed or measured without changing the composition of the substance. a. can be observed with senses l b. shape, color, odor, melting point, freezing point, density l c. properties remain constant for pure substances l

l 3. Chemical Change – a change that occurs when a substance changes composition by forming one or more new substances Fruits/vegetables ripen or rot l Food being digested l Rusting metal l Rotting wood l Two elements combine chemically; silver tarnishing l Burning paper l

l 4. Physical Changes – a change in the physical form/properties of a substance that occurs without a change in composition. Changing the state does not change the composition or mass l Ex: cutting a piece of paper l

Law of Conservation of Mass cannot be created or destroyed; but matter can change from one form to another l Phase changesl – a. freezing – liquid to a solid; water to ice – b. melting – solid to a liquid; ice to water – c. evaporation – liquid to a gas; water to moisture in air – d. condensation – gas to a liquid; liquid on side of a glass, dew on plants – e. sublimation – solid directly to a gas; dry ice, moth balls, candles
 Extensive vs intensive quantity
Extensive vs intensive quantity Physical property and chemical property
Physical property and chemical property 2 properties of matter
2 properties of matter Properties of matter objectives
Properties of matter objectives Measurable properties of matter
Measurable properties of matter General properties of solids
General properties of solids Useful materials at home and ways to use
Useful materials at home and ways to use Properties of liquids
Properties of liquids Matter and materials grade 7
Matter and materials grade 7 Properties used to describe matter
Properties used to describe matter Matter and its properties
Matter and its properties Physical properties of materials grade 7
Physical properties of materials grade 7 Properties of and changes in matter grade 5
Properties of and changes in matter grade 5 General properties of matter
General properties of matter Name two categories used to classify properties of matter
Name two categories used to classify properties of matter Electrical conductivity property of matter
Electrical conductivity property of matter States of matter jeopardy
States of matter jeopardy Chapter 2 properties of matter answer key
Chapter 2 properties of matter answer key Properties of matterwhat is matter?
Properties of matterwhat is matter? Properties of matter vocabulary
Properties of matter vocabulary Properties and characteristics of matter
Properties and characteristics of matter Study of the composition structure and properties
Study of the composition structure and properties Intrinsic physical property
Intrinsic physical property Graphic organizer for matter
Graphic organizer for matter Qualitative physical properties
Qualitative physical properties Properties of water in matter
Properties of water in matter Classification and properties of matter
Classification and properties of matter Matter-properties and changes answer key
Matter-properties and changes answer key Matter waves definition
Matter waves definition Properties of matter jeopardy
Properties of matter jeopardy Bamboo basket properties of matter
Bamboo basket properties of matter General property of matter
General property of matter Concept map about matter
Concept map about matter What are chemical properties of matter
What are chemical properties of matter Electrical properties of matter
Electrical properties of matter Physical properties of notebook paper
Physical properties of notebook paper Physical properties of matter jeopardy
Physical properties of matter jeopardy