CHARACTERISTIC INTRINSIC PROPERTIES Atomic Chemistry Physical Properties Can











- Slides: 21

CHARACTERISTIC INTRINSIC PROPERTIES Atomic Chemistry

Physical Properties • Can be observed or measured without changing the make -up of the matter of the object • Are used to describe the object • Two categories: • non-characterisic • characteristic

Physical Properties • Examples Include: • Appearance • Texture • Color • Odor • Melting Point • Boiling Point • Density • Solubility

Non-Characteristic Properties (NCP) • A physical or chemical property that is NOT unique to one particular substance. • Can be used to describe MANY substances

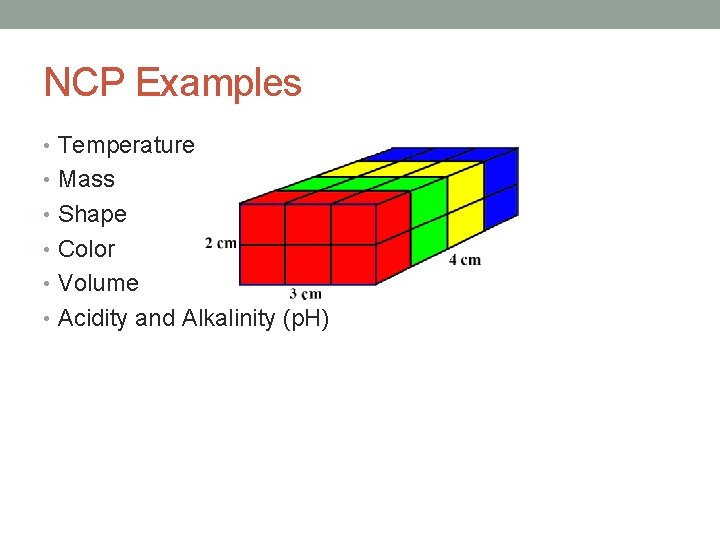
NCP Examples • Temperature • Mass • Shape • Color • Volume • Acidity and Alkalinity (p. H)

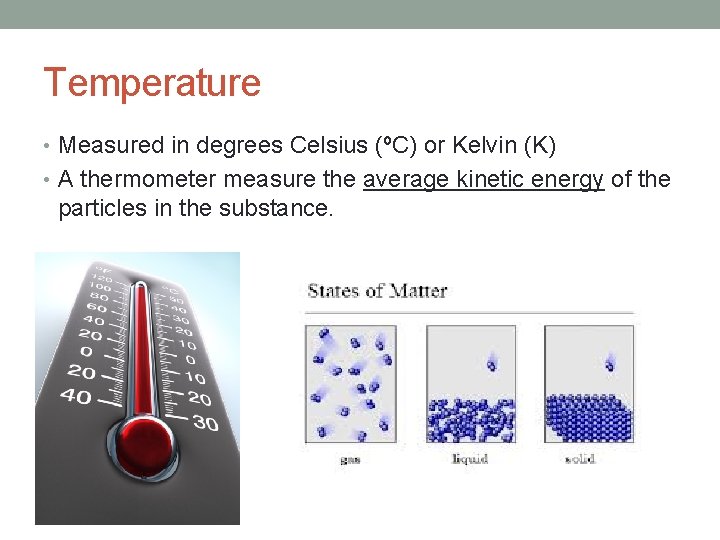
Temperature • Measured in degrees Celsius (ºC) or Kelvin (K) • A thermometer measure the average kinetic energy of the particles in the substance.

Mass

Shape and Color

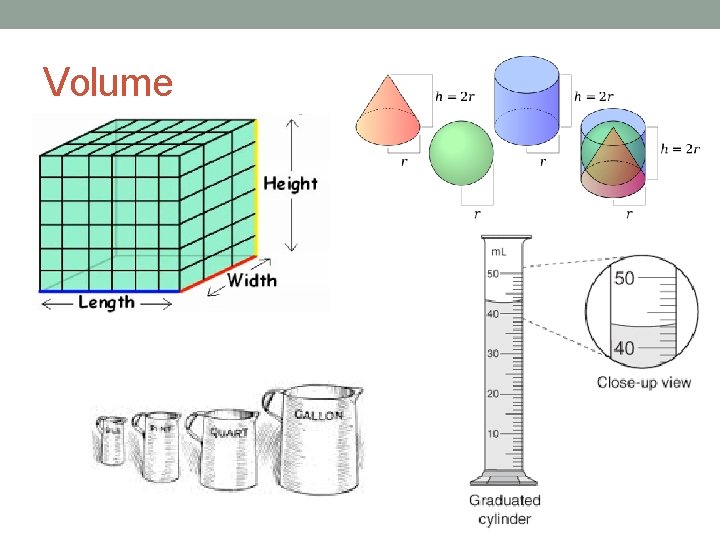
Volume

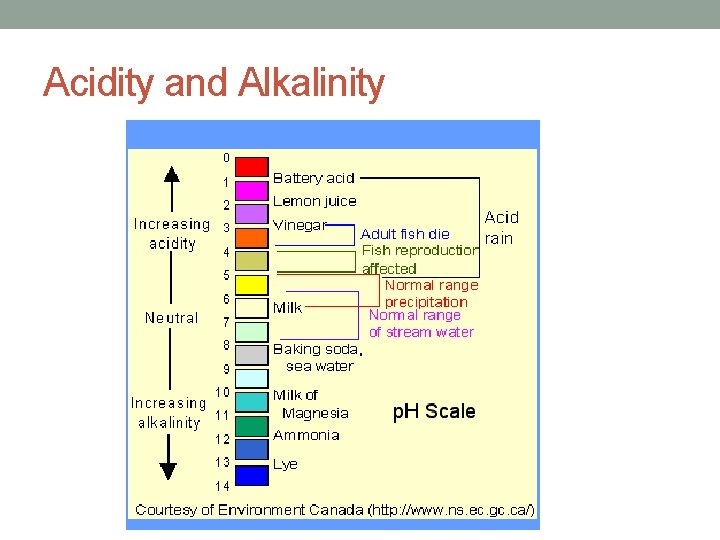
Acidity and Alkalinity

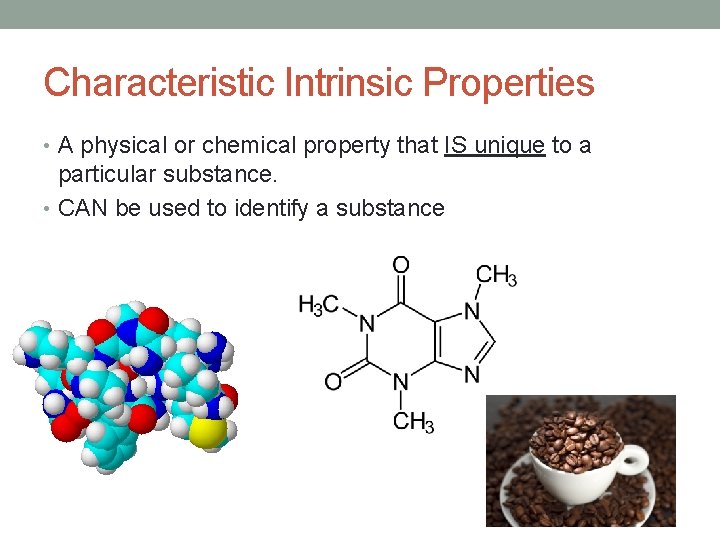
Characteristic Intrinsic Properties • A physical or chemical property that IS unique to a particular substance. • CAN be used to identify a substance

CP Examples • Density: the ratio of mass to volume (how much matter is in a given space) • Magnetism: The force of attraction between a magnet and a magnetic object • Solubility: How well a substance can dissolve in another substance. • Melting Point: Temperature at which substance changes from solid to liquid • Boiling Point: Temperature at which substance changes from liquid to gas

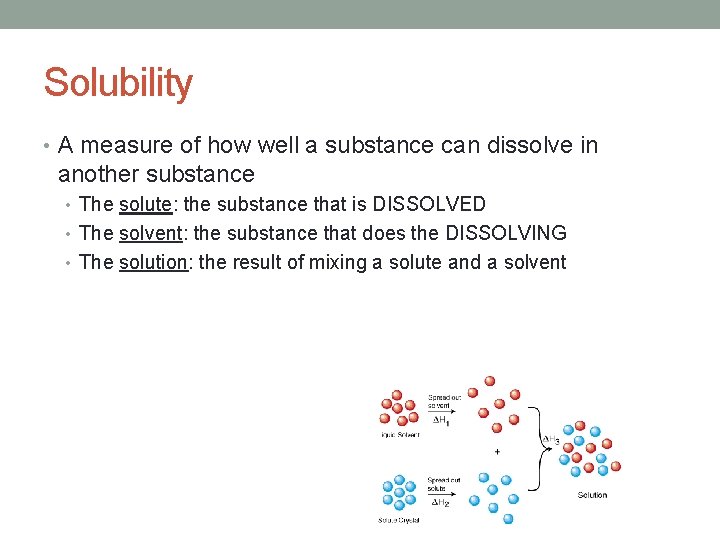
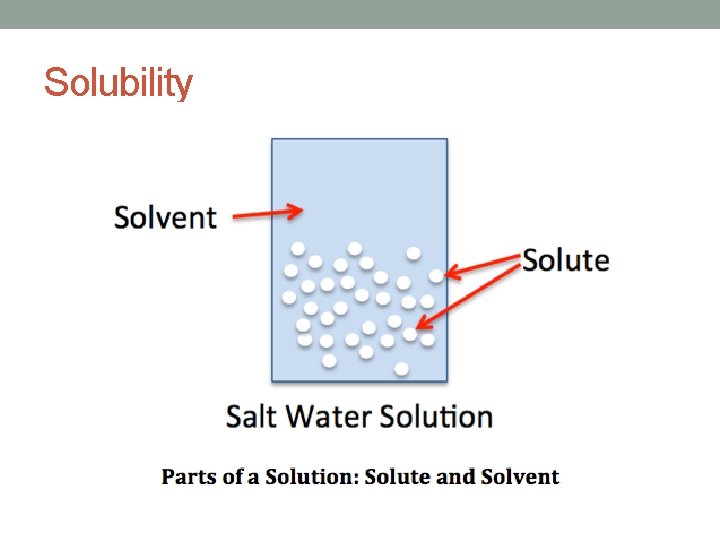
Solubility • A measure of how well a substance can dissolve in another substance • The solute: the substance that is DISSOLVED • The solvent: the substance that does the DISSOLVING • The solution: the result of mixing a solute and a solvent

Solubility

Melting & Boiling Points • Freezing point of H 2 O: 0ºC • Liquid water will freeze to solid ice at 0ºC • Melting point of ice: 0ºC • Solid ice will melt to liquid water at 0ºC • Boiling Point of H 2 O: 100ºC • Liquid water will change into gas at 100ºC

Example • The English Oak • Characteristic Property • Density = 720 kg/m 3 • NCP • Light yellow to brown in color • 25 -30 meters tall

Example #1 • Which of the following properties is an example of a characteristic property? 1. 2. 3. 4. Mass of 346 g Red in color and circular in shape Temperature of 25ºC Boiling Point of 204ºC

Example 2 • Identify ALL of the NCP below: 1. Volume of 200 m. L 2. Square shaped 3. Temperature of 45ºC 4. Freezing point of 204ºC 5. Density of 24 g/m. L

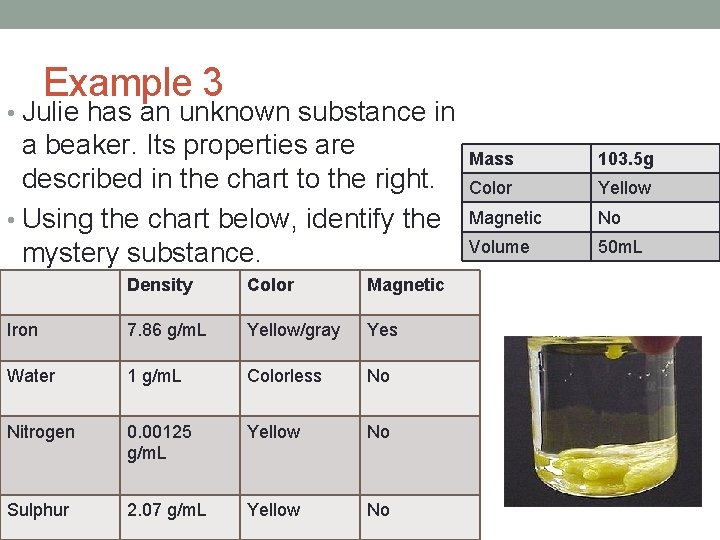
Example 3 • Julie has an unknown substance in a beaker. Its properties are described in the chart to the right. • Using the chart below, identify the mystery substance. Density Color Magnetic Iron 7. 86 g/m. L Yellow/gray Yes Water 1 g/m. L Colorless No Nitrogen 0. 00125 g/m. L Yellow No Sulphur 2. 07 g/m. L Yellow No Mass 103. 5 g Color Yellow Magnetic No Volume 50 m. L


What is the Atomic Theory? • Click here for video!
 Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Intrinsic properties of matter
Intrinsic properties of matter Intrinsic property of nucleus
Intrinsic property of nucleus Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Periodic trends in the periodic table
Periodic trends in the periodic table Periodic trends
Periodic trends Atomic weight of oxygen
Atomic weight of oxygen Distinguish between mass number and atomic mass.
Distinguish between mass number and atomic mass. Atomic number vs atomic radius
Atomic number vs atomic radius Chemical property definition
Chemical property definition Property that can be observed or measured.
Property that can be observed or measured. First ionization energy of calcium
First ionization energy of calcium Ap chemistry chapter 7
Ap chemistry chapter 7 Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity First ionization energy definition ib
First ionization energy definition ib Any characteristic of a material that can be observed
Any characteristic of a material that can be observed Characteristic physical property
Characteristic physical property Examples of non characteristic properties
Examples of non characteristic properties What are measurable properties
What are measurable properties Pure substance examples pictures
Pure substance examples pictures Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry