Organic Chemistry Chapter 24 Copyright The Mc GrawHill


- Slides: 35

Organic Chemistry Chapter 24 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Common Elements in Organic Compounds 24. 1

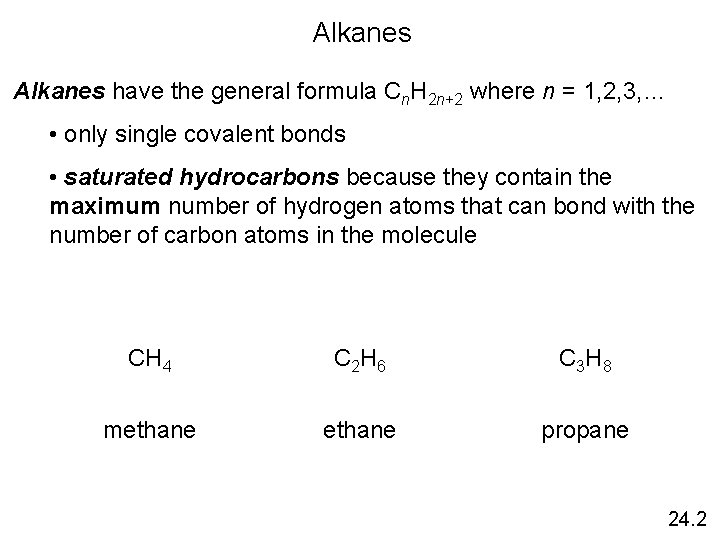
Classification of Hydrocarbons 24. 1

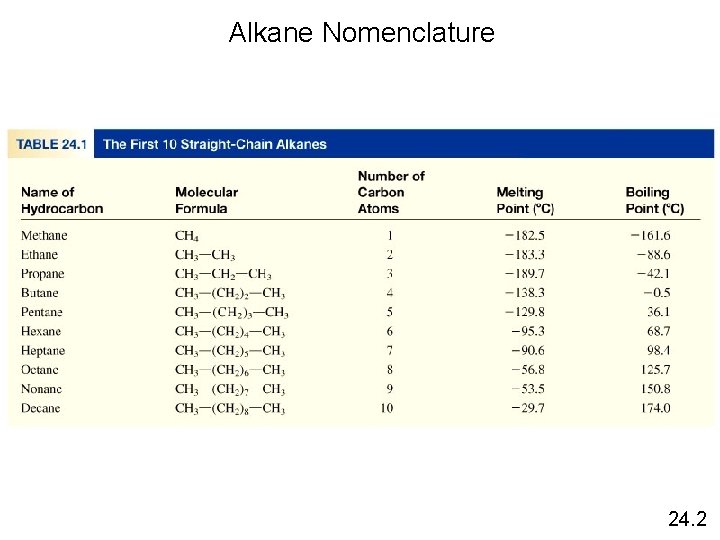
Alkanes have the general formula Cn. H 2 n+2 where n = 1, 2, 3, … • only single covalent bonds • saturated hydrocarbons because they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule CH 4 C 2 H 6 C 3 H 8 methane propane 24. 2

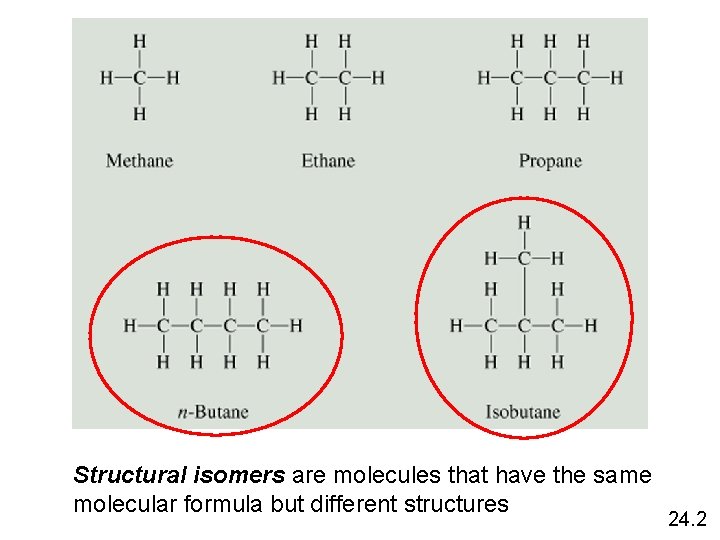
Structural isomers are molecules that have the same molecular formula but different structures 24. 2

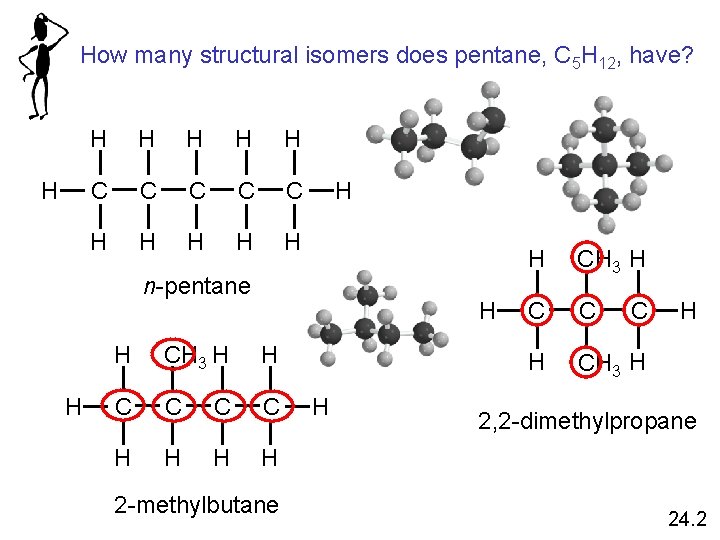
How many structural isomers does pentane, C 5 H 12, have? H H H C C C H H H n-pentane H H H CH 3 H H C C H H 2 -methylbutane H H CH 3 H C C H CH 3 H C H 2, 2 -dimethylpropane 24. 2

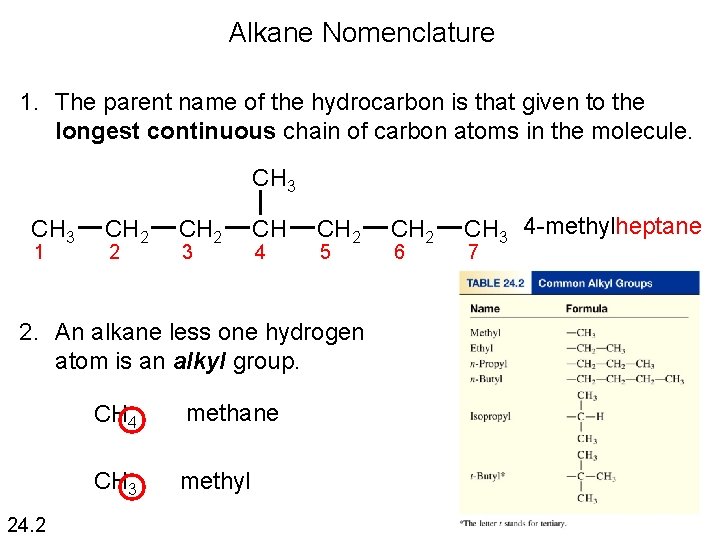
Alkane Nomenclature 1. The parent name of the hydrocarbon is that given to the longest continuous chain of carbon atoms in the molecule. CH 3 1 CH 2 2 CH 2 3 CH 4 CH 2 5 2. An alkane less one hydrogen atom is an alkyl group. 24. 2 CH 4 methane CH 3 methyl CH 2 6 CH 3 4 -methylheptane 7

Alkane Nomenclature 24. 2

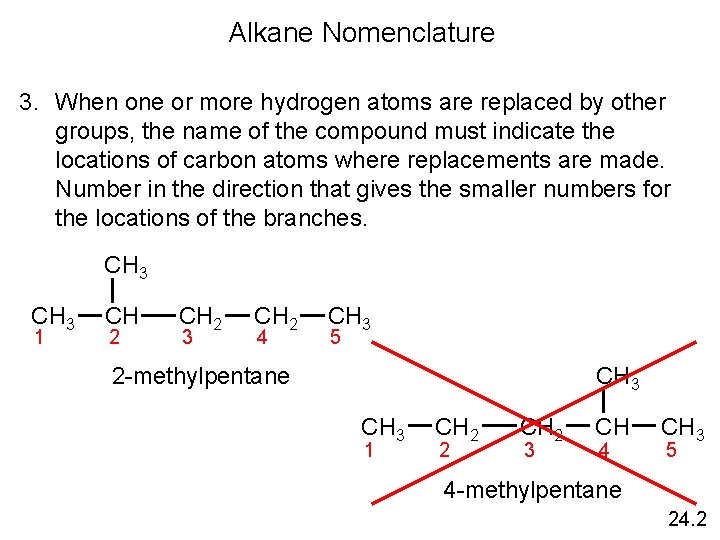
Alkane Nomenclature 3. When one or more hydrogen atoms are replaced by other groups, the name of the compound must indicate the locations of carbon atoms where replacements are made. Number in the direction that gives the smaller numbers for the locations of the branches. CH 3 1 CH 2 3 CH 2 4 CH 3 5 CH 3 2 -methylpentane CH 3 1 CH 2 2 CH 2 3 CH 4 CH 3 5 4 -methylpentane 24. 2

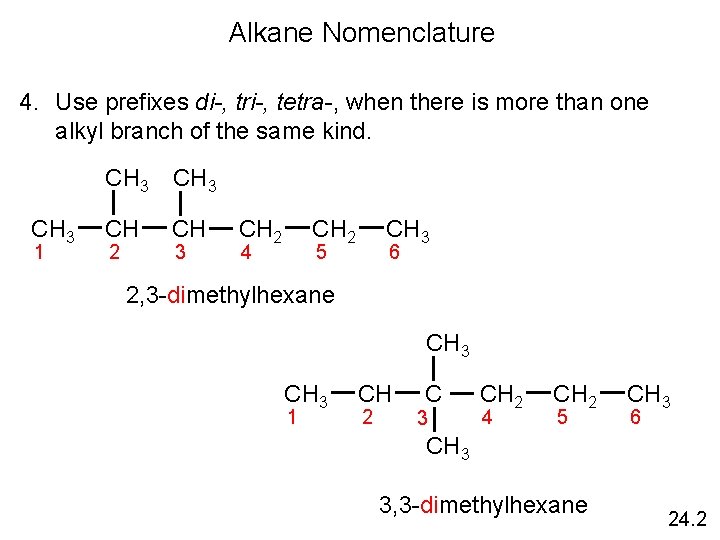
Alkane Nomenclature 4. Use prefixes di-, tri-, tetra-, when there is more than one alkyl branch of the same kind. CH 3 1 CH 3 CH CH 2 3 CH 2 4 CH 3 5 6 2, 3 -dimethylhexane CH 3 1 CH 2 C 3 CH 2 4 CH 2 5 CH 3 6 CH 3 3, 3 -dimethylhexane 24. 2

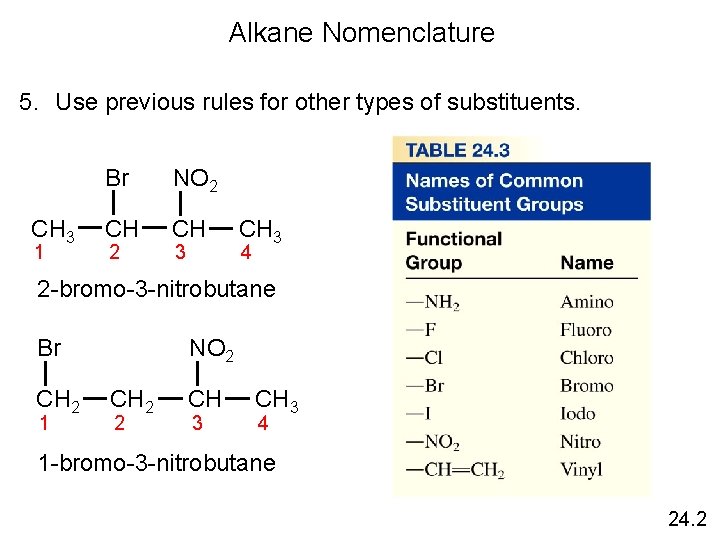
Alkane Nomenclature 5. Use previous rules for other types of substituents. CH 3 1 Br NO 2 CH CH 2 3 CH 3 4 2 -bromo-3 -nitrobutane Br CH 2 1 NO 2 CH 2 2 CH 3 4 1 -bromo-3 -nitrobutane 24. 2

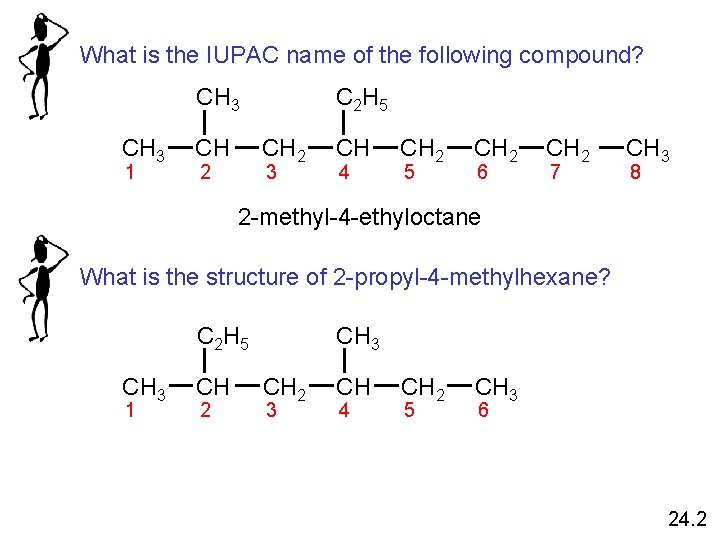
What is the IUPAC name of the following compound? CH 3 1 CH C 2 H 5 CH 2 2 3 CH 4 CH 2 5 CH 2 6 CH 2 7 CH 3 8 2 -methyl-4 -ethyloctane What is the structure of 2 -propyl-4 -methylhexane? C 2 H 5 CH 3 1 CH 2 CH 3 CH 2 3 CH 4 CH 2 5 CH 3 6 24. 2

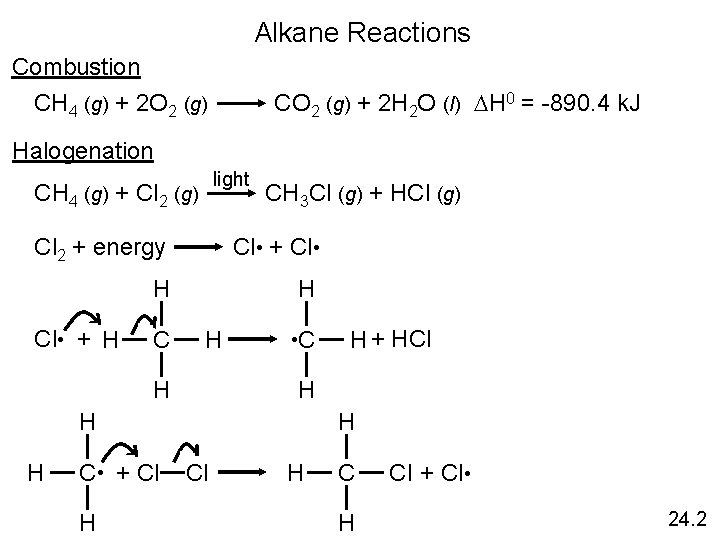
Alkane Reactions Combustion CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (l) DH 0 = -890. 4 k. J Halogenation light CH 4 (g) + Cl 2 (g) CH 3 Cl (g) + HCl (g) Cl 2 + energy Cl • + Cl • H Cl • + H C H H H • C H H H C • + Cl H H + HCl H H Cl + Cl • 24. 2

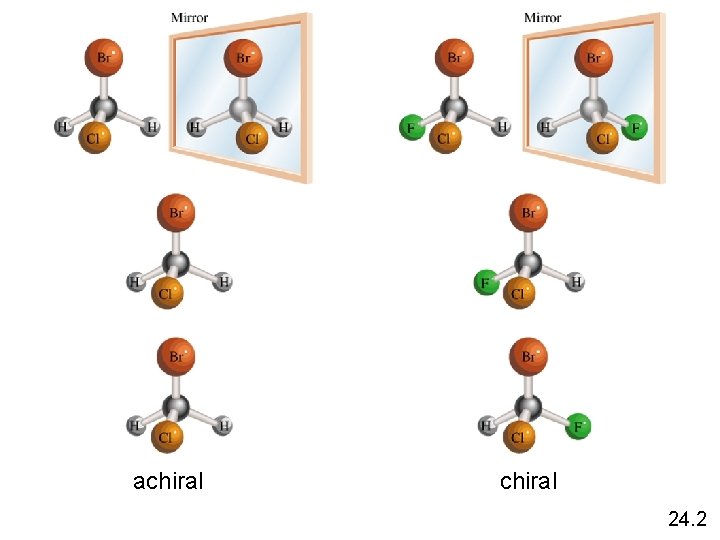
achiral 24. 2

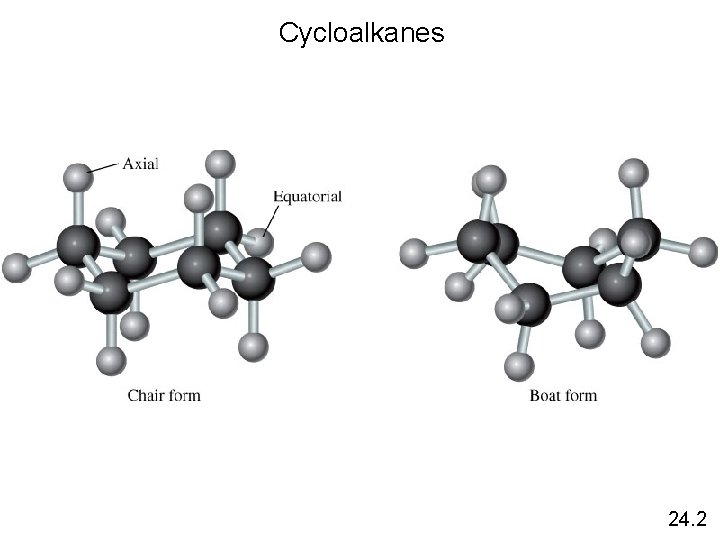
Cycloalkanes Alkanes whose carbon atoms are joined in rings are called cycloalkanes. They have the general formula Cn. H 2 n where n = 3, 4, … 24. 2

Cycloalkanes 24. 2

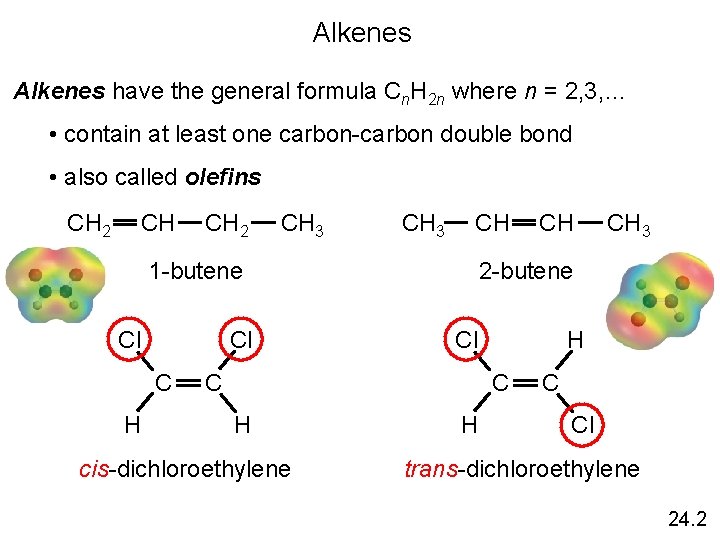
Alkenes have the general formula Cn. H 2 n where n = 2, 3, … • contain at least one carbon-carbon double bond • also called olefins CH CH 2 CH 3 CH 1 -butene Cl Cl C H Cl H C cis-dichloroethylene CH 3 2 -butene C H CH H C Cl trans-dichloroethylene 24. 2

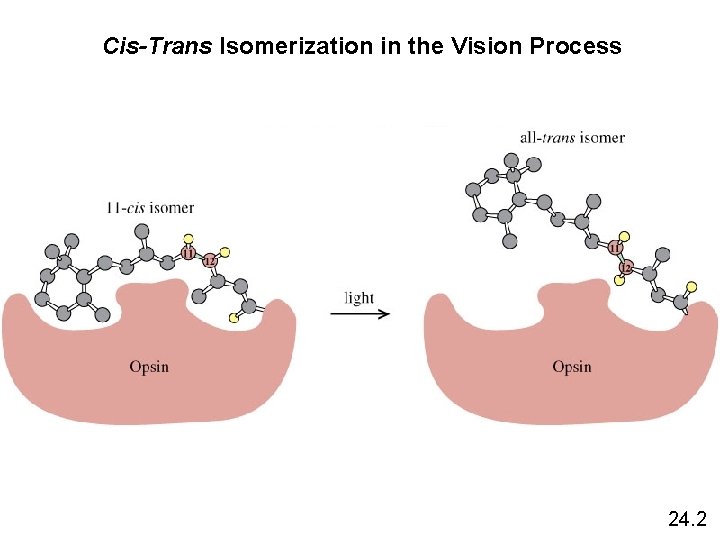
Cis-Trans Isomerization in the Vision Process 24. 2

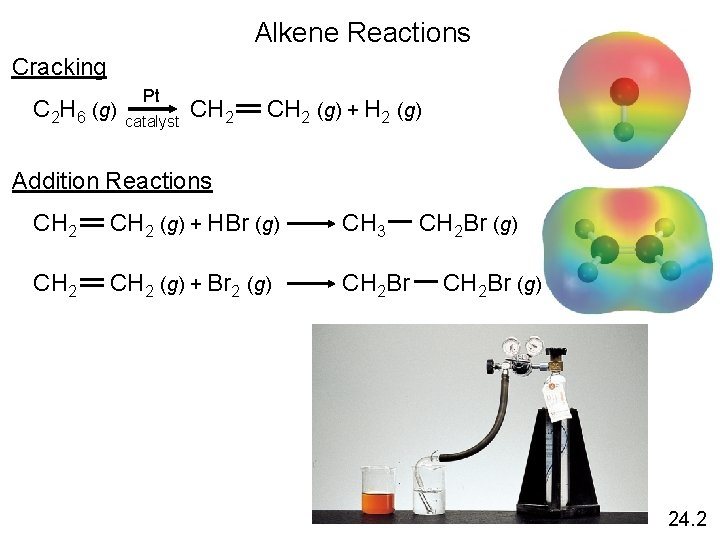
Alkene Reactions Cracking Pt C 2 H 6 (g) CH 2 (g) + H 2 (g) catalyst Addition Reactions CH 2 (g) + HBr (g) CH 3 CH 2 Br (g) CH 2 (g) + Br 2 (g) CH 2 Br (g) 24. 2

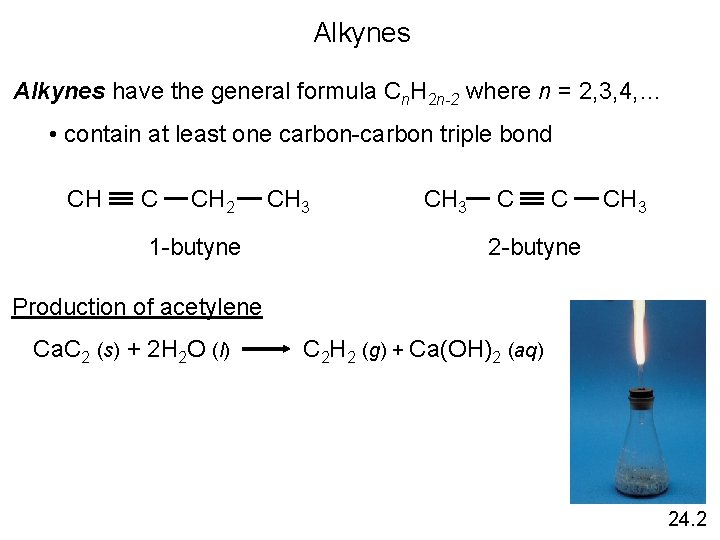
Alkynes have the general formula Cn. H 2 n-2 where n = 2, 3, 4, … • contain at least one carbon-carbon triple bond CH C CH 2 1 -butyne CH 3 C C CH 3 2 -butyne Production of acetylene Ca. C 2 (s) + 2 H 2 O (l) C 2 H 2 (g) + Ca(OH)2 (aq) 24. 2

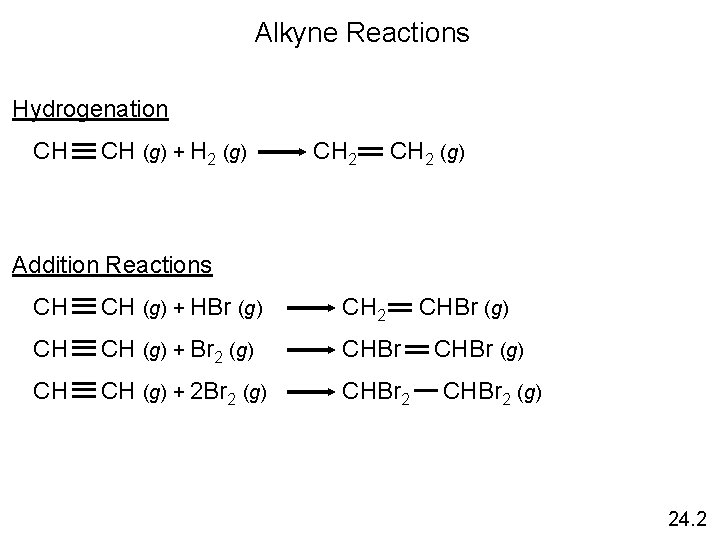
Alkyne Reactions Hydrogenation CH CH (g) + H 2 (g) CH 2 (g) Addition Reactions CH CH (g) + HBr (g) CH 2 CHBr (g) CH CH (g) + Br 2 (g) CHBr (g) CH CH (g) + 2 Br 2 (g) CHBr 2 (g) 24. 2

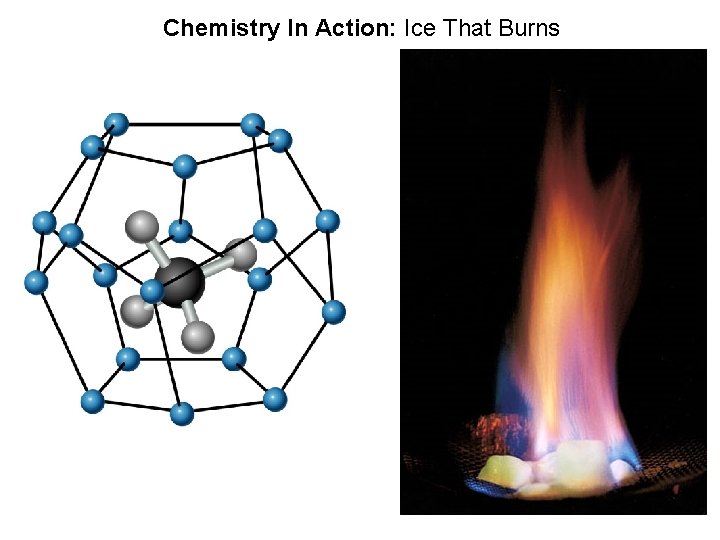
Chemistry In Action: Ice That Burns

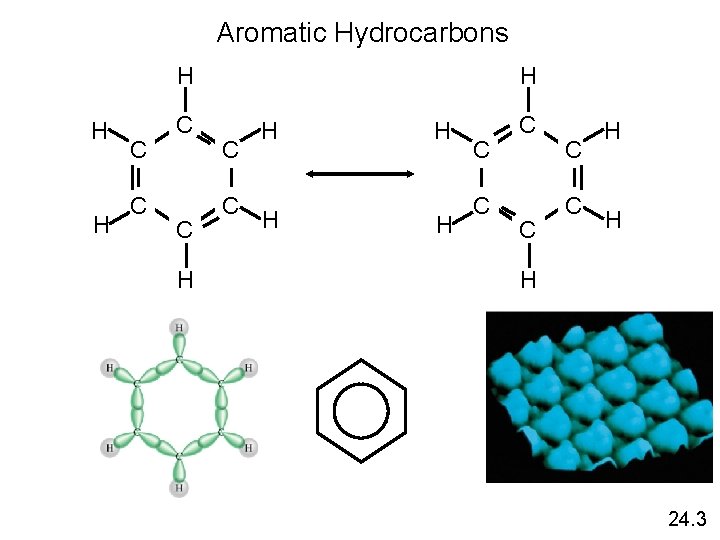
Aromatic Hydrocarbons H H C C H H C C C H H H 24. 3

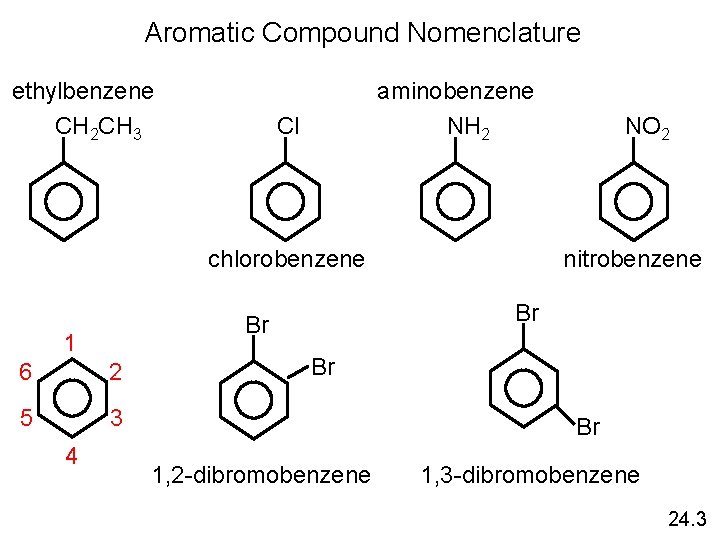
Aromatic Compound Nomenclature ethylbenzene CH 2 CH 3 aminobenzene NH 2 Cl chlorobenzene 6 2 5 3 4 nitrobenzene Br Br 1 NO 2 Br Br 1, 2 -dibromobenzene 1, 3 -dibromobenzene 24. 3

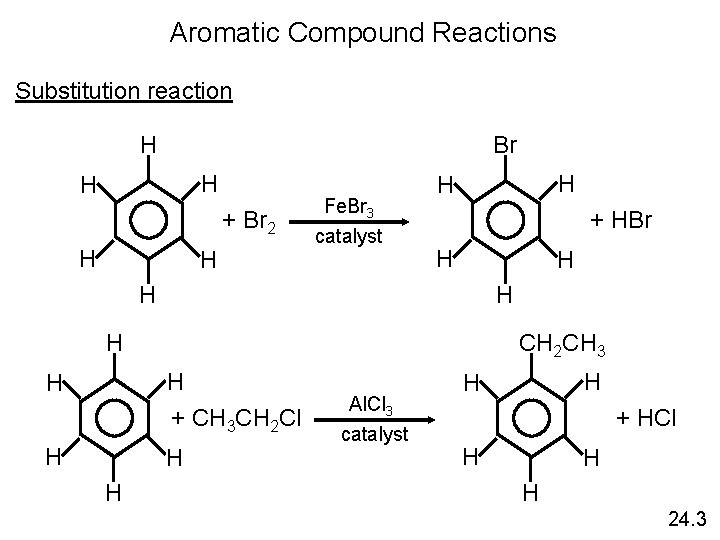
Aromatic Compound Reactions Substitution reaction H Br H H + Br 2 H H Fe. Br 3 catalyst H H + HBr H H H CH 2 CH 3 H + CH 3 CH 2 Cl H H Al. Cl 3 catalyst H H + HCl H H H 24. 3

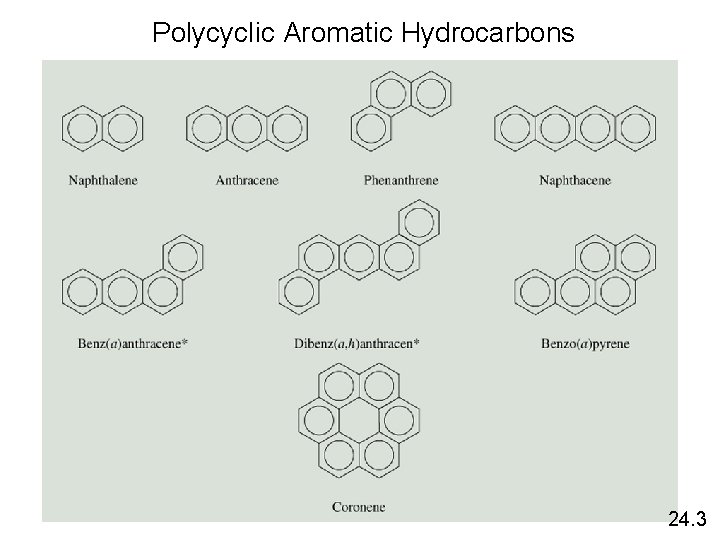
Polycyclic Aromatic Hydrocarbons 24. 3

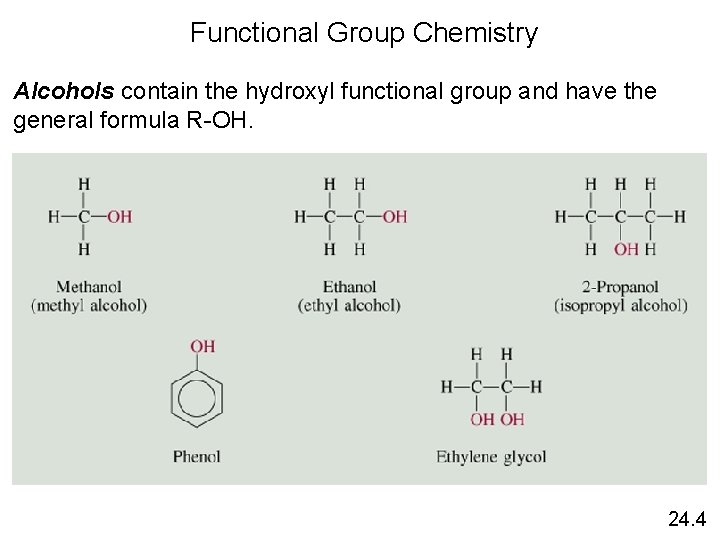
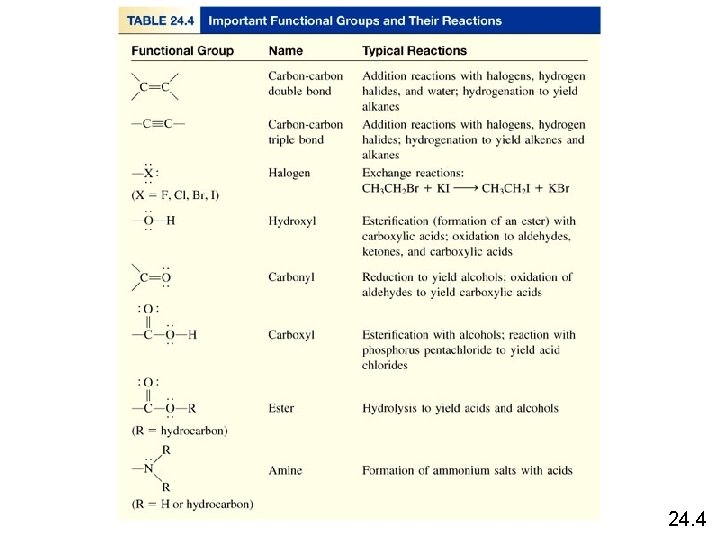
Functional Group Chemistry Alcohols contain the hydroxyl functional group and have the general formula R-OH. 24. 4

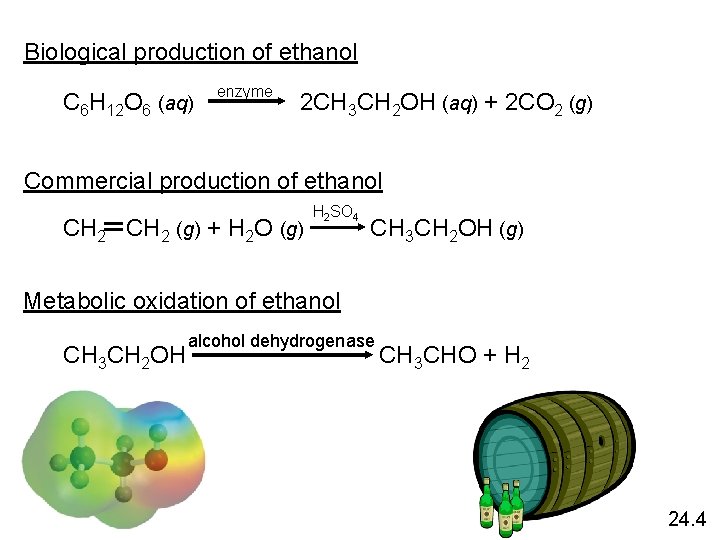
Biological production of ethanol enzyme C 6 H 12 O 6 (aq) 2 CH 3 CH 2 OH (aq) + 2 CO 2 (g) Commercial production of ethanol H 2 SO 4 CH 2 (g) + H 2 O (g) CH 3 CH 2 OH (g) Metabolic oxidation of ethanol alcohol dehydrogenase CH 3 CH 2 OH CH 3 CHO + H 2 24. 4

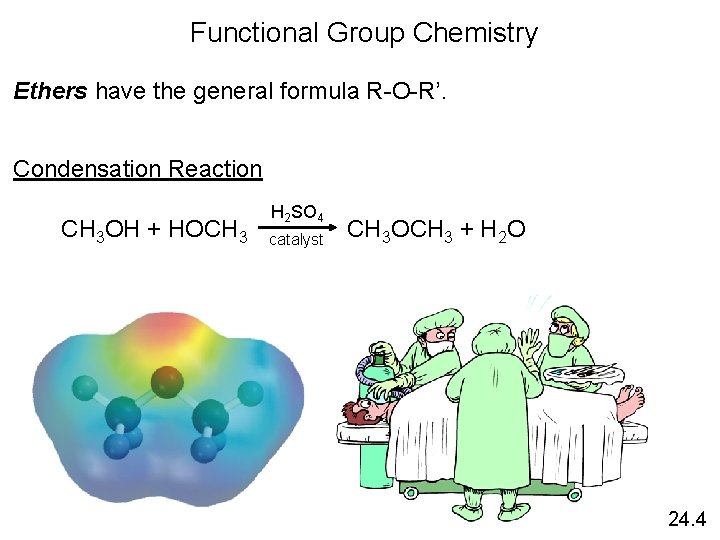
Functional Group Chemistry Ethers have the general formula R-O-R’. Condensation Reaction H 2 SO 4 CH 3 OH + HOCH 3 CH 3 OCH 3 + H 2 O catalyst 24. 4

Functional Group Chemistry O Aldehydes and ketones contain the carbonyl ( ) C functional group. O • aldehydes have the general formula R C H O • ketones have the general formula R C R’ O O O H C H H C CH 3 H 3 C C CH 3 formaldehyde acetone 24. 4

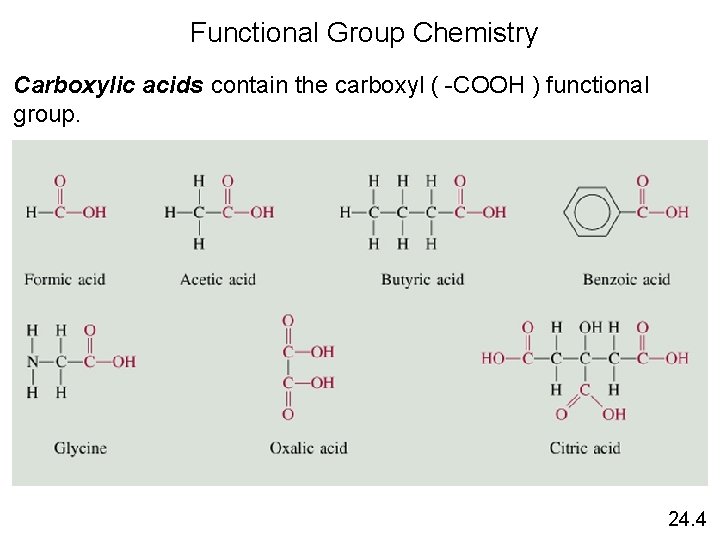
Functional Group Chemistry Carboxylic acids contain the carboxyl ( -COOH ) functional group. 24. 4

Functional Group Chemistry Esters have the general formula R’COOR, where R is a hydrocarbon group. O CH 3 COOH + HOCH 2 CH 3 C O CH 2 CH 3 + H 2 O ethyl acetate 24. 4

Functional Group Chemistry Amines are organic bases with the general formula R 3 N. CH 3 NH 2 + H 2 O RNH 3+ + OH- CH 3 CH 2 NH 2 + HCl CH 3 CH 2 NH 3+Cl- 24. 4

24. 4

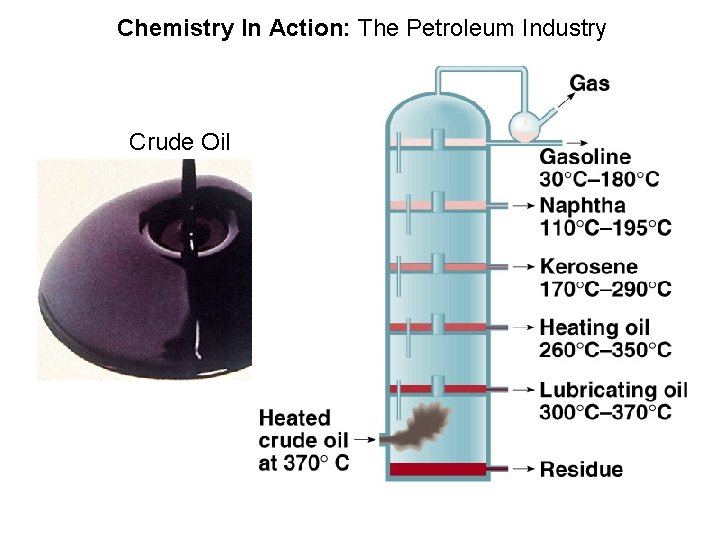
Chemistry In Action: The Petroleum Industry Crude Oil
 Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Organic chemistry chapter 1
Organic chemistry chapter 1 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 chemistry review
Chapter 7 chemistry review Nonene
Nonene Analytical chemistry chapter 1
Analytical chemistry chapter 1 Halohydrin formation
Halohydrin formation Grawhill
Grawhill Grawhill
Grawhill Grawhill
Grawhill Grawhill
Grawhill Mc grawhill
Mc grawhill Single user multitasking os
Single user multitasking os Grawhill
Grawhill Mc grawhill
Mc grawhill Grawhill
Grawhill Cycloalkanes
Cycloalkanes Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry Displayed formula
Displayed formula Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Is alkane an organic compound
Is alkane an organic compound What is the leveling effect organic chemistry
What is the leveling effect organic chemistry Oxo functional group
Oxo functional group Organic chemistry lab report example
Organic chemistry lab report example Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis