Matter Please dont start yet Anything that has















- Slides: 28

Matter Please don’t start … yet! Anything that has mass and takes up space (volume)

There are 5 Physical States or phases of Matter • Bose-Einstein Condensate (BEC) • Solid • Liquid • Gas • Plasma

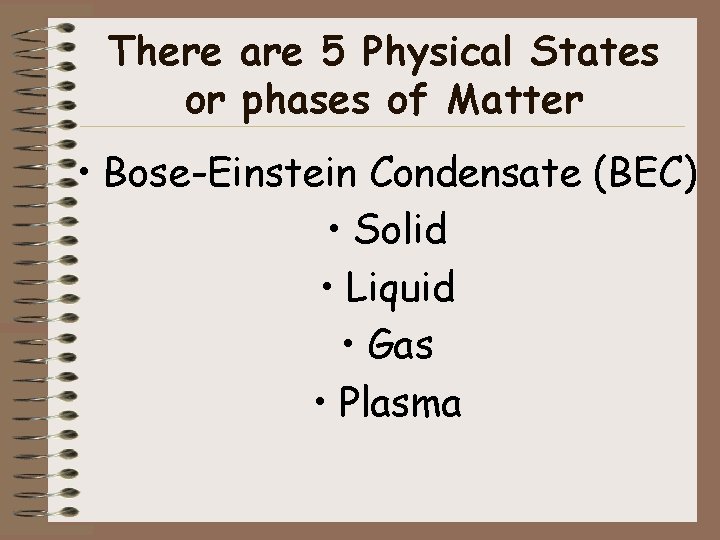
Solid • Particles are tightly compact • Particles vibrate without the ability to move freely • Definite shape and volume • • Copy and paste interactive into chrome: http: //www. footprintsscience. com/index. php? module=2&type=State s of matter§ion=Section 1&info=7

‘Solid’ Animation 1. Did you watch and interact with the animation? So what was the graph showing? 2. What do the liquid particles do that the solid particles can’t? 3. Explain why this can happen? 4. What else should you write down from the animation about a solid?

Self and Teacher check (SOLID) • Have you made notes on BOTH organizers? (NO … go back to the beginning!) • Have you answered ALL the questions on the previous slide? (NO … do so!) • If you’re reading this … put your hand up to get your teacher’s permission to move onto liquids • In order to get permission you’ll need to show what you’ve found out about solids so far.

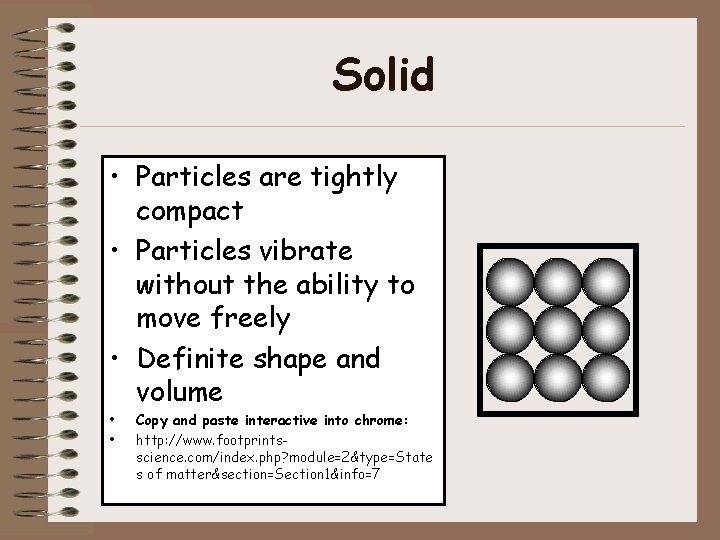
Liquid • Particles are tightly compact, but able to move around close to each other • No definite shape, but definite volume • • Same interactive as solids: http: //www. footprintsscience. com/index. php? module=2&type =States of matter§ion=Section 1&info=7

‘Liquid’ Animation 1. So which state or phase has the most ‘organized’ particles, solid or liquid? 2. Can liquid particles move? Describe the movement. 3. What else should you write down from the animation about a liquid?

Self and Teacher check (LIQUID) • Have you made notes on BOTH organizers? (NO … go back to the beginning of liquids!) • Have you answered ALL the questions on the previous slide? (NO … do so!) • If you’re reading this … put your hand up to get your teacher’s permission to move onto gases. • In order to get permission you’ll need to show what you’ve found out about liquids so far.

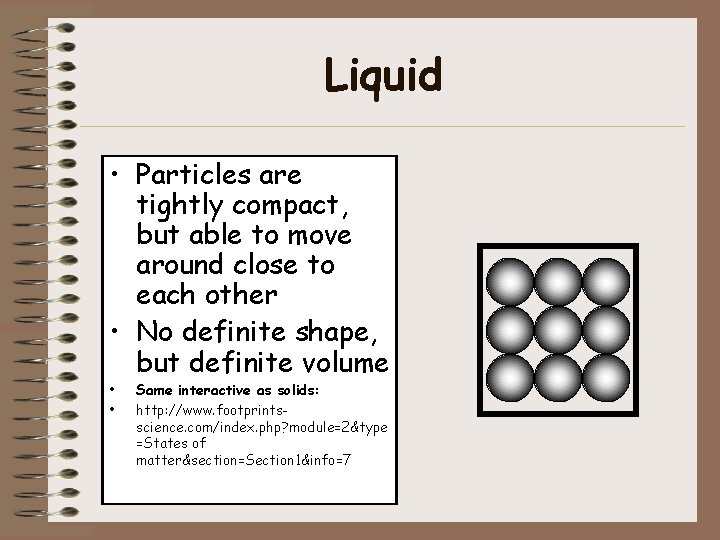
Gas • Particles can easily spread out or move close together • Particles move freely and with a lot of energy • No definite shape or volume • • Same interactive as solids: http: //www. footprintsscience. com/index. php? module=2&type =States of matter§ion=Section 1&info=7

‘Gas’ Animation 1. What happens as the temperature increases? 2. Why do you think there is a flat line on the graph between a liquid and a gas? 3. What does it mean?

Self and Teacher check (GAS) • Have you made notes BOTH organizers? (NO … go back to the beginning of gases!) • Have you answered ALL the questions on the previous slide? (NO … do so!) • If you’re reading this … put your hand up to get your teacher’s permission to move onto Plasma and BEC • In order to get permission you’ll need to show what you’ve found out about gases so far.

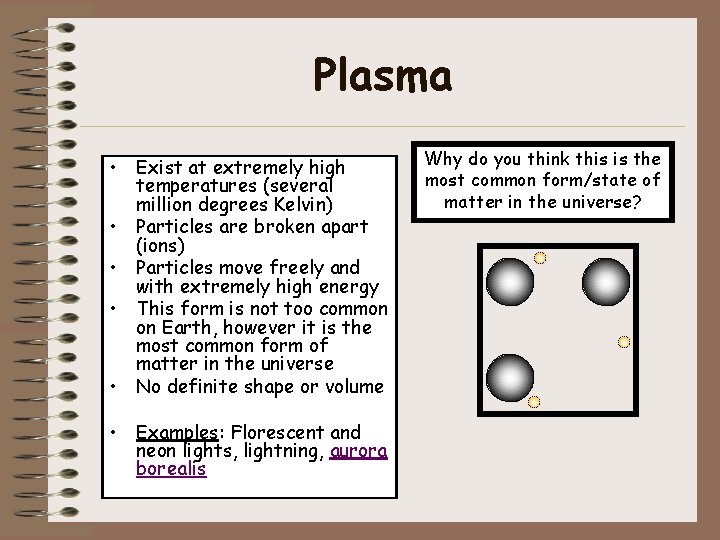
Plasma • Exist at extremely high temperatures (several million degrees Kelvin) • Particles are broken apart (ions) • Particles move freely and with extremely high energy • This form is not too common on Earth, however it is the most common form of matter in the universe • No definite shape or volume • Examples: Florescent and neon lights, lightning, aurora borealis Why do you think this is the most common form/state of matter in the universe?

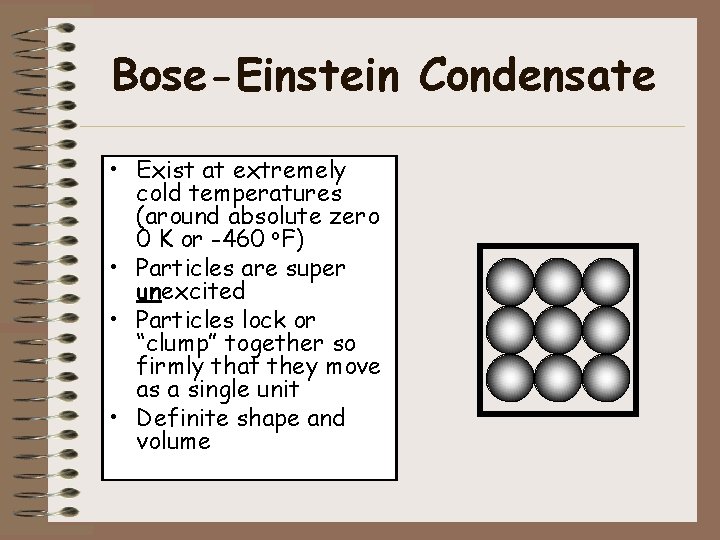
Bose-Einstein Condensate • Exist at extremely cold temperatures (around absolute zero 0 K or -460 o. F) • Particles are super unexcited • Particles lock or “clump” together so firmly that they move as a single unit • Definite shape and volume

Plasma and Bose-Einstein Condensate 1. What is plasma? 2. Why is not just gas? 3. What is Bose-Einstein Condensate? 4. When does it occur?

Self and Teacher check (Plasma and BEC) • Have you made notes on BOTH organizers? (NO … go back to the beginning of Plasma!) • Have you answered ALL the questions on the previous slide? (NO … do so!) • If you’re reading this … put your hand up to get your teacher’s permission to move onto matter continuum. • In order to get permission you’ll need to show what you’ve found out about Plasma and BEC so far.

Energy and the States or Phases of Matter • The physical states of matter result from the amount of energy the particles composing the matter have. Basically, more energy means more movement for the particles and less energy means less movement. If you were to compare an ice cube and the steam created from boiling water, which would you think has more energy?

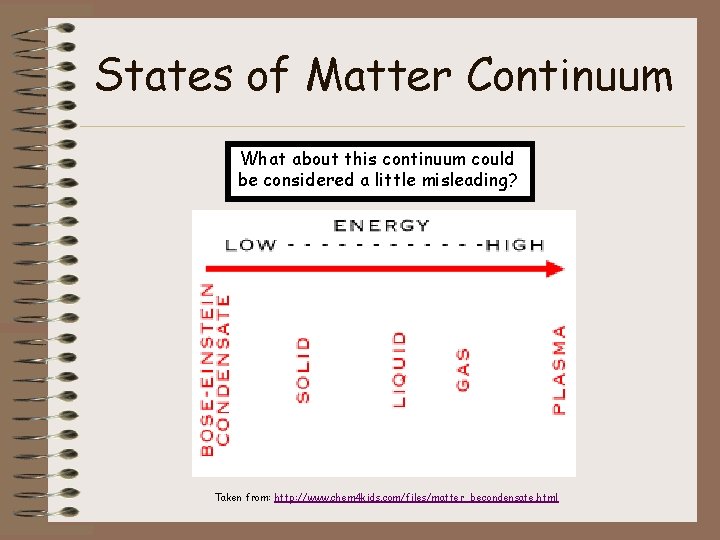
States of Matter Continuum What about this continuum could be considered a little misleading? Taken from: http: //www. chem 4 kids. com/files/matter_becondensate. html

States of Matter Continuum • Add to your organizers once you’ve interacted with the website below. • States of matter continuum interactive • http: //www. pbs. org/wgbh/nova/physics/states-of-matter. html 1. How can a gas be changed into a liquid? 2. Which state has the most energy? 3. What happens to the water molecules when you heat them up? 4. What about when you increase the

Self and Teacher check (States of Matter Continuum) • Have you made notes on BOTH organizers? (NO … go back to the beginning of the continuum!) • Have you answered ALL the questions on the previous slide? (NO … do so!) • If you’re reading this … put your hand up to get your teacher’s permission to move onto changes in matter. • In order to get permission you’ll need to show what you’ve found out about matter continuum so far.

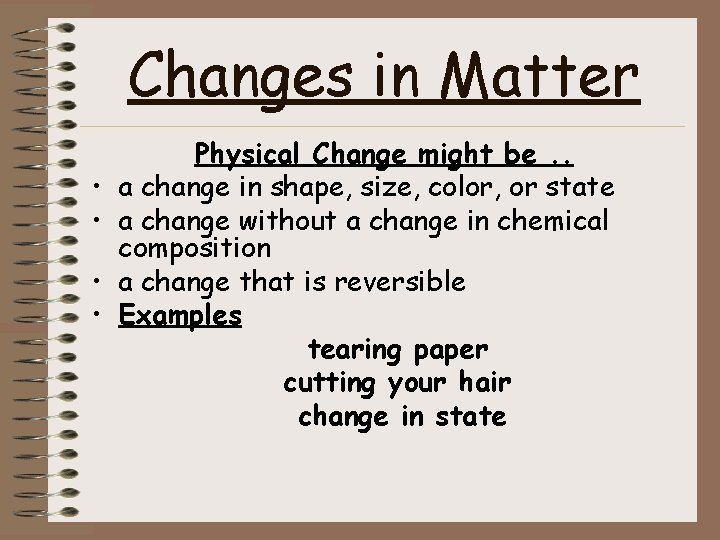
Changes in Matter • • Physical Change might be. . a change in shape, size, color, or state a change without a change in chemical composition a change that is reversible Examples tearing paper cutting your hair change in state

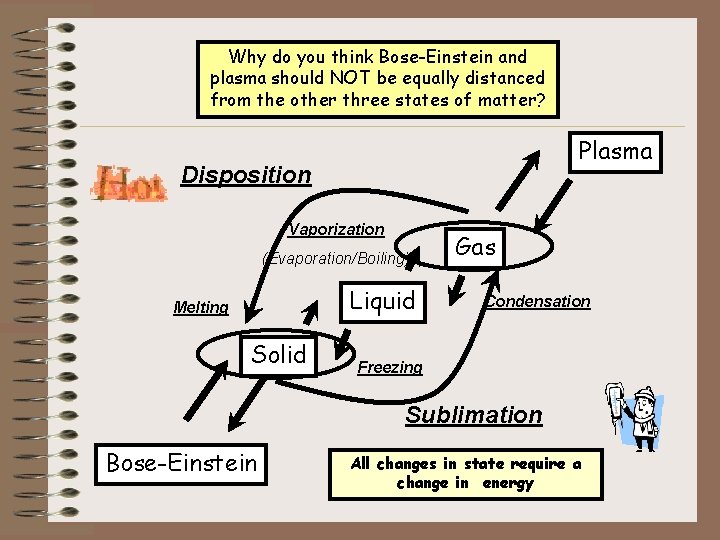
Why do you think Bose-Einstein and Changes in States plasma should NOT be equally distanced from the(Physical other three. Changes) states of matter? Plasma Disposition Vaporization (Evaporation/Boiling) Liquid Melting Solid Gas Condensation Freezing Sublimation Bose-Einstein All changes in state require a change in energy

Changes in state or phase 1. What’s the difference between evaporation and condensation? 2. What is needed to change state or phase? 3. What’s the answer to the yellow ‘boxed’ question on the previous slide?

Self and Teacher check (Changes in state or phase) • Have you made notes? (NO … go back to the beginning!) • Have you answered ALL the questions? (NO … do so!) • If you’re reading this … put your hand up to get your teacher’s permission to move onto the summary • In order to get permission you’ll need to show what you’ve found out about changes in state so far.

States of Matter Summary I States or phases of Matter Summary I https: //authoring. concord. org/activities/1074/single_page/f 3 b 2 bc 43 -1 eee-4114 -955305 f 784847929 1. Complete the interactive 2. What state of matter has strong attractions between atoms? 3. What state of matter will have weak attractions at room temperature?

States of Matter Summary II States or phases of Matter Changes Summary II http: //www. chem 4 kids. com/files/matter_changes. html This is what happens when energy is added and taken away

States of Matter Summary II 1. How can oxygen be made into a solid? (two ways) 2. What are freezing and melting points? Are they the same? 3. What is sublimation? 4. Why does adding energy help change the state of matter? 5. What was on part II?

States or phases of Matter • What other information can you find out from the summary information that you didn’t have notes about already? • Now watch the video tutorial and add any further notes to your organizers. • States of matter video tutorial • https: //www. dropbox. com/s/tuvg 4 k 0 rzv 14 r 6 z/lesson 4 states of matter. mp 4? dl=0

Self and Teacher check (Changes in state SUMMARY) • Have you made notes? (NO … go back to the beginning of the summary!) • Have you answered ALL the questions? (NO … do so!) • If you’re reading this … put your hand up to get your teacher’s permission to complete the organizer. • In order to get permission you’ll need to show what other information you’ve found out.