Matter What is matter Anything that has mass

















- Slides: 23

Matter

What is matter? Anything that has mass and takes up space Please give me some examples of matter in our room:

Properties of Matter MASS = The amount of matter that makes up that object › Does not depend on shape of object VOLUME = amount of space something takes up › Depends on its shape › Measure in cm 3 › Length x width x height

Properties of Matter Would the mass of a clay ball change if the clay is spread into the shape of a pancake? A. Yes B. No What is the volume of a block of wood that measures 1 cm by 4 cm by 2 cm? A. B. C. D. 1 cm 3 7 cm 3 8 cm 3 10 cm 3

Properties of Matter WEIGHT = the measure of the pull of gravity on an object Will vary if pull of gravity changes Weight on Earth is 6 times greater than on the moon DENSITY = measure of the amount of matter in a given space Mass per unit of volume mass Density = volume An object with a mass of 30 -grams and volume of 15 cm 3 has a density of ? ?

Properties of Matter You have two blocks of metal that are the same size. Their densities are 9 g/cm 3 and 9. 5 g/cm 3? Are they the same metal? A. Yes B. No A block of a substance is 2 cm by 1 cm by 5 cm. It has a mass of 6. 8 -grams. What is it’s density and what is it? (use table on p. 369) A. B. C. D. Rubber - 1. 1 g/cm 3 White Oak – 0. 68 g/cm 3 Silver 10. 5 g/cm 3 Gold 19. 32 g/cm 3

Physical Properties PHYSICAL PROPERTIES = those that can be seen or measured › Conducts electricity › Shiny or solid CHEMICAL PROPERTIES = how the substances changes to new substances when it reacts with something else › Flammable › Does not burn Chart on page 371

Properties of Matter Which is which: Zinc is a metal that is a shiny solid that burns with a blue green flame. A. Physical B. Chemical

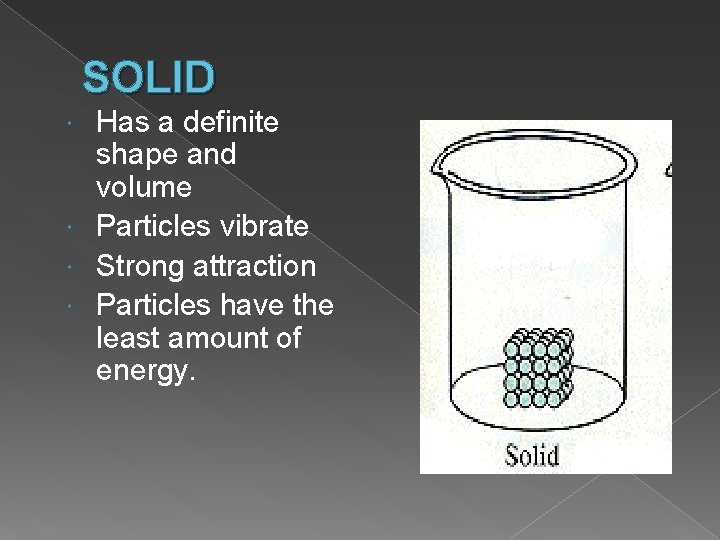
SOLID Has a definite shape and volume Particles vibrate Strong attraction Particles have the least amount of energy.

LIQUID Volume stays the same Will take the shape of the container it is in Particles have enough energy to push other particles out of the way. Some attraction This causes the "flow" of a liquid.

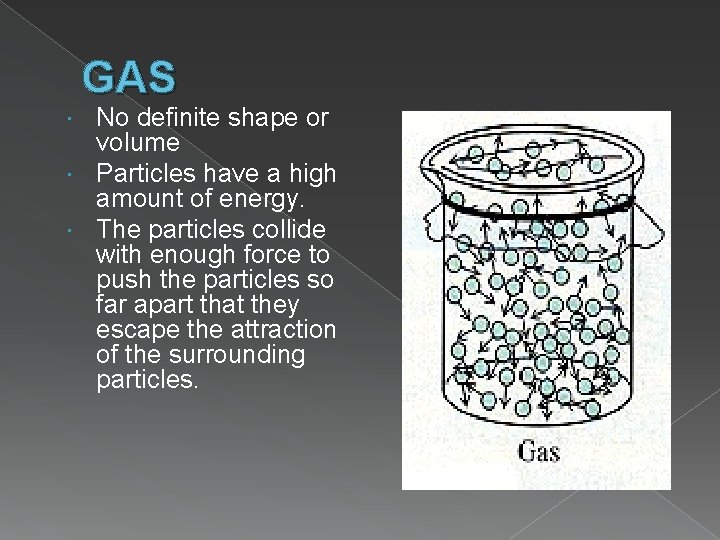
GAS No definite shape or volume Particles have a high amount of energy. The particles collide with enough force to push the particles so far apart that they escape the attraction of the surrounding particles.

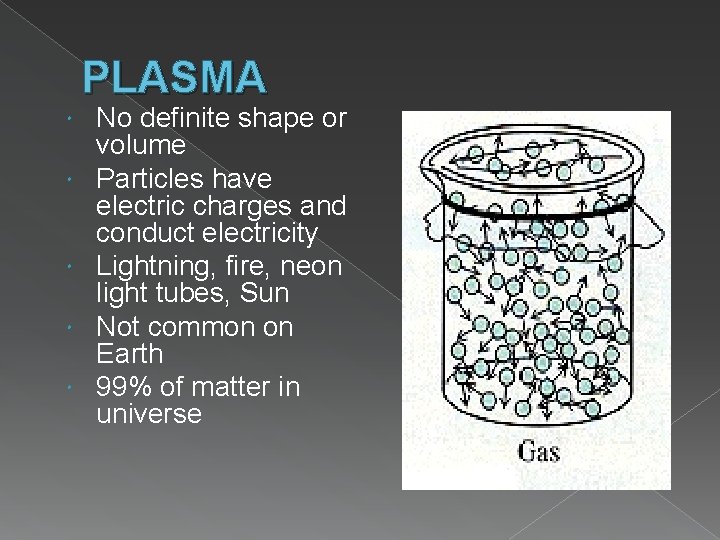
PLASMA No definite shape or volume Particles have electric charges and conduct electricity Lightning, fire, neon light tubes, Sun Not common on Earth 99% of matter in universe

Writing For Science Make a Google Document and put it in your science folder Explain the difference between a solid, a liquid and a gas.

How do states of matter change? Water: (vapor, water, ice) Solid to liquid = 1. heat flows to ice 2. ice melts because particles have enough energy to escape the solid’s pull 3. Becomes liquid

How do states of matter change? Water: liquid to gas 1. As liquid water boils 2. Particles gain enough energy to escape the pull of the liquid and change to a gas

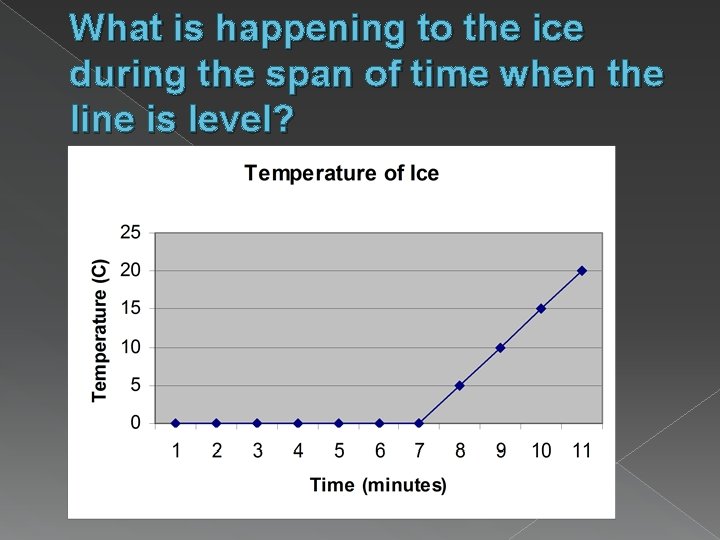
Melting and Freezing The state of matter depends on 1. its temperature 2. amount of attraction between its particles MELTING = when substance changes from solid to liquid › Result of particles gaining heat

Melting and Freezing FREEZING = occurs when substance changes from liquid to solid At freezing point the particles move more slowly – temperature is lower In pure substances, melting and freezing occur at the same temperature

What is happening to the ice during the span of time when the line is level?

Boiling and Evaporation BOILING = when all particles in a liquid have enough energy to change to a gas Water at 100°C Oxygen at -183°C Particles speed up as they heat up At boiling point particles gain enough energy to break free to a gas

Boiling and Evaporation EVAPORATION = the change of state from a liquid to a gas at the surface of a liquid Evaporation produces a temperature drop since it removes faster/warmer moving particles

Condensation Water vapor (gas) is always present in air (humidity) When these faster particles collide with cool surface (glass of ice water) › They lose energy › Come closer together › Water in liquid form Condensation = change in state from gas to liquid

Physical Changes When the appearance of a substance changes but its properties stay the same. › Text page 376

Chemical Changes One or more substances change into a completely new substance › Text page 376