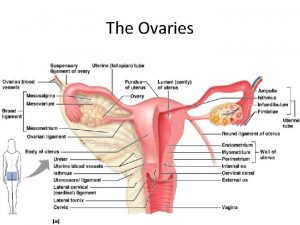
MALIGNANT DISORDERS OF THE OVARIES Assoc Prof Gazi


























- Slides: 60

MALIGNANT DISORDERS OF THE OVARIES Assoc. Prof. Gazi YILDIRIM, M. D. Yeditepe University, Medical Faculty Dept of Ob&Gyn

Objectives n To define n n Ovarian cancer To learn Risk factors for ovarian cancer n Prognostic factor for ovarian cancer n Diagnosis of ovarian cancer n n To manage n A woman with ovarian cancer

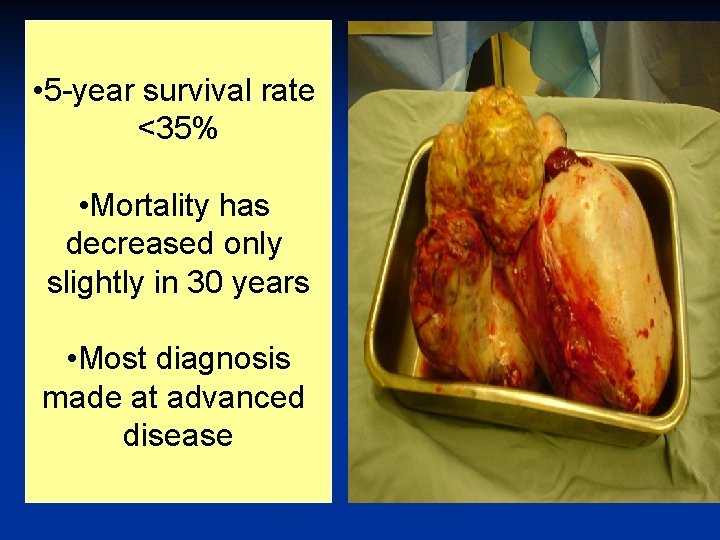
n The 5. most common cancer in women n The 5. most frequent cause of cancer death n Lifetime risk 1/70





• 5 -year survival rate <35% • Mortality has decreased only slightly in 30 years • Most diagnosis made at advanced disease

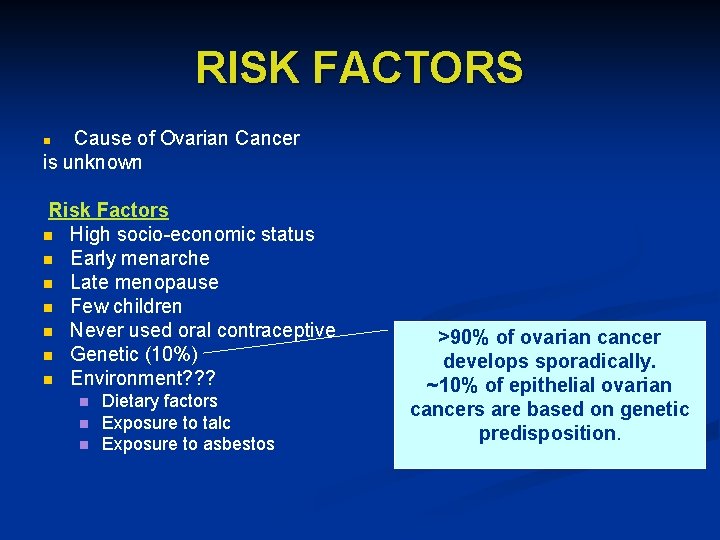
RISK FACTORS Cause of Ovarian Cancer is unknown n Risk Factors n High socio-economic status n Early menarche n Late menopause n Few children n Never used oral contraceptive n Genetic (10%) n Environment? ? ? n n n Dietary factors Exposure to talc Exposure to asbestos >90% of ovarian cancer develops sporadically. ~10% of epithelial ovarian cancers are based on genetic predisposition.

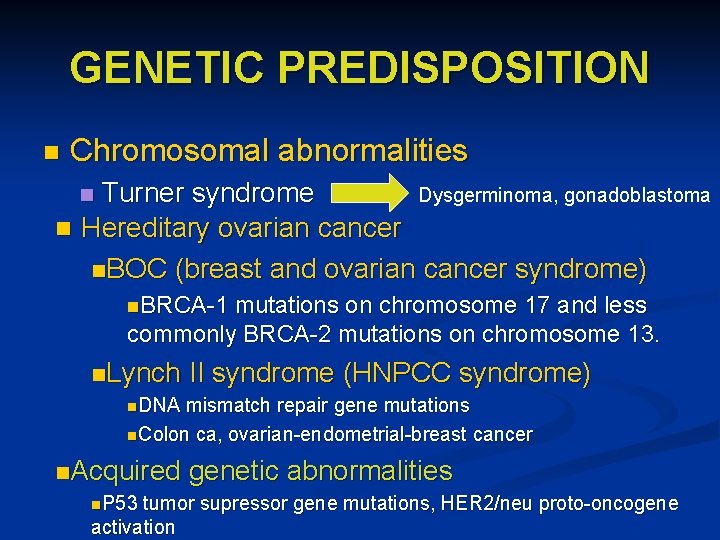
GENETIC PREDISPOSITION n Chromosomal abnormalities Turner syndrome Dysgerminoma, gonadoblastoma n Hereditary ovarian cancer n. BOC (breast and ovarian cancer syndrome) n n. BRCA-1 mutations on chromosome 17 and less commonly BRCA-2 mutations on chromosome 13. n. Lynch II syndrome (HNPCC syndrome) n. DNA mismatch repair gene mutations n. Colon ca, ovarian-endometrial-breast cancer n. Acquired genetic abnormalities n. P 53 tumor supressor gene mutations, HER 2/neu proto-oncogene activation

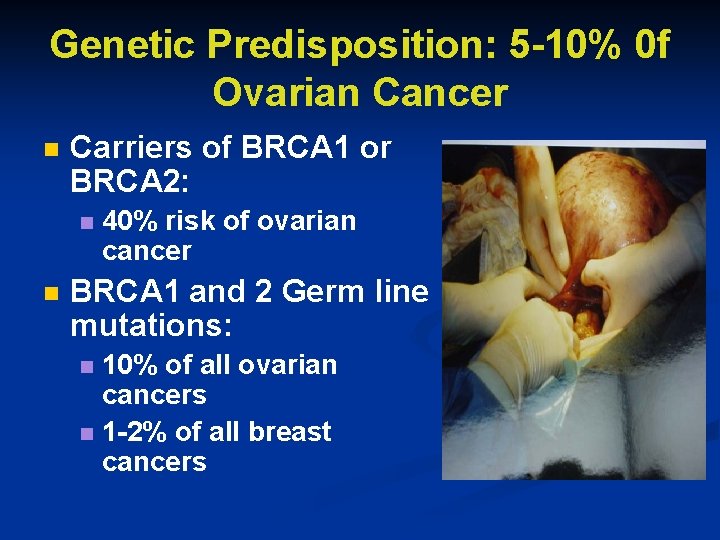
Genetic Predisposition: 5 -10% 0 f Ovarian Cancer n Carriers of BRCA 1 or BRCA 2: n n 40% risk of ovarian cancer BRCA 1 and 2 Germ line mutations: 10% of all ovarian cancers n 1 -2% of all breast cancers n

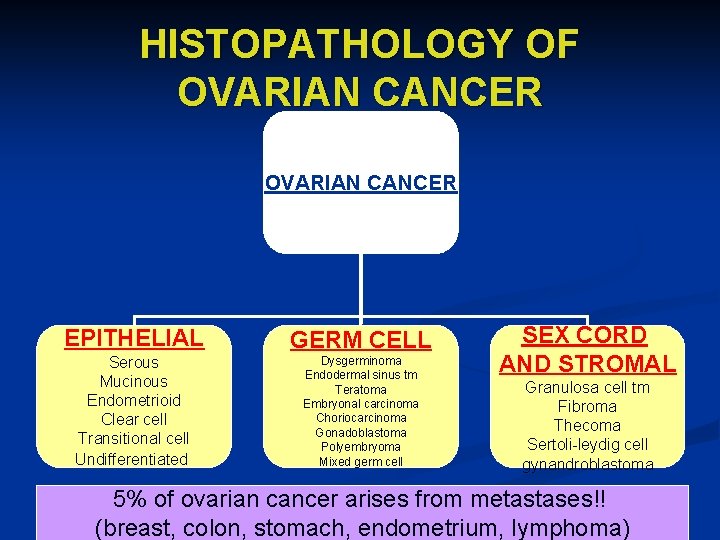
HISTOPATHOLOGY OF OVARIAN CANCER EPITHELIAL GERM CELL Serous Mucinous Endometrioid Clear cell Transitional cell Undifferentiated Dysgerminoma Endodermal sinus tm Teratoma Embryonal carcinoma Choriocarcinoma Gonadoblastoma Polyembryoma Mixed germ cell SEX CORD AND STROMAL Granulosa cell tm Fibroma Thecoma Sertoli-leydig cell gynandroblastoma 5% of ovarian cancer arises from metastases!! (breast, colon, stomach, endometrium, lymphoma)

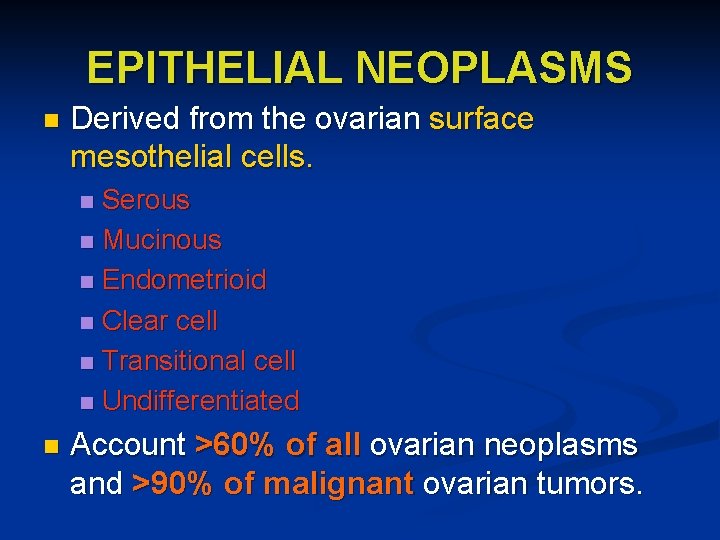
EPITHELIAL NEOPLASMS n Derived from the ovarian surface mesothelial cells. Serous n Mucinous n Endometrioid n Clear cell n Transitional cell n Undifferentiated n n Account >60% of all ovarian neoplasms and >90% of malignant ovarian tumors.

Serous Neoplasms n Most common malignant tumor of the ovary. n n 35 -50% of all epithelial tumors. Bilateral in 40 -60 of cases. Extraovarian spread at the time of diagnosis in 85% of cases. Cut section: solid areas, areas of hemorrhage, necrosis, cyst wall invasion and adhesions to adjacent structures.

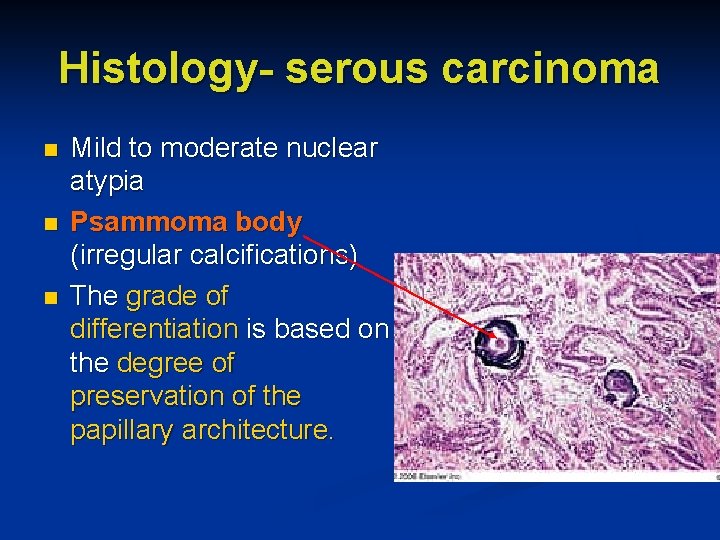
Histology- serous carcinoma n n n Mild to moderate nuclear atypia Psammoma body (irregular calcifications) The grade of differentiation is based on the degree of preservation of the papillary architecture.

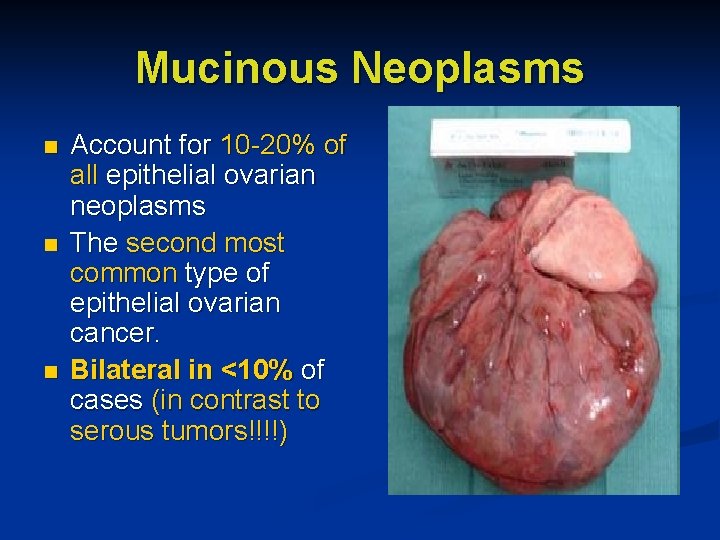
Mucinous Neoplasms n n n Account for 10 -20% of all epithelial ovarian neoplasms The second most common type of epithelial ovarian cancer. Bilateral in <10% of cases (in contrast to serous tumors!!!!)

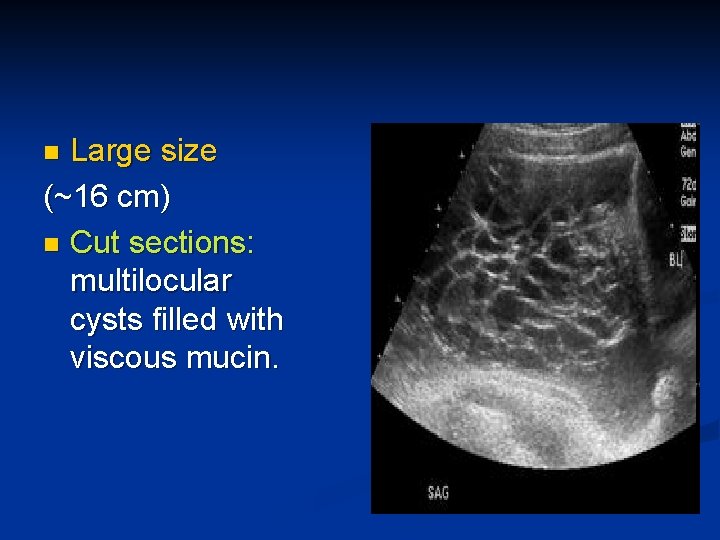
Large size (~16 cm) n Cut sections: multilocular cysts filled with viscous mucin. n

Histology- mucinous carcinoma Composed predominantly of intestinal-like cells that invade surrounding stroma. n Invasive tumors exhibit marked histologic variability from area to area within the Extensive sampling required !! tumor. n The differentiation is based on the preservation of the glandlike architecture of the tumor. n

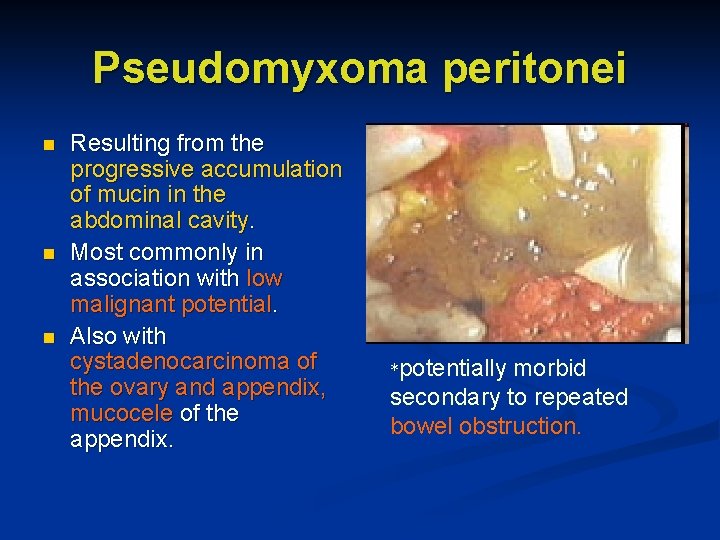
Pseudomyxoma peritonei n n n Resulting from the progressive accumulation of mucin in the abdominal cavity. Most commonly in association with low malignant potential. Also with cystadenocarcinoma of the ovary and appendix, mucocele of the appendix. *potentially morbid secondary to repeated bowel obstruction.

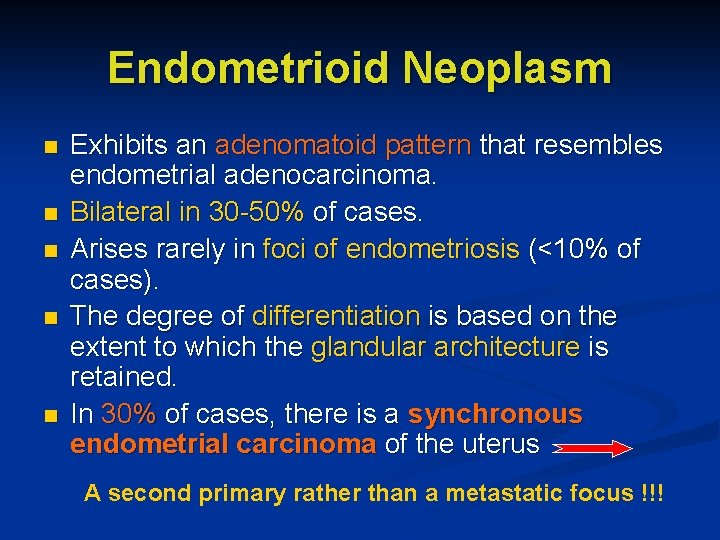
Endometrioid Neoplasm n n n Exhibits an adenomatoid pattern that resembles endometrial adenocarcinoma. Bilateral in 30 -50% of cases. Arises rarely in foci of endometriosis (<10% of cases). The degree of differentiation is based on the extent to which the glandular architecture is retained. In 30% of cases, there is a synchronous endometrial carcinoma of the uterus A second primary rather than a metastatic focus !!!

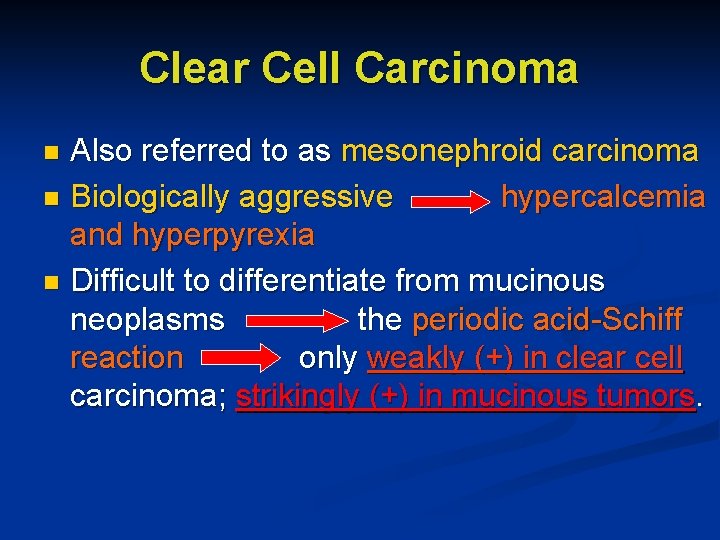
Clear Cell Carcinoma Also referred to as mesonephroid carcinoma n Biologically aggressive hypercalcemia and hyperpyrexia n Difficult to differentiate from mucinous neoplasms the periodic acid-Schiff reaction only weakly (+) in clear cell carcinoma; strikingly (+) in mucinous tumors. n

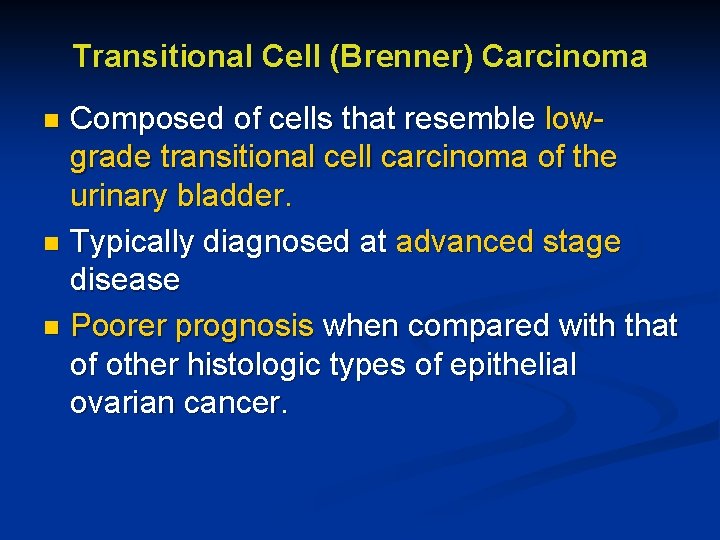
Transitional Cell (Brenner) Carcinoma Composed of cells that resemble lowgrade transitional cell carcinoma of the urinary bladder. n Typically diagnosed at advanced stage disease n Poorer prognosis when compared with that of other histologic types of epithelial ovarian cancer. n

Undifferentiated Carcinoma <10% of epithelial neoplasms. n Characterized by the absence of any distinguishing microscopic features that permit its placement in one of the other histologic categories. n

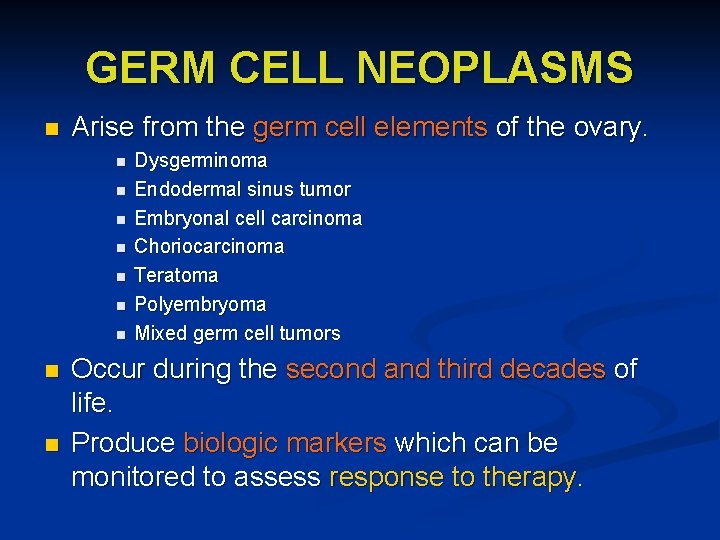
GERM CELL NEOPLASMS n Arise from the germ cell elements of the ovary. n n n n n Dysgerminoma Endodermal sinus tumor Embryonal cell carcinoma Choriocarcinoma Teratoma Polyembryoma Mixed germ cell tumors Occur during the second and third decades of life. Produce biologic markers which can be monitored to assess response to therapy.

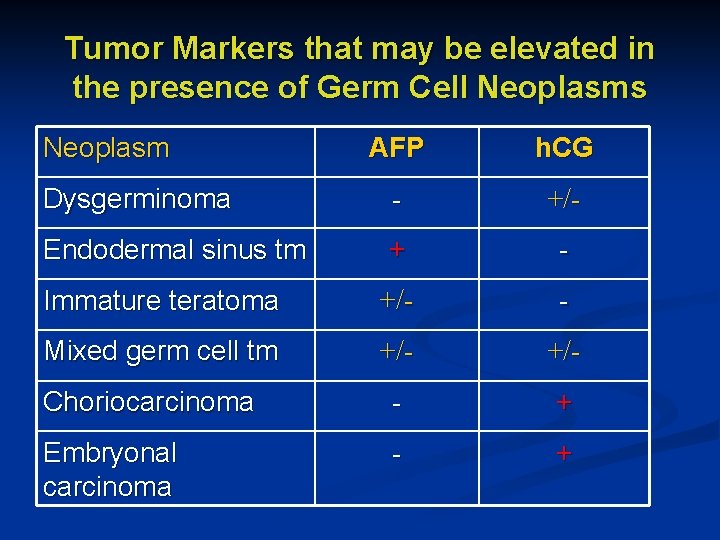
Tumor Markers that may be elevated in the presence of Germ Cell Neoplasms Neoplasm AFP h. CG Dysgerminoma - +/- Endodermal sinus tm + - Immature teratoma +/- - Mixed germ cell tm +/- Choriocarcinoma - + Embryonal carcinoma - +

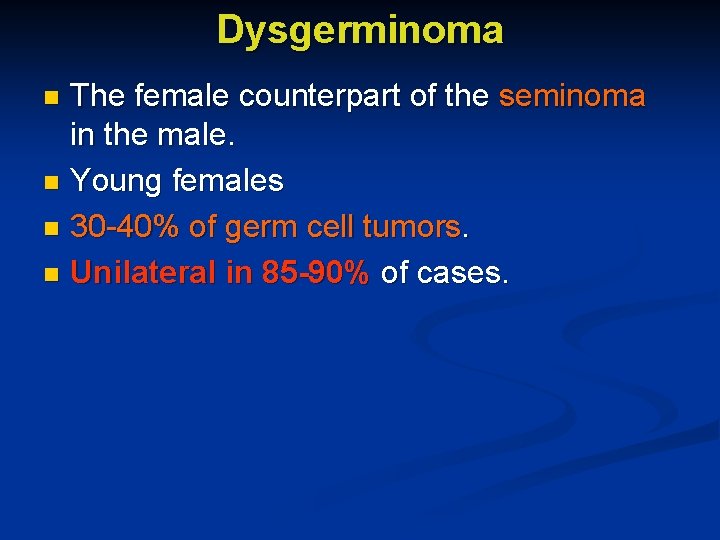
Dysgerminoma The female counterpart of the seminoma in the male. n Young females n 30 -40% of germ cell tumors. n Unilateral in 85 -90% of cases. n

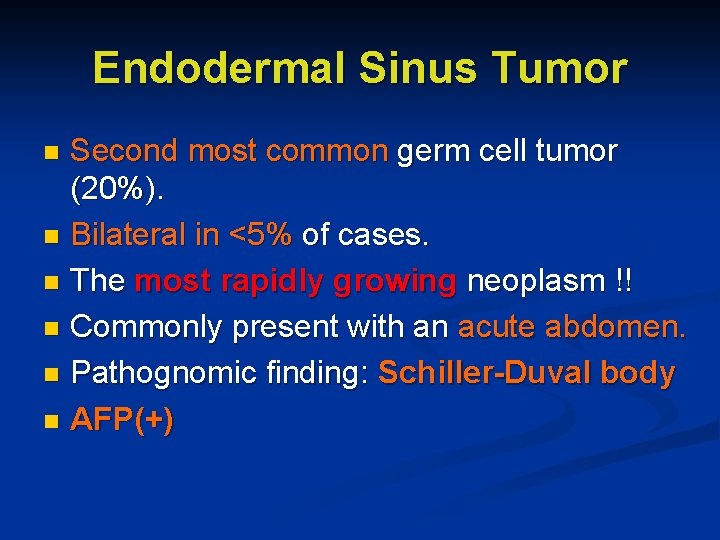
Endodermal Sinus Tumor Second most common germ cell tumor (20%). n Bilateral in <5% of cases. n The most rapidly growing neoplasm !! n Commonly present with an acute abdomen. n Pathognomic finding: Schiller-Duval body n AFP(+) n

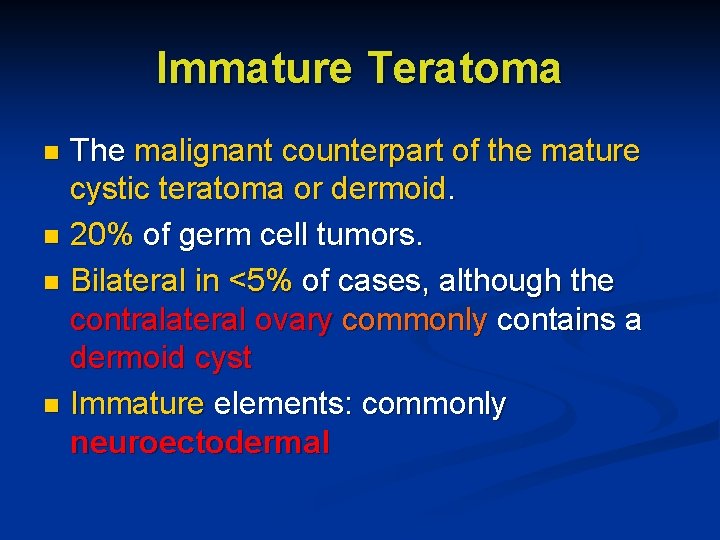
Immature Teratoma The malignant counterpart of the mature cystic teratoma or dermoid. n 20% of germ cell tumors. n Bilateral in <5% of cases, although the contralateral ovary commonly contains a dermoid cyst n Immature elements: commonly neuroectodermal n

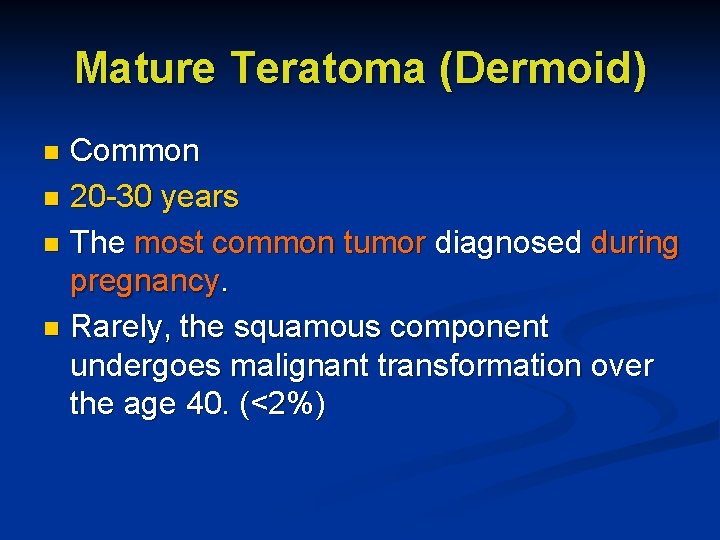
Mature Teratoma (Dermoid) Common n 20 -30 years n The most common tumor diagnosed during pregnancy. n Rarely, the squamous component undergoes malignant transformation over the age 40. (<2%) n

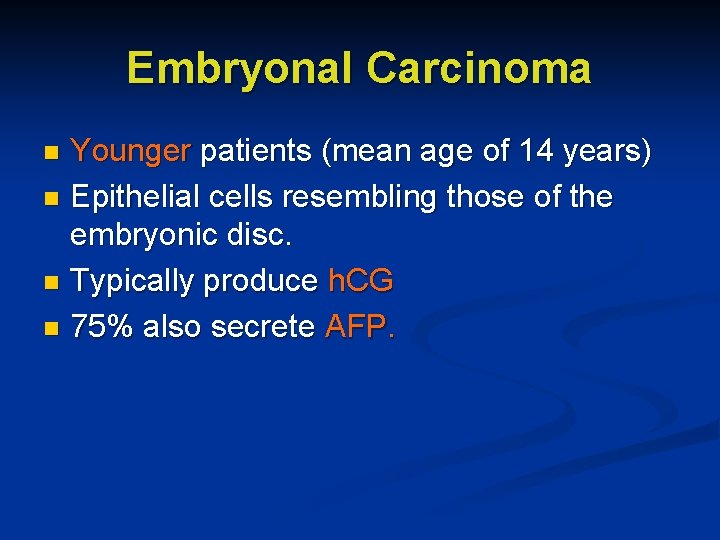
Embryonal Carcinoma Younger patients (mean age of 14 years) n Epithelial cells resembling those of the embryonic disc. n Typically produce h. CG n 75% also secrete AFP. n

Choriocarcinoma Primary ovarian choriocarcinoma arises from a germ cell similar in appearance to gestational choriocarcinoma. Nongestational tumors: poorer prognosis * The detection of other germ cell components indicates nongestational tumors! n

Gonadoblastoma Rare tumor composed of nests of germ cells and sex cord derivatives. n More common in the right ovary. n Usually during the second decade of life. n Found in patients with abnormal gonadal development in the presence of a Y chromosome. n

Mixed Germ Cell Tumors 10% of germ cell neoplasms. n Contain ≥ 2 germ cell elements. n Dysgerminoma and endodermal sinus tumor occur together most frequently. n

SEX CORD-STROMAL TUMORS Heterogeneus group of rare neoplasms originating from the ovarian matrix. cells within matrix have potential for hormon production. Signs and symptoms of estrogen or androgen excess. n

Granulosa Cell Tumors 1 -2% of all ovarian tumors. n The most common malignant tumors of the sex cord-stromal tumors. n Hyperestrogenism Precocious puberty n in young girls n Call-exner bodies Endometrial hyperplasia and vaginal bleeding in postmenopausal women

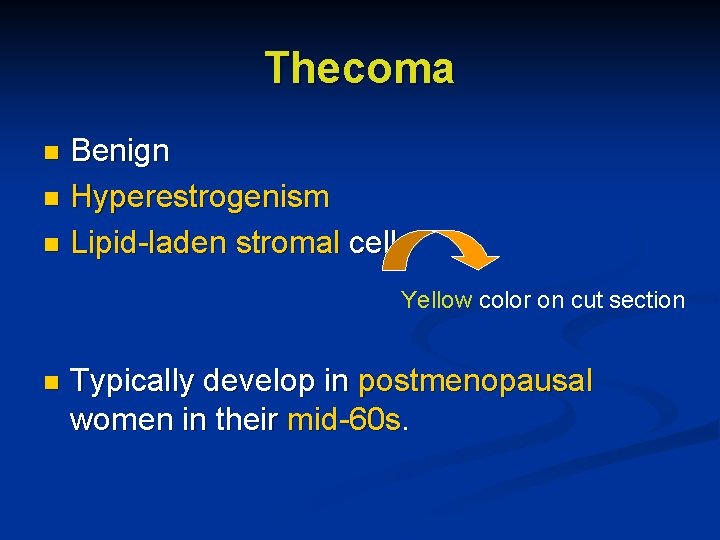
Thecoma Benign n Hyperestrogenism n Lipid-laden stromal cells n Yellow color on cut section n Typically develop in postmenopausal women in their mid-60 s.

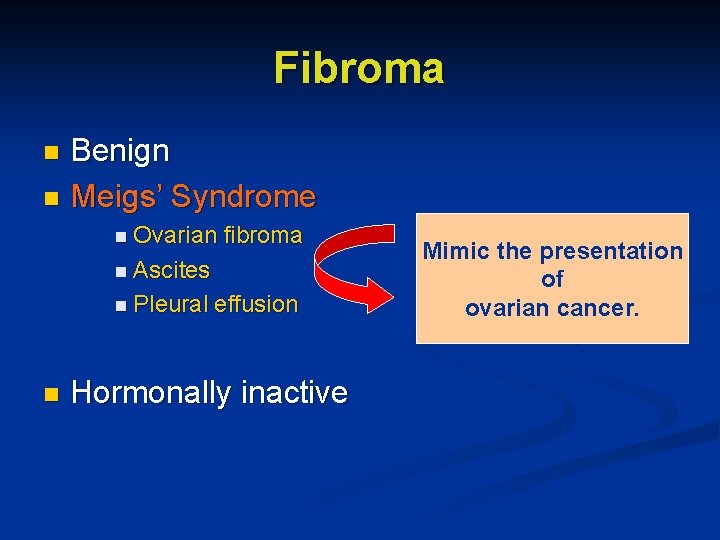
Fibroma Benign n Meigs’ Syndrome n n Ovarian fibroma n Ascites n Pleural n effusion Hormonally inactive Mimic the presentation of ovarian cancer.

Sertoli-Leydig Cell Tumors Rare n Consist of testicular structures at different stages of development. n Usually virilizing n During the third decade of life n Rarely bilateral n

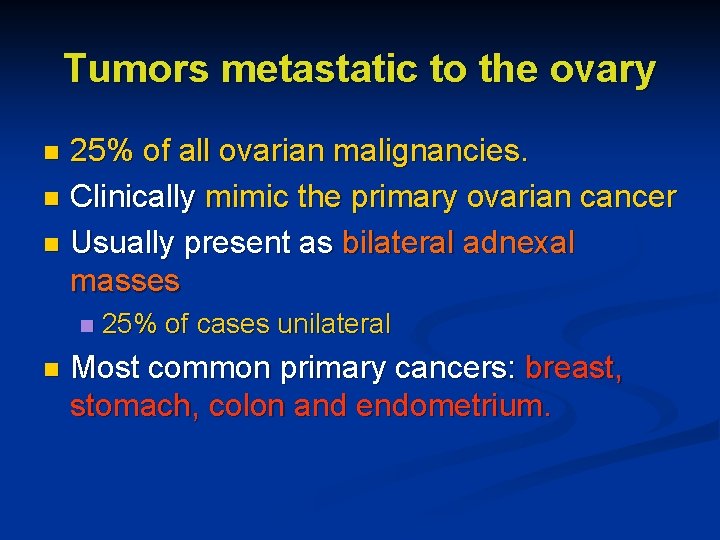
Tumors metastatic to the ovary 25% of all ovarian malignancies. n Clinically mimic the primary ovarian cancer n Usually present as bilateral adnexal masses n n n 25% of cases unilateral Most common primary cancers: breast, stomach, colon and endometrium.

SYMPTOMS n Vague and non-specific !! Abdominal bloating n Indigestion, dyspepsia n Altered bowel habits n Menstruel abnormalities n Pelvic fullness n Pain n

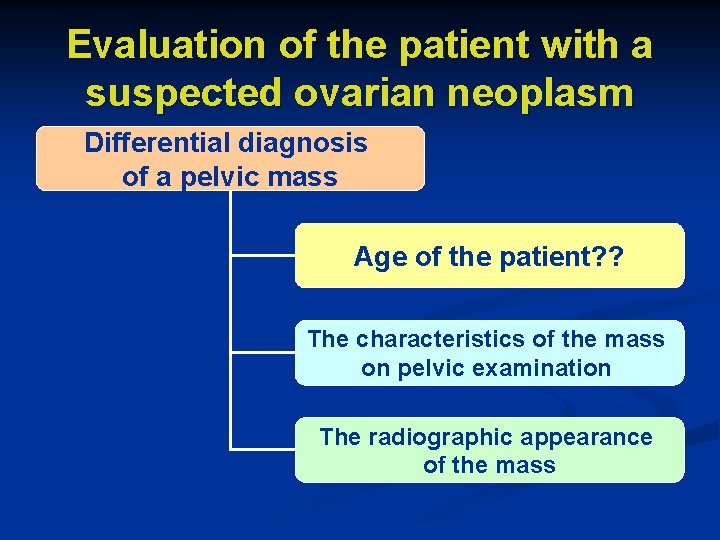
Evaluation of the patient with a suspected ovarian neoplasm Differential diagnosis of a pelvic mass Age of the patient? ? The characteristics of the mass on pelvic examination The radiographic appearance of the mass

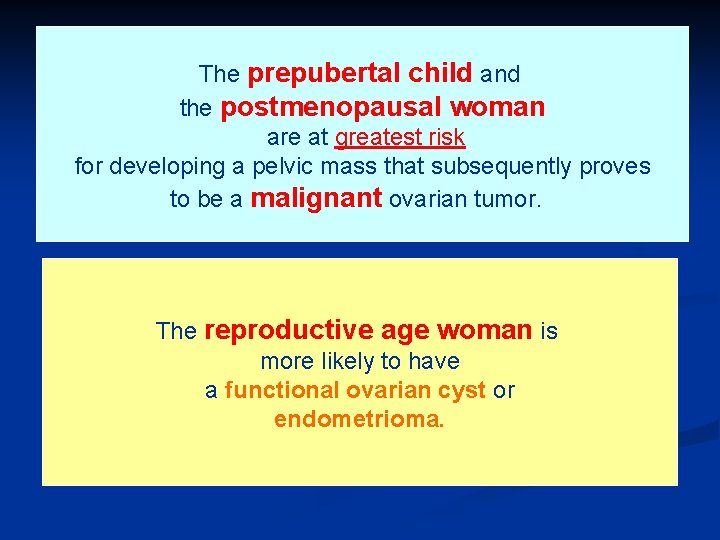
The prepubertal child and the postmenopausal woman are at greatest risk for developing a pelvic mass that subsequently proves to be a malignant ovarian tumor. The reproductive age woman is more likely to have a functional ovarian cyst or endometrioma.

Physical Examination n n Perform a comprehensive examination. Attention to the lymph-node-bearing areas n n Particularly the supraclavicular and inguinal areas. Examination of the abdomen n n Abdominal distention The presence of flank fullness and shifting dullness Tympanitic percussion note over the lateral abdomen a large mass displacing the bowel to the periphery. central tympanitic percussion note ascites

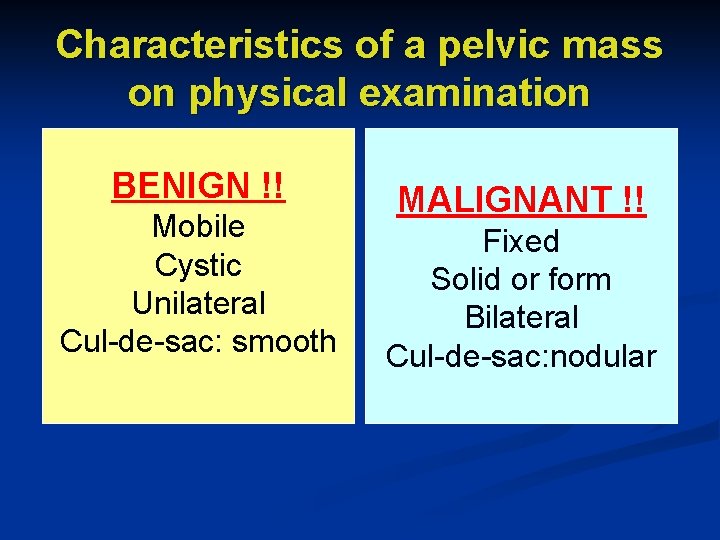
Characteristics of a pelvic mass on physical examination BENIGN !! Mobile Cystic Unilateral Cul-de-sac: smooth MALIGNANT !! Fixed Solid or form Bilateral Cul-de-sac: nodular

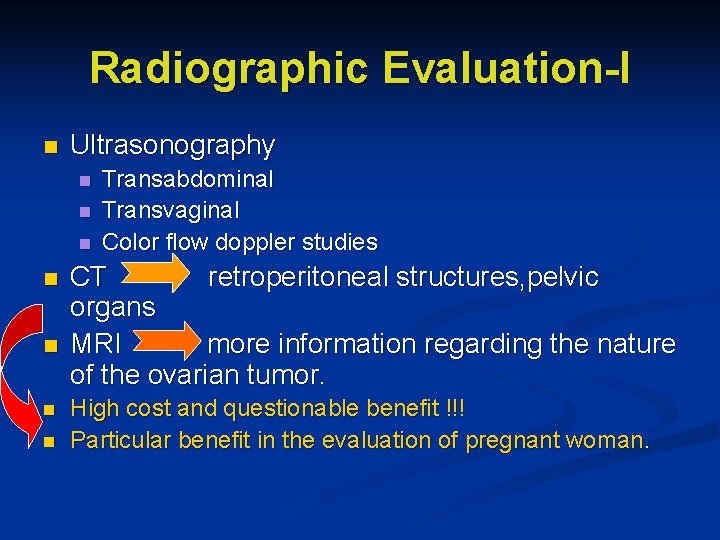
Radiographic Evaluation-I n Ultrasonography n n n n Transabdominal Transvaginal Color flow doppler studies CT retroperitoneal structures, pelvic organs MRI more information regarding the nature of the ovarian tumor. High cost and questionable benefit !!! Particular benefit in the evaluation of pregnant woman.

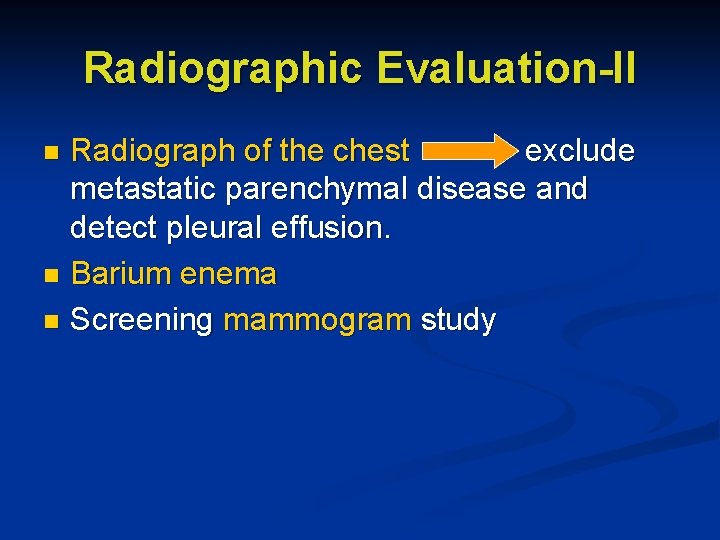
Radiographic Evaluation-II Radiograph of the chest exclude metastatic parenchymal disease and detect pleural effusion. n Barium enema n Screening mammogram study n

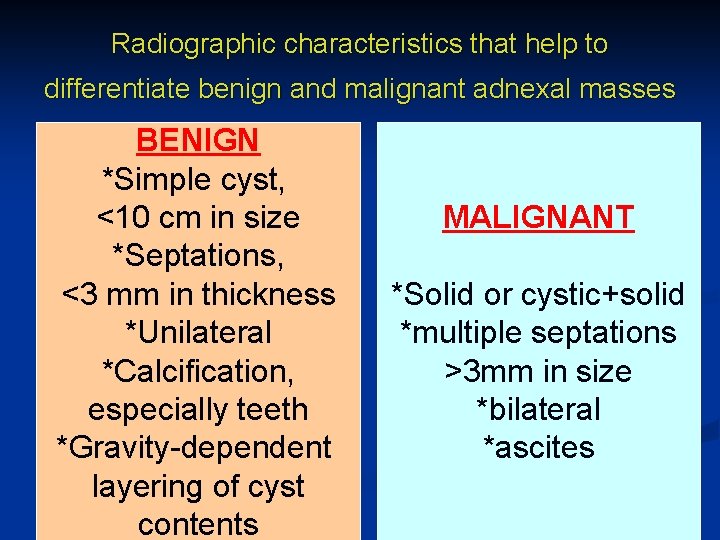
Radiographic characteristics that help to differentiate benign and malignant adnexal masses BENIGN *Simple cyst, <10 cm in size *Septations, <3 mm in thickness *Unilateral *Calcification, especially teeth *Gravity-dependent layering of cyst contents MALIGNANT *Solid or cystic+solid *multiple septations >3 mm in size *bilateral *ascites

PROGNOSTIC FACTORS n n n Stage !! Grade Cell-type of tumor Residual disease after surgery Disease volume prior to any surgical debulking Age of woman >70 n Performance status

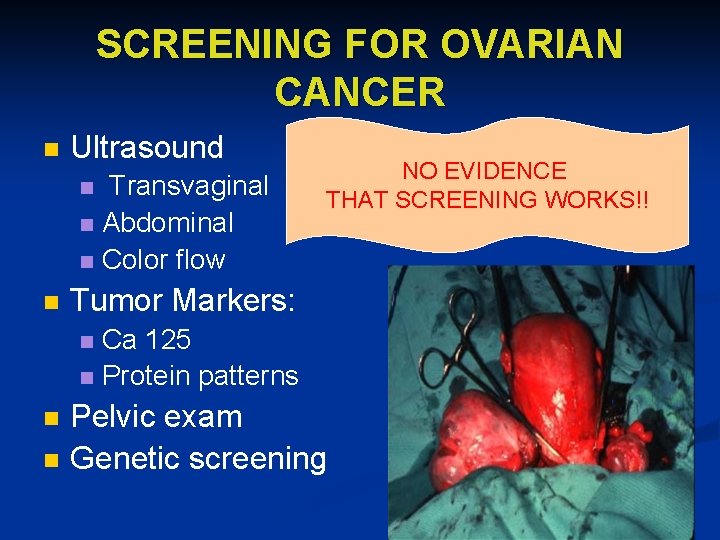
SCREENING FOR OVARIAN CANCER n Ultrasound Transvaginal n Abdominal n Color flow n n NO EVIDENCE THAT SCREENING WORKS!! Tumor Markers: Ca 125 n Protein patterns n n n Pelvic exam Genetic screening

SURGICAL TREATMENT of epithelial overian cancer n n n Surgery: the cornerstone of therapy debulking: remove as much of the cancer as possible the less cancer left after primary surgery the better the outcome n the best outcome is when there is no residual disease n

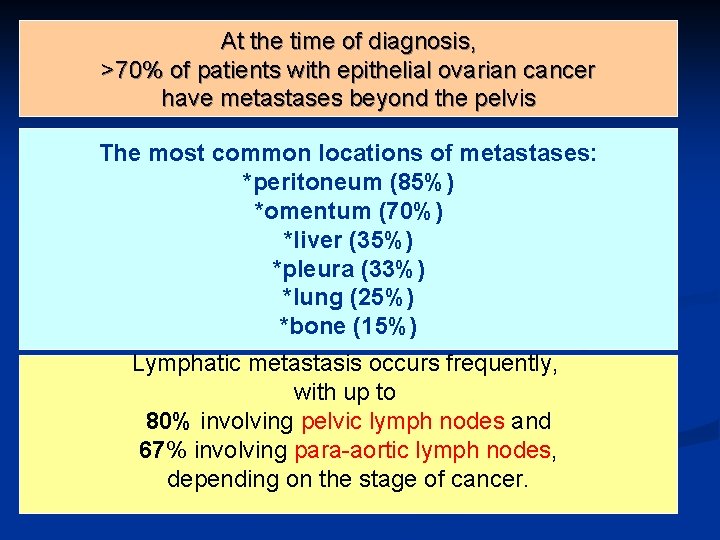
At the time of diagnosis, >70% of patients with epithelial ovarian cancer have metastases beyond the pelvis n . The most common locations of metastases: *peritoneum (85%) *omentum (70%) *liver (35%) *pleura (33%) *lung (25%) *bone (15%) Lymphatic metastasis occurs frequently, with up to 80% involving pelvic lymph nodes and 67% involving para-aortic lymph nodes, depending on the stage of cancer.

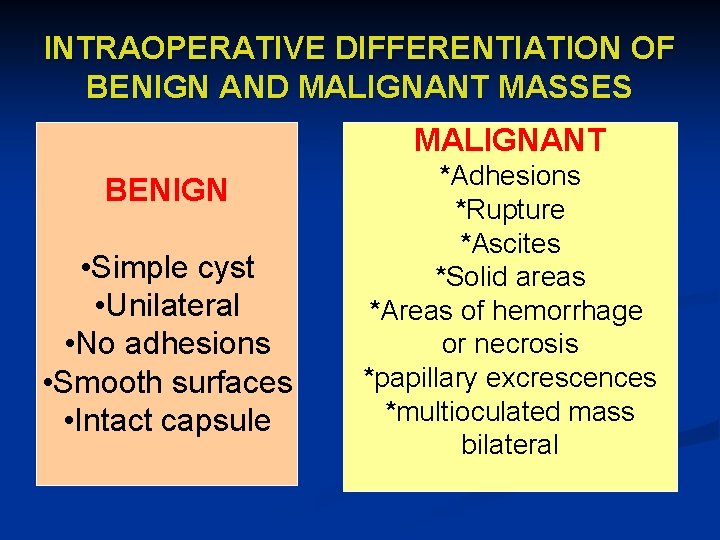
INTRAOPERATIVE DIFFERENTIATION OF BENIGN AND MALIGNANT MASSES MALIGNANT BENIGN • Simple cyst • Unilateral • No adhesions • Smooth surfaces • Intact capsule *Adhesions *Rupture *Ascites *Solid areas *Areas of hemorrhage or necrosis *papillary excrescences *multioculated mass bilateral

Procedures in the surgical staging of ovarian cancer n n n n Sample of ascites or peritoneal washings from the paracolic gutters and pelvic and subdiaphragmatic surface for cytology Complete abdominal exploration Intact removal of tumor Hysterectomy Infracolic omentectomy Biopsies of abdominal peritoneal implants; if present, random biopsies from the paracolic gutter peritoneum, pelvic peritoneum, and right subdiaphragmatic peritoneal surface Pelvic and para-aortic lymph node biopsies Cytoreductive surgery to remove all visible disease

FIGO staging of ovarian cancer n Stage 1: growth limited to ovaries n n 1 a: one ovary involved 1 b: both ovaries involved 1 c: 1 a or 1 b and ovarian surface tm, ruptured capsule, malignant ascites, or peritoneal cytology (+) for malignant cells Stage 2: extension of the tm from the ovary to the pelvis n n n 2 a: extension to the uterus or fallopian tube 2 b: extension to other pelvic tissues 2 c: 2 a or 2 b and ovarian surface tm, ruptured capsule, malignant ascites, or peritoneal cytology (+) for malignant cells

n Stage 3: disease extension to the abdominal cavity n n 3 a: abdominal peritoneal surfaces with microscopic metastases 3 b: tm metastases < 2 cm in size 3 c: tm metastases > 2 cm in size or metastatic disease in the pelvic, paraaortic or inguinal lymph nodes Stage 4: distant metastatic disease n n Malignant pleural effusion Pulmonary parenchymal metastases Liver or splenic parenchymal metastases (not surface implants) Metastases to the supraclavicular lymph nodes or skin

SURGICAL TREATMENT of germ cell neoplasms Early stage at the time of diagnosis n Low incidence of bilaterality n Young age of patients n Fertility sparing surgery by removal the involved adnexa

CHEMOTHERAPY of epithelial ovarian cancer All other patients, except stage Ia and grade I tumors, should undergo systemic chemotherapy. n Agents against epithelial ovarian cancer: n Cisplatin n Carboplatin n Cyclophosphamide n Paclitaxel n Combination therapies !!

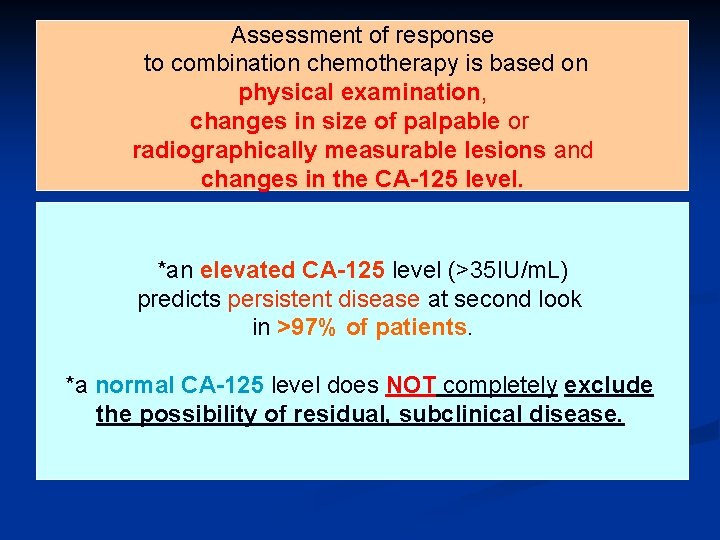
Assessment of response to combination chemotherapy is based on physical examination, changes in size of palpable or radiographically measurable lesions and changes in the CA-125 level. *an elevated CA-125 level (>35 IU/m. L) predicts persistent disease at second look in >97% of patients. *a normal CA-125 level does NOT completely exclude the possibility of residual, subclinical disease.

Chemotherapy-associated toxicities n n n n n Cisplatin: nephrotoxicity, neurotoxicity, ototoxicity Carboplatin: thrombocytopenia, neutropenia Cyclophosphamide: hemorrhagic cystitis, pulmonary fibrosis Paclitaxel: myelosupression Altretamine: peripheral neuropathy Etoposide: myelosupression Bleomycine: pulmonary fibrosis Doxorubicin: cardiac toxicity Vincristine: neuropathy Ifosfamide: hemorrhagic cystitis, central neurotoxicity

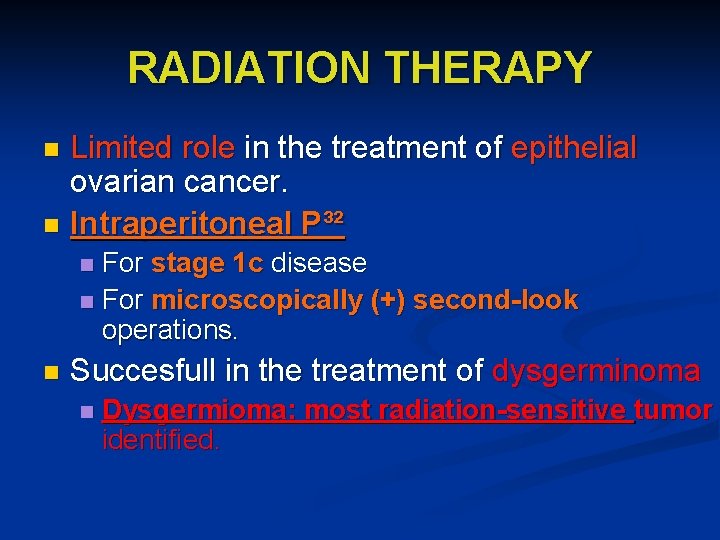
RADIATION THERAPY Limited role in the treatment of epithelial ovarian cancer. n Intraperitoneal P³² n For stage 1 c disease n For microscopically (+) second-look operations. n n Succesfull in the treatment of dysgerminoma n Dysgermioma: most radiation-sensitive tumor identified.
 Common lisp assoc
Common lisp assoc Angusonline
Angusonline Mesovariu
Mesovariu Functions of overies
Functions of overies Distinguish between ova, ovaries, and ovulation
Distinguish between ova, ovaries, and ovulation Squid classification
Squid classification Neuroleptic malignant syndrome
Neuroleptic malignant syndrome Malignant hypertension ppt
Malignant hypertension ppt Malignant neoplasm of liver
Malignant neoplasm of liver Cataractectomy
Cataractectomy Schizophrenia disorganized behavior
Schizophrenia disorganized behavior Hypokalemia
Hypokalemia Hypertensive urgency vs emergency
Hypertensive urgency vs emergency Benign or malignant
Benign or malignant Prostate adenocarcinoma perineural invasion
Prostate adenocarcinoma perineural invasion Malignant and benign tumors
Malignant and benign tumors Hypertensive urgency vs emergency
Hypertensive urgency vs emergency Malignant neoplasm of the blood-forming organs
Malignant neoplasm of the blood-forming organs Malignant htn
Malignant htn Malignant neuroleptic syndrome
Malignant neuroleptic syndrome Carcinoma in situ
Carcinoma in situ Malignant social psychology
Malignant social psychology Histological features of malignant cells
Histological features of malignant cells Malignant mesothelioma
Malignant mesothelioma Risk factors for malignant melanoma
Risk factors for malignant melanoma Benign and malignant tumor
Benign and malignant tumor Basedoxifene
Basedoxifene Gazi teknik siteler
Gazi teknik siteler Ertuğrul gazi orta okulu
Ertuğrul gazi orta okulu Rahman biology classes
Rahman biology classes Kan gazı örneği
Kan gazı örneği Etm gazi
Etm gazi Gazi tto
Gazi tto Ayhan ural kimdir
Ayhan ural kimdir P değeri yorumlama
P değeri yorumlama Gazi üniversitesi tez yazım kılavuzu
Gazi üniversitesi tez yazım kılavuzu Pv nrt r sabiti
Pv nrt r sabiti Erichtonios
Erichtonios Kan gazı örneği
Kan gazı örneği Gazi ma
Gazi ma Fotoşimik reaksiyon
Fotoşimik reaksiyon Ahi kart para yükleme
Ahi kart para yükleme Koray akkan gazi
Koray akkan gazi Saftekin gazi ortaokulu
Saftekin gazi ortaokulu Sera etkisi
Sera etkisi Sintez gazi
Sintez gazi Gazi şbp
Gazi şbp Gazi etm
Gazi etm Oksijen gazı
Oksijen gazı Etm gazi
Etm gazi Bacak venleri
Bacak venleri Kreni gardo bandu gazi
Kreni gardo bandu gazi Karbürleyici alev
Karbürleyici alev đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Bổ thể
Bổ thể Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hát lên người ơi
Hát lên người ơi