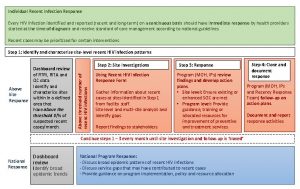
HIV IIIPrevention of HIV infection Allison Liddell MD














































- Slides: 58

HIV IIIPrevention of HIV infection Allison Liddell, MD Monday, January 24 th, 2005

HIV Curriculum n n n HIV I-Diagnosis and HAART HIV II-Complications of HIV/AIDS HIV III-Prevention of HIV infection

HIV history n Date of first published case? n n n June 5, 1981 MMWR five cases of PCP among previously healthy young men in What city? n Los Angeles. All of the men were described as "homosexuals"; two had died. Who wrote it? n Where? n n Local clinicians and the Epidemic Intelligence Service (EIS) Officer stationed at the Los Angeles County Department of Public Health editorial note stated that the histories suggested a "cellular-immune dysfunction related to a common exposure" and a "disease acquired through sexual contact. "

HIV history n n n CDC's investigation drug unit, the sole distributor of pentamidine, therapy for PCP, began to receive requests for the drug from physicians also to treat young men June 1981, CDC developed an investigative team Within 18 months, epidemiologists conducted studies and prepared MMWR reports that identified all of the major risks factors for acquired immnodeficiency syndrome (AIDS).



HIV Prevention n CDC initiative: Advancing HIV Prevention: New Strategies for a Changing Epidemic n n reducing barriers to early diagnosis increasing access to quality medical care, treatment ongoing prevention services emphasizes the use of proven public health approaches to n n n appropriate routine screening identification of new cases partner counseling and referral increased availability of sustained treatment prevention services for the infected


Barrier Methods. Do they work? n n must be used correctly and consistently Latex condoms are highly effective in preventing transmission of HIV. Well documented. reduce the risk of other STDs associated with a lower rate of cervical cancer, an HPV-associated disease. n n n condoms lubricated with spermicides are no more effective epidemiologic studies of STDs, other than HIV, are characterized by methodological limitations inconclusiveness of epidemiologic data about condom effectiveness for other STDs indicates that more research is needed--not that latex condoms do not work

n Epidemiologic studies that are conducted in real-life settings, where one partner is infected with HIV and the other partner is not, demonstrate conclusively that the consistent use of latex condoms provides a high degree of protection.

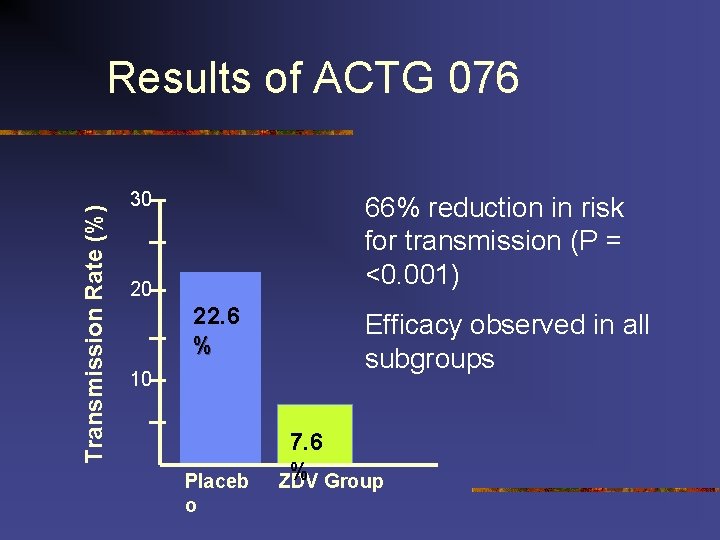
Vertical Transmission n 91% of all AIDS cases reported among U. S. children February 1994 PACTG Protocol 076 documented that ZDV chemoprophylaxis could reduce perinatal HIV-1 transmission by nearly 70% transmission rates can be reduced to less than 2% (Cooper 2002) compared with approximately 25% when no interventions are given (Connor 1994).

Transmission Rate (%) Results of ACTG 076 30 66% reduction in risk for transmission (P = <0. 001) 20 22. 6 % 10 Placeb o Efficacy observed in all subgroups 7. 6 % Group ZDV


Vertical Transmission n n n n perinatally acquired AIDS cases declined 90% in the US during 1992 -2000 (912 cases to 90 cases) CDC unpublished data Lifetime treatment cost for perinatally infected infants $51. 8$68. 5 million n assumes 280 -370 perinatal infections per year n lifetime cost of $185, 000 per infant (Mrus 2004). 90% of mothers were voluntarily tested for HIV before birth for 1999 -2001. 79% of HIV-infected women and infants received antiretroviral therapy prenatally 77% at labor/delivery 92% neonatally 8% of the HIV-infected pregnant women identified had not received prenatal care (CDC 2004). Another study concluded that approximately 14% of HIV-infected pregnant women do not receive any prenatal care

HIV in pregnancy n n pregnancy is not a reason to defer standard therapy unique considerations n n need to alter dosage as a result of physiologic changes associated with pregnancy potential for adverse shortor long-term effects on the fetus and newborn n conflicting data on asso. between HAART and preterm delivery to prevent perinatal transmission, ZDV chemoprophylaxis should be incorporated into the antiretroviral regimen AZT alone may be considered for prophylaxis of perinatal transmission in pregnant women with HIV RNA <1, 000 copies/m. L

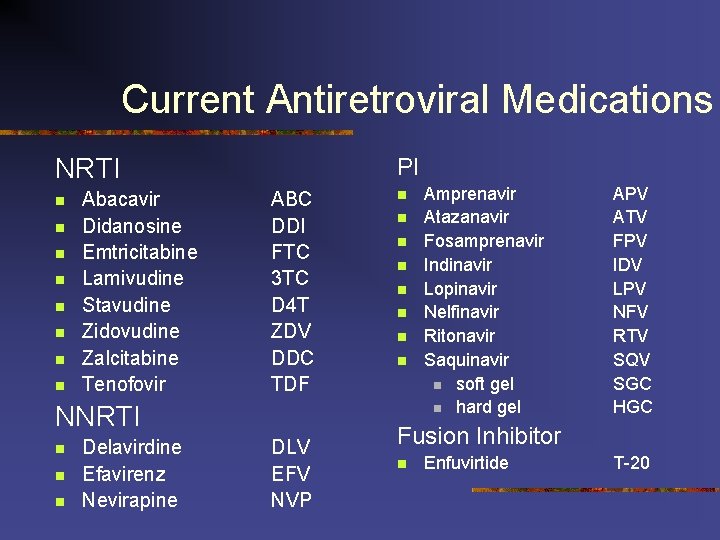
Current Antiretroviral Medications NRTI n n n n Abacavir Didanosine Emtricitabine Lamivudine Stavudine Zidovudine Zalcitabine Tenofovir PI ABC DDI FTC 3 TC D 4 T ZDV DDC TDF NNRTI n n n Delavirdine Efavirenz Nevirapine DLV EFV NVP n n n n Amprenavir Atazanavir Fosamprenavir Indinavir Lopinavir Nelfinavir Ritonavir Saquinavir n soft gel n hard gel APV ATV FPV IDV LPV NFV RTV SQV SGC HGC Fusion Inhibitor n Enfuvirtide T-20

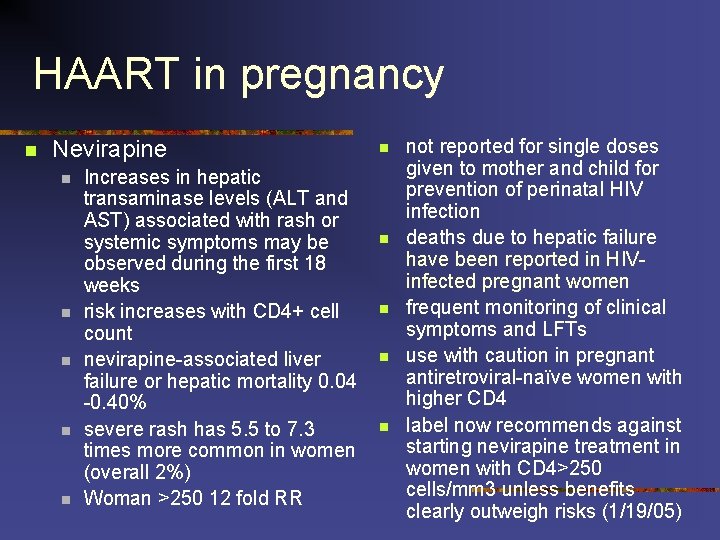
HAART in pregnancy n Nevirapine n n n Increases in hepatic transaminase levels (ALT and AST) associated with rash or systemic symptoms may be observed during the first 18 weeks risk increases with CD 4+ cell count nevirapine-associated liver failure or hepatic mortality 0. 04 -0. 40% severe rash has 5. 5 to 7. 3 times more common in women (overall 2%) Woman >250 12 fold RR n n not reported for single doses given to mother and child for prevention of perinatal HIV infection deaths due to hepatic failure have been reported in HIVinfected pregnant women frequent monitoring of clinical symptoms and LFTs use with caution in pregnant antiretroviral-naïve women with higher CD 4 label now recommends against starting nevirapine treatment in women with CD 4>250 cells/mm 3 unless benefits clearly outweigh risks (1/19/05)

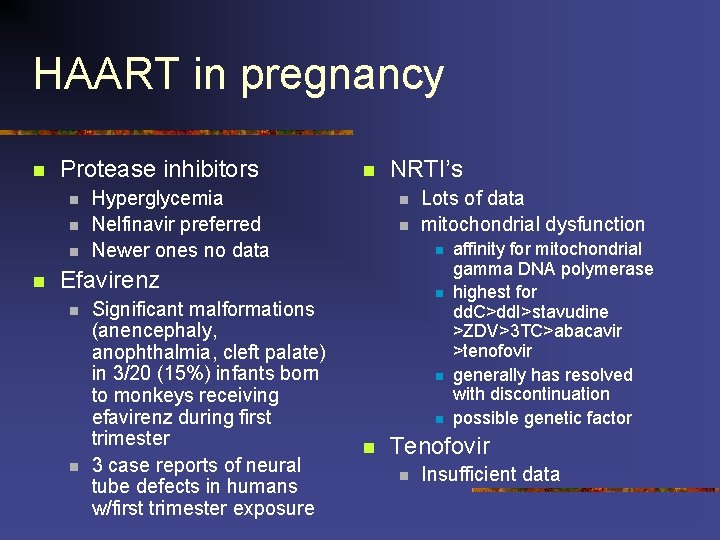
HAART in pregnancy n Protease inhibitors n n n Hyperglycemia Nelfinavir preferred Newer ones no data NRTI’s n n n Efavirenz n n Significant malformations (anencephaly, anophthalmia, cleft palate) in 3/20 (15%) infants born to monkeys receiving efavirenz during first trimester 3 case reports of neural tube defects in humans w/first trimester exposure Lots of data mitochondrial dysfunction n n affinity for mitochondrial gamma DNA polymerase highest for dd. C>dd. I>stavudine >ZDV>3 TC>abacavir >tenofovir generally has resolved with discontinuation possible genetic factor Tenofovir n Insufficient data

Prenatal screening n In 2003, CDC recommended that HIV testing be included in the standard battery of prenatal tests and procedures, with notification to pregnant women that the test would be performed and could be declined (CDC 2003 b). n n similar to recommendations by the Institute of Medicine (IOM 1999) American College of Obstetricians and Gynecologists (AAP, ACOG 1999). n American Academy of Pediatrics (AAP, ACOG 1999).

Key Strategies Universal, routine HIV screening of all pregnant women Universal, routine retesting in the third trimester if: 1. 2. HIV seroprevalence (>0. 5%) or high risk 1. 2. 3. 4. 5. 6. 3. 4. history of sexually transmitted diseases (STDs) sex for money or drugs multiple sex partners during pregnancy illicit drugs sex partner(s) known to be HIV+ or at high risk, signs and symptoms of seroconversion) Universal, routine rapid HIV testing among untested women on arrival rapid HIV testing of newborns whose mothers were not previously screened for HIV Appropriate treatment for pregnant women determined to be HIVinfected and prophylaxis for their infants.

Prevention of transmission n 3 -part regimen n n oral ZDV initiated at 14 -34 weeks' gestation intravenous ZDV during labor oral ZDV to infant for 6 weeks after delivery No breastfeeding if safe alternatives available

HIV prevention in the workplace n Key is good policies and procedures and education, education NIOSH

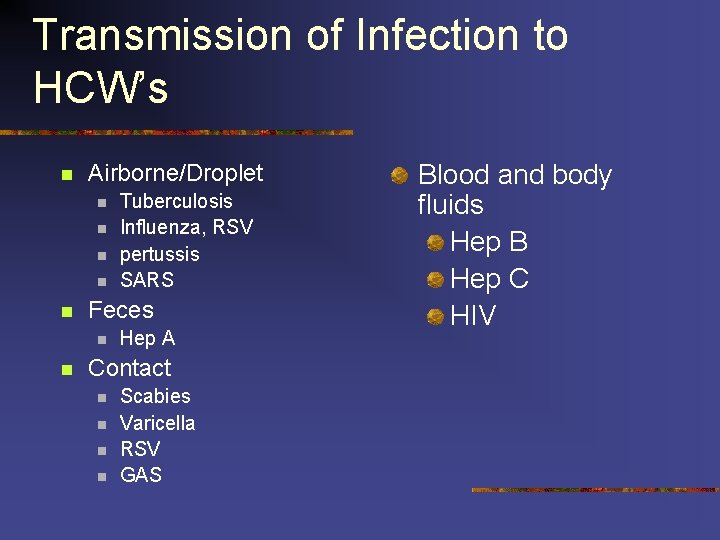
Transmission of Infection to HCW’s n Airborne/Droplet n n n Feces n n Tuberculosis Influenza, RSV pertussis SARS Hep A Contact n n Scabies Varicella RSV GAS Blood and body fluids Hep B Hep C HIV

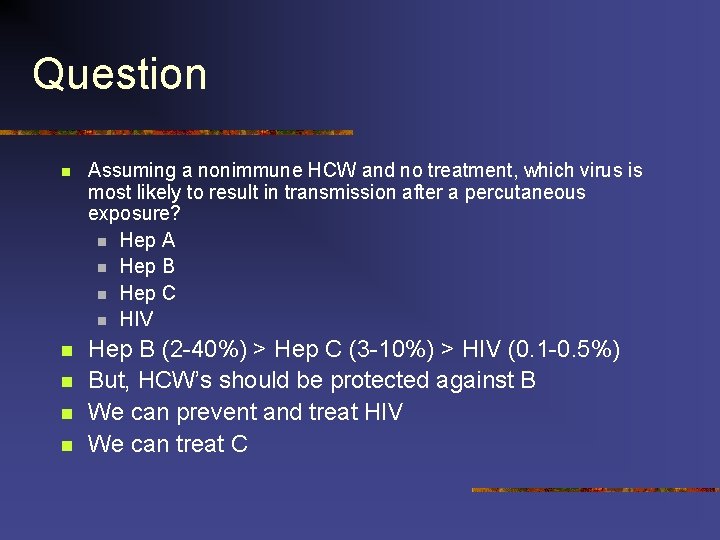
Question n Assuming a nonimmune HCW and no treatment, which virus is most likely to result in transmission after a percutaneous exposure? n Hep A n Hep B n Hep C n HIV n Hep B (2 -40%) > Hep C (3 -10%) > HIV (0. 1 -0. 5%) But, HCW’s should be protected against B We can prevent and treat HIV We can treat C n n n

Bloodborne pathogen exposure n n n Nurses most common Physicians, others underreport Risk factors n n Hollow-bore device Visible blood Depth of injury Patient factors n Prevention n n Safety devices Training n n n procedures no recapping proper disposal






Recommendations and Reports January 21, 2005 / 54(RR 02); 1 -20 Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug Use, or Other Nonoccupational Exposure to HIV in the United States Recommendations from the U. S. Department of Health and Human Services. htm Link to Recommendations

Nonoccupational Exposures n n Voluntary sex Sharing needles Accidental injury Blood transfusion n Sexual assault n n n MMWR January 21, 2005 / 54(RR 02); 1 -20 13% of adult women report having been raped (60% before age 18) 5% more than once 5% of reported rapes in ER involved men assaulting men National Crime Victimization Survey 1999 n In >12 yo, 11. 6 % men only 3 documented cases of HIV infection resulting from rape

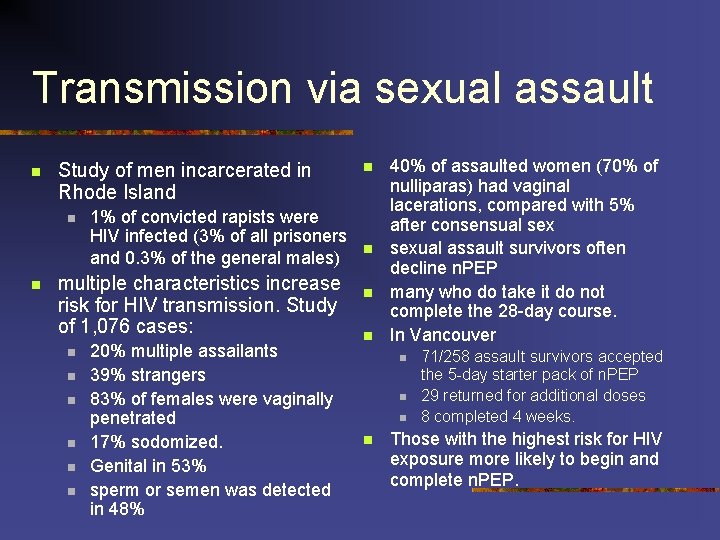
Transmission via sexual assault n Study of men incarcerated in Rhode Island n n 1% of convicted rapists were HIV infected (3% of all prisoners and 0. 3% of the general males) multiple characteristics increase risk for HIV transmission. Study of 1, 076 cases: n n n 20% multiple assailants 39% strangers 83% of females were vaginally penetrated 17% sodomized. Genital in 53% sperm or semen was detected in 48% n n 40% of assaulted women (70% of nulliparas) had vaginal lacerations, compared with 5% after consensual sexual assault survivors often decline n. PEP many who do take it do not complete the 28 -day course. In Vancouver n n 71/258 assault survivors accepted the 5 -day starter pack of n. PEP 29 returned for additional doses 8 completed 4 weeks. Those with the highest risk for HIV exposure more likely to begin and complete n. PEP.

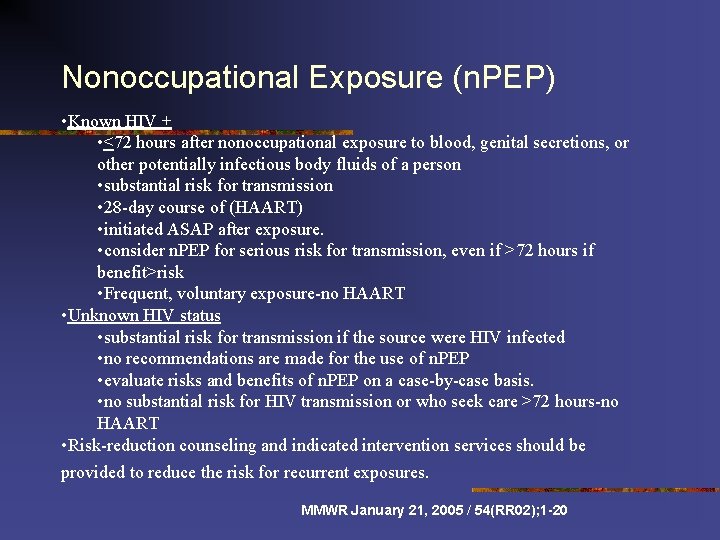
Nonoccupational Exposure (n. PEP) • Known HIV + • <72 hours after nonoccupational exposure to blood, genital secretions, or other potentially infectious body fluids of a person • substantial risk for transmission • 28 -day course of (HAART) • initiated ASAP after exposure. • consider n. PEP for serious risk for transmission, even if >72 hours if benefit>risk • Frequent, voluntary exposure-no HAART • Unknown HIV status • substantial risk for transmission if the source were HIV infected • no recommendations are made for the use of n. PEP • evaluate risks and benefits of n. PEP on a case-by-case basis. • no substantial risk for HIV transmission or who seek care >72 hours-no HAART • Risk-reduction counseling and indicated intervention services should be provided to reduce the risk for recurrent exposures. MMWR January 21, 2005 / 54(RR 02); 1 -20

Concerns about n. PEP n n increases in risk behavior-not supported by data Toxicity Selection of resistance Cost effectiveness MMWR January 21, 2005 / 54(RR 02); 1 -20

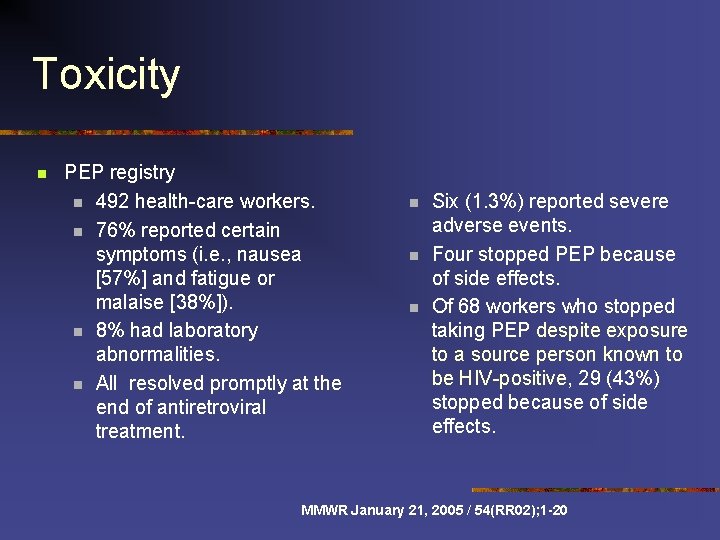
Toxicity n PEP registry n 492 health-care workers. n 76% reported certain symptoms (i. e. , nausea [57%] and fatigue or malaise [38%]). n 8% had laboratory abnormalities. n All resolved promptly at the end of antiretroviral treatment. n n n Six (1. 3%) reported severe adverse events. Four stopped PEP because of side effects. Of 68 workers who stopped taking PEP despite exposure to a source person known to be HIV-positive, 29 (43%) stopped because of side effects. MMWR January 21, 2005 / 54(RR 02); 1 -20

Toxicity n U. S. n. PEP surveillance registry, among. n 107 exposures. n initial regimen stopped or modified in 22%; 50% due to side effects. n serious side effects have been reported (e. g. , nephrolithiasis and hepatitis). n Nevirapine 1997 --2000. n 22 severe ADRs for PEP or n. PEP reported to FDA. n 12 severe hepatotoxicity (one transplant), 14 severe skin reactions, 4 both. n risk of nevirapinecontaining regimen for occupational PEP outweighs benefits. n nevirapine should not be used for n. PEP. MMWR January 21, 2005 / 54(RR 02); 1 -20

Selection of Resistance n n n “probably rare” PEP failures have been documented after at least one sexual and 21 occupational exposures n 3/4 AZT only n Only 4 3+ drugs n 1 had 3 TC mutation, but source unknown Consider resistance testing if patient does seroconvert MMWR January 21, 2005 / 54(RR 02); 1 -20

Cost effectiveness n US study n n cost-effective only with known HIV+ source or after unprotected receptive anal intercourse with a homosexual or bisexual man of unknown serostatus. n (already doing n. PEP) n >50% did not fit criteria (e. g. , for exposure to intact skin). n use of nonindicated n. PEP doubled the cost per HIV infection prevented ($530, 000 vs. $230, 000) French study n n n. PEP cost-saving for unprotected receptive anal intercourse with known HIV + partner and for receptive anal intercourse with a homosexual or bisexual partner of unknown serostatus not cost-effective for penilevaginal sex, insertive anal intercourse, or other exposures considered. British Columbia study n n Even if n. PEP is cost-effective for highest risk exposures, behavioral interventions more cost-effective. Emphasizes the importance of providing risk-avoidance and risk-reduction counseling to reduce the occurrence of future HIV exposures. MMWR January 21, 2005 / 54(RR 02); 1 -20

Barriers to n. PEP n n n Failure to report Cost to patient Harder to test source

Evaluation of Exposure n n Blood is key source Infected saliva very low risk Rapidly test and interview source (? viral load, HAART, prior resistance) If experts not immediately available, do not delay n n n Facilitate adherence Frank, nonjudgemental counseling about risk behaviors Treatment for other blood borne or sexually transmitted infections Emergency contraception Referral for psychiatric services




Question 35 yo WM found HIV+ on insurance exam. Only symptom is occasional night sweats. Thrush on exam. CD 4 260. Viral load 1550. Management? Begin treatment with a 3 -drug regimen and start PCP prophylaxis.

Question n 25 yo WM in ER for fever/cough x 2 weeks. HIV+ in prison for 4+ years, now on parole. Decreased BS in right mid lung, sat 98% RA, RML infiltrate on CXR. You admit. Plan? Airborne isolation, rx for CAP and collect sputa

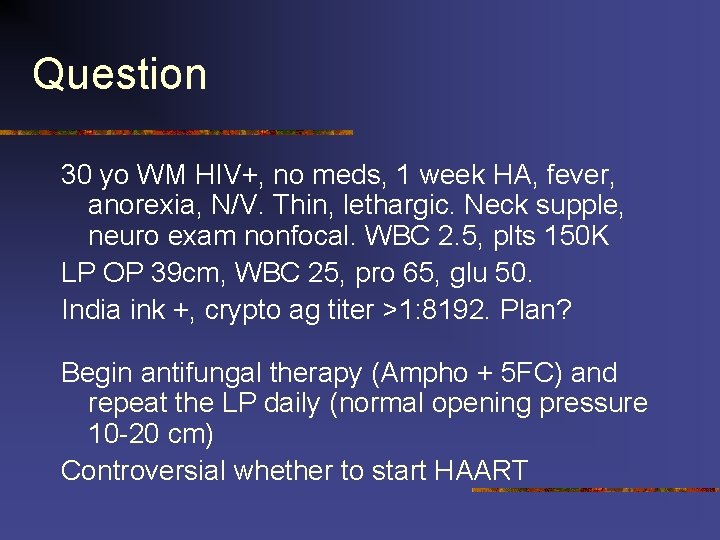
Question 30 yo WM HIV+, no meds, 1 week HA, fever, anorexia, N/V. Thin, lethargic. Neck supple, neuro exam nonfocal. WBC 2. 5, plts 150 K LP OP 39 cm, WBC 25, pro 65, glu 50. India ink +, crypto ag titer >1: 8192. Plan? Begin antifungal therapy (Ampho + 5 FC) and repeat the LP daily (normal opening pressure 10 -20 cm) Controversial whether to start HAART

Question 36 yo WM HIV+ 10 years, no HAART in 5 yrs, to ER w/new onset seizures. 2 weeks memory loss, odd behavior. Confused, disoriented. MRI single ringenhancing lesion left cerebral hemisphere arising in basal ganglia, with significant mass effect and midline shift. Admit, steroids, CD 4=17, toxo Ig. M neg, Ig. G+, CMV Ig. M neg, Ig. G+. Next step? Start empiric pyrimethamine/sulfa (or clinda). No LP.

Question 25 yo BF HIV+ for 2 years, now in 8 th week of pregnancy. Asympto, CD 4>700, viral loads <1000. NO HAART ever. Plan? AZT only starting beginning of second trimester, then routine perinatal AZT.

Question 38 yo LAM with chronic HIV admit w/pneumonia. Migrant worker from Mexico. Bilateral interstitial infiltrates, no HAART, no history of OIs. Hypoxic, intubated. Worsens on PCP rx, bronch shows long larvae. Dx? Strongyloides stercoralis

Question 29 yo AIDS and TB. 3 TB drugs and abacavir/lamivudine/efavirenz started. Improves, then at week 4 comes in with huge fluctuant cervical nodes, fever, palpable spleen, pleural effusion. Aspirate of node no organisms. Next step? a) b) c) d) e) Add ethambutol Substitute tenofovir for abacavir Lymph node biopsy Thoracentesis Treat symptomatically, consider steroids

Question 47 yo WM w/chronic HIV on PI regimen for 2 years. Presents with increasing abdominal girth. Undetectable, good CD 4 recovery. Feels well. 10 lb weight loss, exam has large dorsocervical fat pad, extremity wasting and protruding abdomen with hepatomegaly and striae. Diagnosis? HIV-associated lipodystrophy

Question 34 yo WF new HIV. Fatigue, mild anorexia, but weight stable. CD 4 230, viral load 99, 000. abacavir/3 TC/efavirenz started. One week later, rash, nausea, nonproductive cough and fever to 38. 9. Symptoms wax and wane, feels best first thing in the morning and early evening. Plan? Substitute another drug for abacavir and watch closely

Question Employee needlestick from IDU’r with multiple sex partners, but no bad behavior in a year. Never tested. Plan? 1. 2. 3. 4. 5. Begin 3 drug HAART immediately and continue for 2 months Wait on results of testing Begin 3 drugs then stop if negative Obtain viral load testing on source and employee now and in 6 weeks Obtain baseline testing of both source and employee, now and again in 6 weeks and 6 months.

Question 24 yo sexually active WF requests HIV test. EIA +, Western blot + in 1 band (p 24). Viral load is 324. Interpretation? Indeterminate. Single band nondiagnostic and very low viral load could be false +. Repeat in 6 weeks, 3 months and 6 months (and counsel)

Question 37 yo WM HIV+, on HAART 3 years since presenting with CMV retinitis. CD 4 gone from 12 to 480 over 18 months. He has been undetectable for a year. He is on Bactrim, azithromycin and valganciclovir. What can you stop? n All 3 prophylactic drugs.

Question n What virus presents as a rash in kids, arthritis in adults? How does it present in HIV patients? n Parvovirus B 19 Severe anemia n

Name that HIV drug… n n n n Causes bone marrow suppression? Pancreatitis? Absolutely contraindicated in pregnancy? Severe flu-like hypersensitivity reaction, fatal on rechallenge? diabetes Rash and hepatitis? Peripheral neuropathy? Nightmares? Nephrolithiasis? Lactic acidosis? Lipodystrophy? Ingrown toenails? Worst diarrhea? hyperlipidemia n Zidovudine Didanosine, stavudine n Efavirenz n Abacavir PIs Nevirapine Didanosine, stavudine Efavirenz Indinavir Stavudine, didanosine, etc. PIs and RTIs indinavir Nelfinavir PIs n n n
 Daintree rainforest average rainfall
Daintree rainforest average rainfall Eric liddell quotes
Eric liddell quotes Edith liddell
Edith liddell Jerry allison
Jerry allison Jerry allison
Jerry allison Graham allison models
Graham allison models Who are considered the father of new criticism
Who are considered the father of new criticism Dr allison dean
Dr allison dean Allison mankin
Allison mankin Symbols in the poisonwood bible
Symbols in the poisonwood bible Allison morgan ts
Allison morgan ts Shower curtain blows inward
Shower curtain blows inward Allison corbat
Allison corbat Monitor model
Monitor model Dr allison clarke
Dr allison clarke Kirsten norris
Kirsten norris Allison cowie
Allison cowie Allison
Allison Allison bloodworth
Allison bloodworth Allison rossett
Allison rossett Allison rislov
Allison rislov Allison keegan
Allison keegan Avatrombopag
Avatrombopag Tracy allison mediation
Tracy allison mediation Well ordering principle
Well ordering principle Allison maclaurin
Allison maclaurin Allison beth quillin
Allison beth quillin Allison bentley
Allison bentley Allison lackie
Allison lackie Willow sweeney
Willow sweeney Alisson tayler
Alisson tayler Bilali bounama
Bilali bounama Allison gauss
Allison gauss Allison
Allison Jerry allison
Jerry allison Allison horst
Allison horst Professor robert allison
Professor robert allison Allison vaillancourt
Allison vaillancourt El club de los 5
El club de los 5 Allison mumbleau
Allison mumbleau Jerry allison
Jerry allison The king of all media
The king of all media Dr lee allison
Dr lee allison Allison hamlin
Allison hamlin Takedown book allison van diepen
Takedown book allison van diepen Allison zmuda
Allison zmuda Garry allison
Garry allison Allison bloodworth
Allison bloodworth Holman frenia allison
Holman frenia allison Allison fansler
Allison fansler Mount allison university bookstore
Mount allison university bookstore Allison mattingly
Allison mattingly Chuck allison
Chuck allison Cbic recertification
Cbic recertification Conclusion of infection control
Conclusion of infection control Infection controlcare home
Infection controlcare home Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Environmental controls infection control
Environmental controls infection control Kidney infection
Kidney infection