HIVAIDS An Update Allison M Liddell MD Infectious










































































- Slides: 101

HIV/AIDS An Update Allison M. Liddell, MD Infectious. Care January 2 nd, 2006

HIV Curriculum z. Year 1: HIV Pathogenesis and Antiretroviral Therapy z. Year 2: Opportunistic Infections z. Year 3: HIV miscellaneous issues: Postexposure prophylaxis, pregnancy, neurologic disease, wasting syndrome, new drugs, HIV stigma, other special groups.

HIV Background z Late 1970’s Retroviruses described (HTLV) z 1981: AIDS recognized z Kaposi’s sarcoma, PCP appeared z risk groups identified z 1982: theory of HIV z 1983: HIV-1 identified z blood supply screening z 1983 AZT approved z 1994 PI’s approved z 1994 PACTG-076


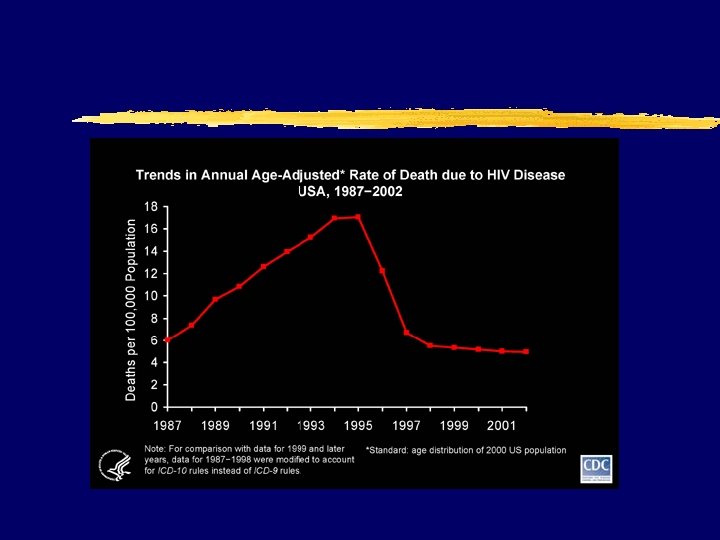
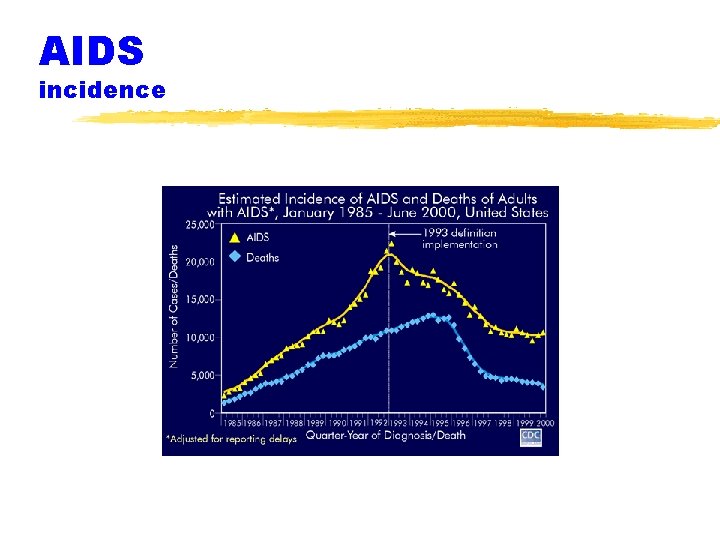
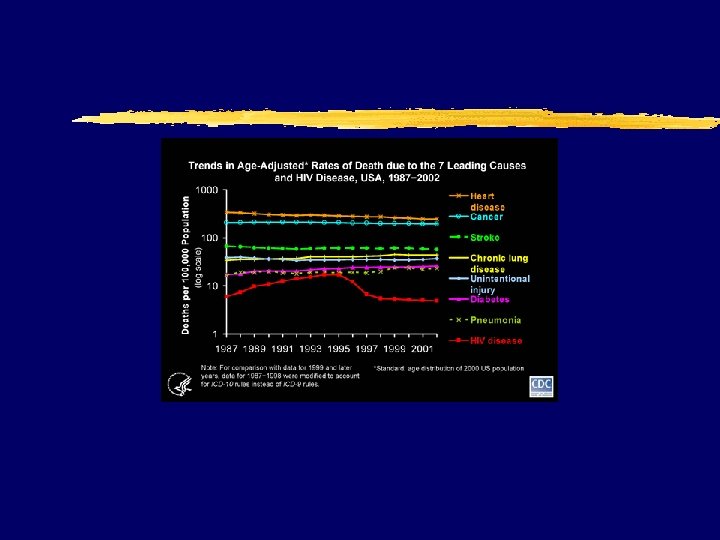
AIDS incidence








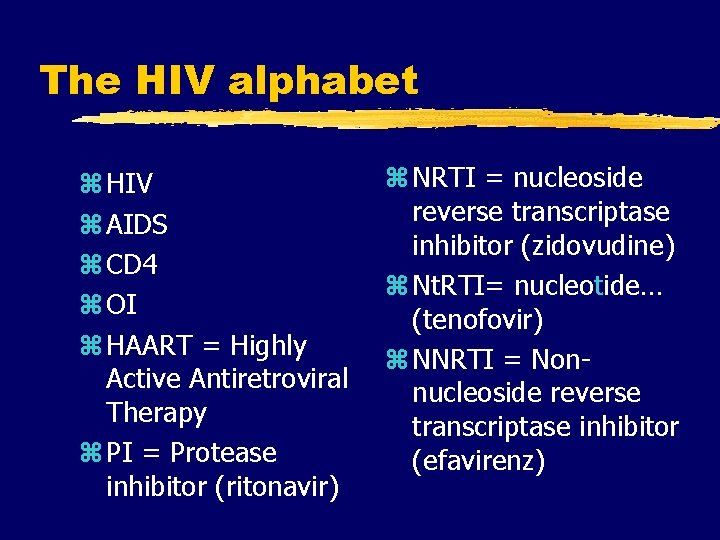
The HIV alphabet z HIV z AIDS z CD 4 z OI z HAART = Highly Active Antiretroviral Therapy z PI = Protease inhibitor (ritonavir) z NRTI = nucleoside reverse transcriptase inhibitor (zidovudine) z Nt. RTI= nucleotide… (tenofovir) z NNRTI = Nonnucleoside reverse transcriptase inhibitor (efavirenz)

AIDS case definition z CDC: 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR 1992; 41(no. RR-17). z includes all HIV-infected persons who have less than 200 CD 4+ T-lymphocytes/u. L or a CD 4+ T-lymphocyte percent of total lymphocytes less than 14 z added pulmonary tuberculosis, invasive cervical cancer, or recurrent pneumonia to list of >20 AIDS defining illnesses

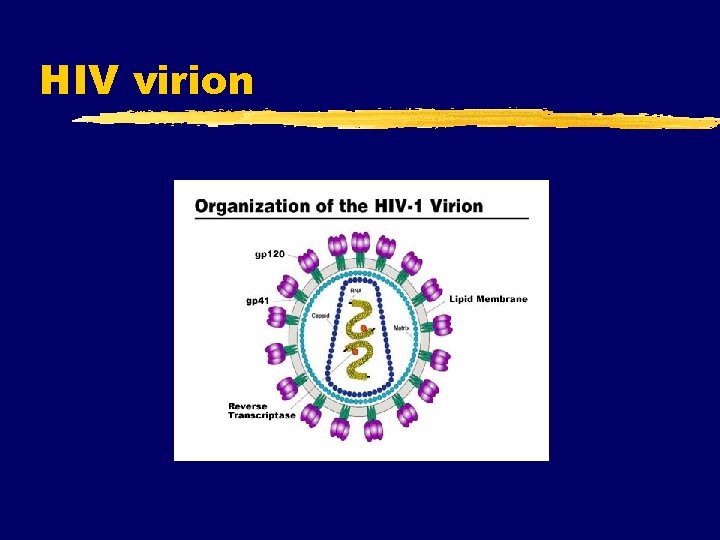
HIV virion

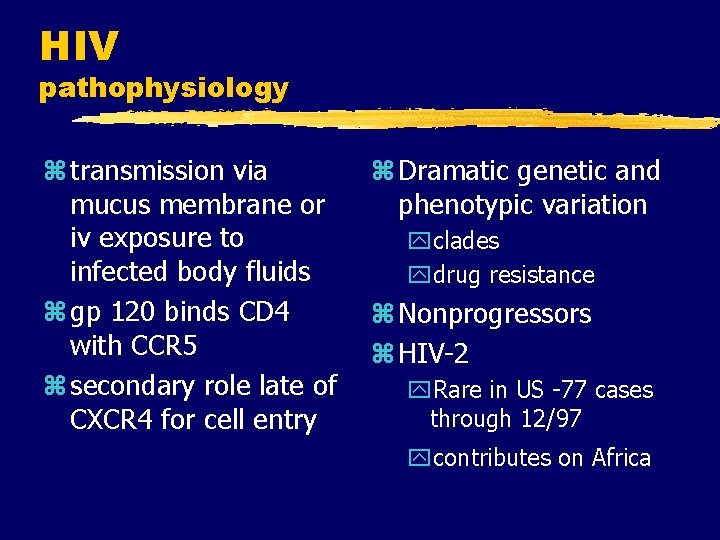
HIV pathophysiology z transmission via mucus membrane or iv exposure to infected body fluids z gp 120 binds CD 4 with CCR 5 z secondary role late of CXCR 4 for cell entry z Dramatic genetic and phenotypic variation yclades ydrug resistance z Nonprogressors z HIV-2 y. Rare in US -77 cases through 12/97 ycontributes on Africa

HIV pathogenesis

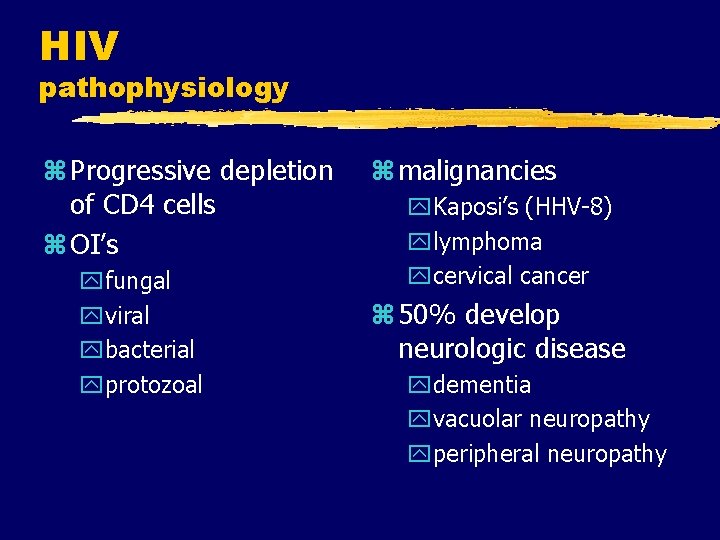
HIV pathophysiology z Progressive depletion of CD 4 cells z OI’s yfungal yviral ybacterial yprotozoal z malignancies y. Kaposi’s (HHV-8) ylymphoma ycervical cancer z 50% develop neurologic disease ydementia yvacuolar neuropathy yperipheral neuropathy

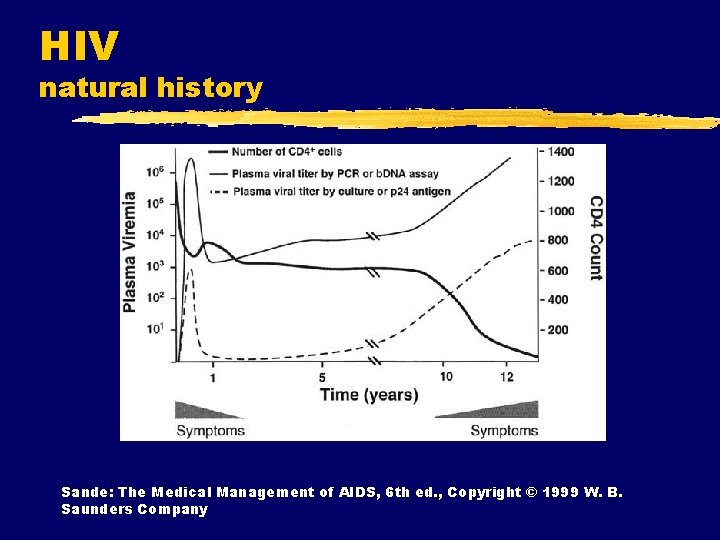
HIV natural history Sande: The Medical Management of AIDS, 6 th ed. , Copyright © 1999 W. B. Saunders Company

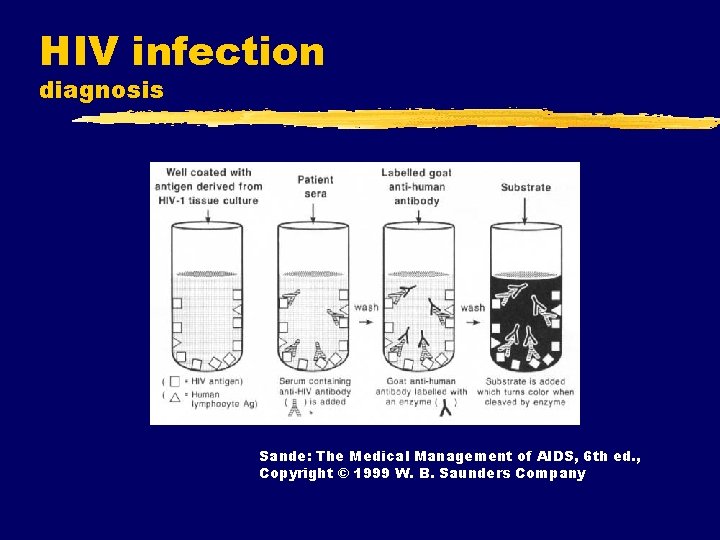
HIV infection diagnosis Sande: The Medical Management of AIDS, 6 th ed. , Copyright © 1999 W. B. Saunders Company

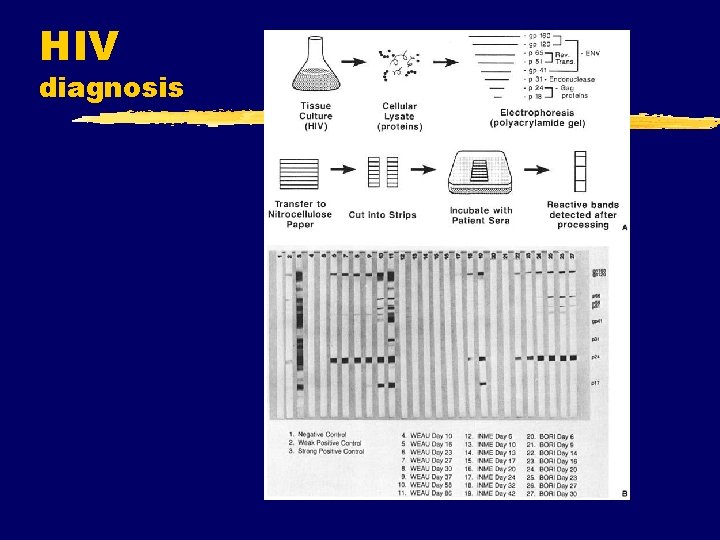
HIV diagnosis

Four FDA-approved Rapid HIV Tests Ora. Quick Advance - whole blood - oral fluid - plasma Uni-Gold Recombigen - whole blood - serum/plasma Reveal G 2 - serum - plasma Multispot - serum/plasma - HIV-2

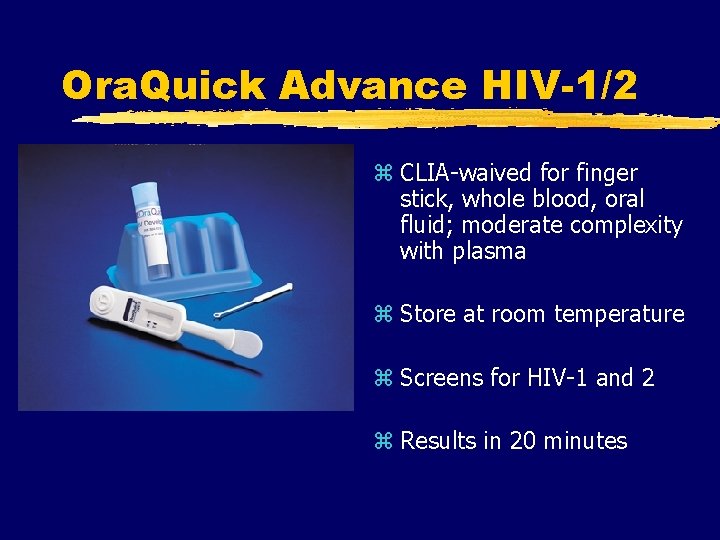
Ora. Quick Advance HIV-1/2 z CLIA-waived for finger stick, whole blood, oral fluid; moderate complexity with plasma z Store at room temperature z Screens for HIV-1 and 2 z Results in 20 minutes

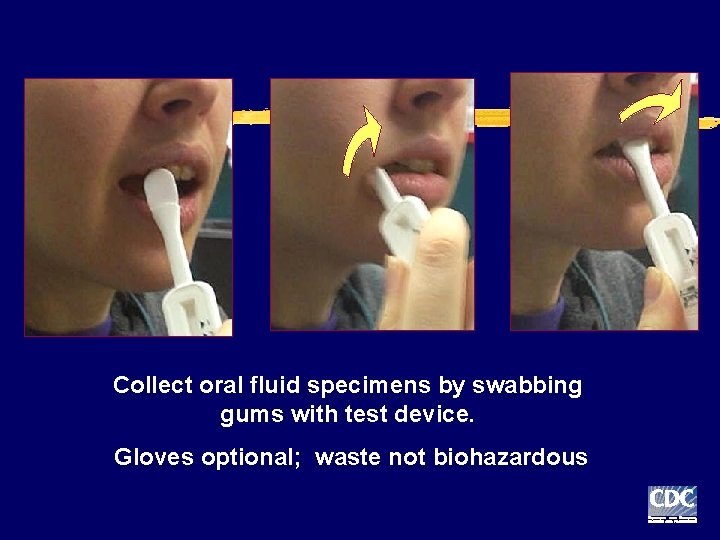
Collect oral fluid specimens by swabbing gums with test device. Gloves optional; waste not biohazardous

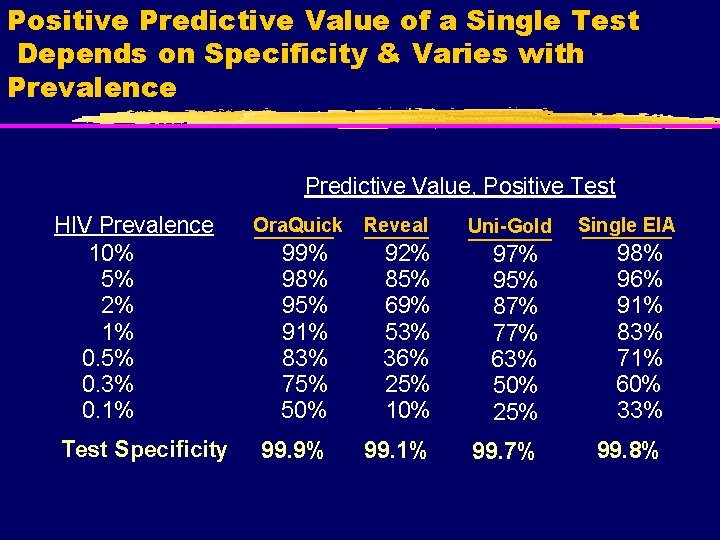
Positive Predictive Value of a Single Test Depends on Specificity & Varies with Prevalence Predictive Value, Positive Test HIV Prevalence 10% 5% 2% 1% 0. 5% 0. 3% 0. 1% Test Specificity Ora. Quick Reveal Uni-Gold Single EIA 99% 98% 95% 91% 83% 75% 50% 92% 85% 69% 53% 36% 25% 10% 97% 95% 87% 77% 63% 50% 25% 98% 96% 91% 83% 71% 60% 33% 99. 9% 99. 1% 99. 7% 99. 8%

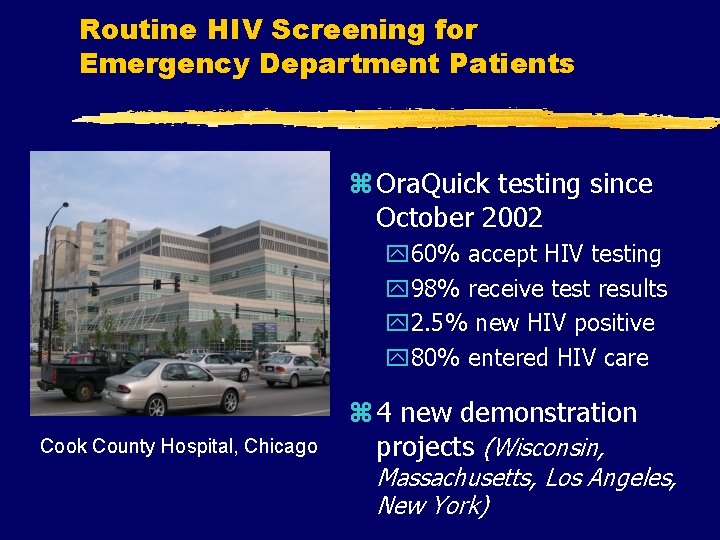
Routine HIV Screening for Emergency Department Patients z Ora. Quick testing since October 2002 y 60% accept HIV testing y 98% receive test results y 2. 5% new HIV positive y 80% entered HIV care Cook County Hospital, Chicago z 4 new demonstration projects (Wisconsin, Massachusetts, Los Angeles, New York)

Characteristics Rapid Test Positive Patients

Advancing HIV Prevention: New Strategies for a Changing Epidemic z Four priorities: 1. Make voluntary HIV testing a routine part of medical care 2. Implement new models for diagnosing HIV infections outside medical settings 3. Prevent new infections by working with persons diagnosed with HIV and their partners 4. Further decrease perinatal HIV transmission MMWR April 18, 2003

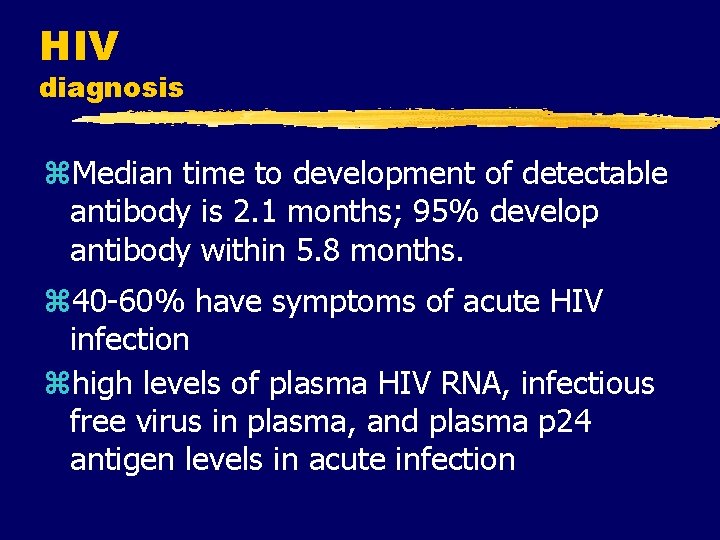
HIV diagnosis z. Median time to development of detectable antibody is 2. 1 months; 95% develop antibody within 5. 8 months. z 40 -60% have symptoms of acute HIV infection zhigh levels of plasma HIV RNA, infectious free virus in plasma, and plasma p 24 antigen levels in acute infection






Acute HIV Infection: Treatment Possible benefits: Possible risks: z Decrease the severity of acute disease z Alter the viral “set point” z Reduce the rate of mutation z Preserve immune function z Reduce risk of viral transmission z Drug-related adverse effects on quality of life z Earlier emergence of drug resistance z Limitation of future treatment options z Potential need for indefinite treatment

HIV Chronic Infection z Establish relationship z Establish stage of disease z Counsel regarding prognosis and transmission (including vertical) z Screen for coexisting OI y RPR y toxo y hepatitis y tuberculosis y ROS y neurologic exam y skin exam y PAP smear, GC/chlaamydia z Lipids z Resistance testing

AIDS Drug therapy


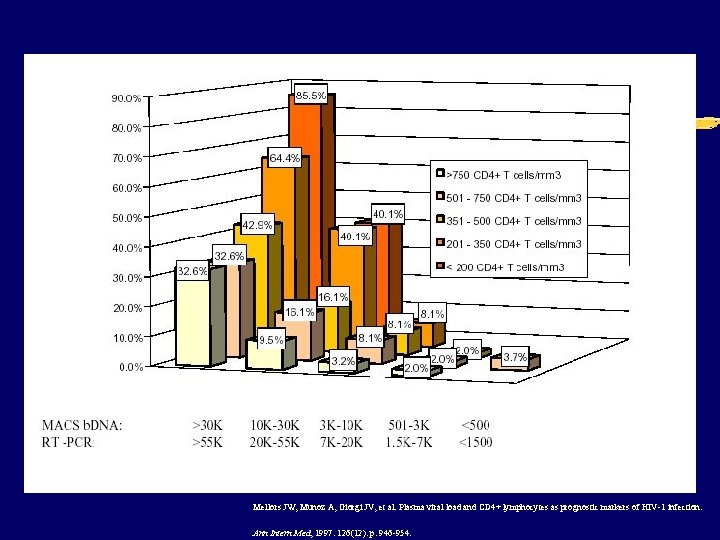
Prognosis per CD 4 3 year probability AIDS/death (%) vl<105 vl>105 0 -49 16 20 5 -99 12 16 100 -199 9. 3 12 200 -349 4. 7 6. 1 >350 3. 4 5. 4

Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD 4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med, 1997. 126(12): p. 946 -954.



Indications for Initiation of Therapy: Chronic Infection z. Defer therapy for patients with viral load <100, 000 and CD 4 >350 (2005 recs)

HIV treatment considerations z Goals y Maximal and durable viral suppression (by 16 -24 wks) y Restoration and/or preservation of immunologic function x. CD 4 rise 100 -150/year y Improved quality of life y Reduction of HIV-related morbidity and mortality z Toxicity z drug interactions z disease interactions z pharmacokinetics z Adherence z HIV expertise y>20 pts asso w/improved outcomes y>50 even better

HIV treatment considerations Tools to Achieve Goals of Therapy · Maximize adherence to the antiretroviral regimen · Address substance abuse, depression, lack of social support · Rational sequencing of drugs · Preservation of future treatment options · Reserve 2 classes · Use of resistance testing in selected clinical settings

Antiretroviral agents z. NRTIs y. Always use at least 2 z. NNRTIs z. Protease inhibitors z. Fusion inhibitors

HIV Drug therapy

Inhibition of Fusion HIV-1 gp 41 CCR-5 gp 120 CD 4 gp 41 ENF

Current Antiretroviral Medications NRTI z z z z Abacavir Didanosine Emtricitabine Lamivudine Stavudine Zidovudine Zalcitabine Tenofovir NNRTI z Delavirdine z Efavirenz z Nevirapine PI ABC DDI FTC 3 TC D 4 T ZDV DDC TDF DLV EFV NVP Amprenavir Atazanavir Fosamprenavir Indinavir Lopinavir Nelfinavir Ritonavir Saquinavir y soft gel y hard gel y tablet z Tipranavir z z z z Fusion Inhibitor z Enfuvirtide APV ATV FPV IDV LPV NFV RTV SQV SGC HGC INV TPV T-20

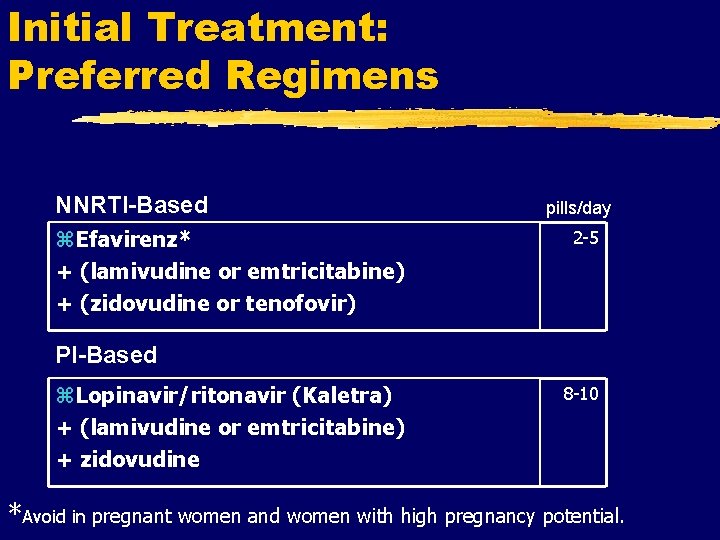
Initial Treatment: Preferred Regimens NNRTI-Based z. Efavirenz* + (lamivudine or emtricitabine) + (zidovudine or tenofovir) pills/day 2 -5 PI-Based z. Lopinavir/ritonavir (Kaletra) + (lamivudine or emtricitabine) + zidovudine 8 -10 *Avoid in pregnant women and women with high pregnancy potential.

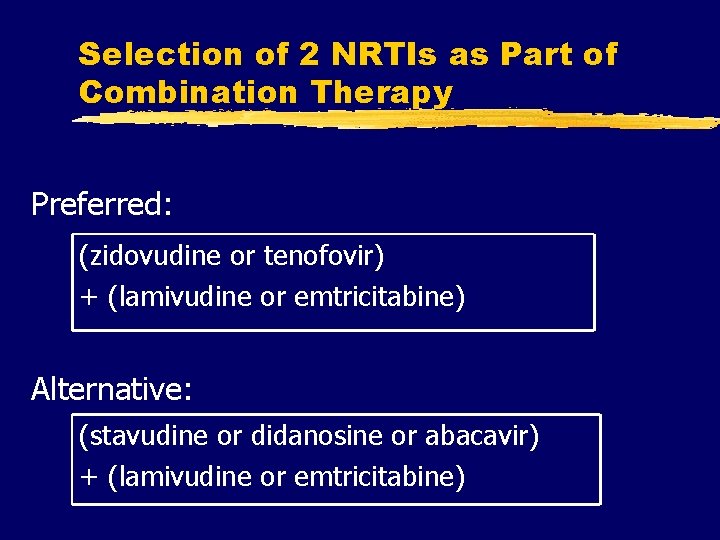
Selection of 2 NRTIs as Part of Combination Therapy Preferred: (zidovudine or tenofovir) + (lamivudine or emtricitabine) Alternative: (stavudine or didanosine or abacavir) + (lamivudine or emtricitabine)

Antiretroviral Medications: Not Recommended in Initial Treatment (1) High rate of virologic failure z Didanosine + tenofovir + NNRTI Inferior antiviral activity z Delavirdine High incidence of toxicities z z Zidovudine + zalcitabine Saquinavir (unboosted) Stavudine + didanosine Ritonavir used as sole PI

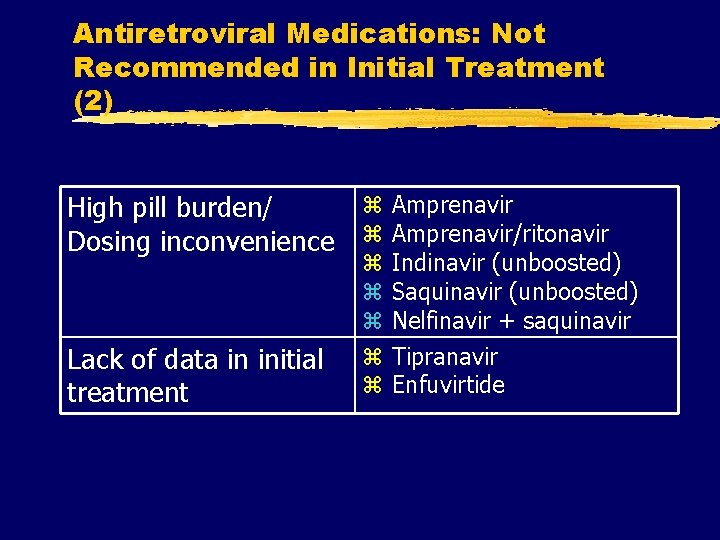
Antiretroviral Medications: Not Recommended in Initial Treatment (2) High pill burden/ Dosing inconvenience Lack of data in initial treatment z z z z Amprenavir/ritonavir Indinavir (unboosted) Saquinavir (unboosted) Nelfinavir + saquinavir Tipranavir Enfuvirtide

Antiretroviral Medications: Should not be offered at any time z. Regimens not recommended: y. Monotherapy (except possibly in prevention of perinatal HIV transmission) y. Dual NRTI therapy y. Didanosine + tenofovir + NNRTI y 3 -NRTI regimen of abacavir + tenofovir + lamivudine y 3 -NRTI regimen of didanosine + tenofovir + lamivudine

Antiretroviral Medications: Should not be offered at any time z. Antiretroviral components not recommended: y. Efavirenz in pregnancy and in women with high potential for pregnancy* y. Nevirapine initiation in women with CD 4 >250 cells/mm 3 or men with CD 4 >400 cells/mm 3 * Women with high pregnancy potential are those who are trying to conceive or who are not using effective and consistent contraception.

HIV RTI’s

HIV NNRTI’s

Adverse Effects: PIs z All PIs: y. Hyperlipidemia y. Insulin resistance and diabetes y. Lipodystrophy y. Elevated liver function tests y. Possible increased bleeding risk in hemophiliacs y. Drug-drug interactions

Adverse Effects: PIs z Amprenavir and fosamprenavir - GI intolerance, rash z Atazanavir - hyperbilirubinemia, PR interval prolongation z Indinavir - nephrolithiasis, GI intolerance z Lopinavir/ritonavir - GI intolerance z Nelfinavir - diarrhea z Ritonavir - GI intolerance, hepatitis z Tipranavir – GI intolerance, rash, hyperlipidemia, liver toxicity

HIV Drug Interactions z. Most important with NNRTI’s and PI’s z. Always look up drug interactions every time you prescribe a drug to a patient on HAART Just do it!!!

Adverse reactions Fusion inhibitors z. Enfuvirtide y. Injection site reaction y. Hypersensitivity y. Eosinophilia y. Possible increased pneumonia








HAART failure z. Resistance testing z. Maintain even partial suppression complete cessation associated w/rapid progression to AIDS/death









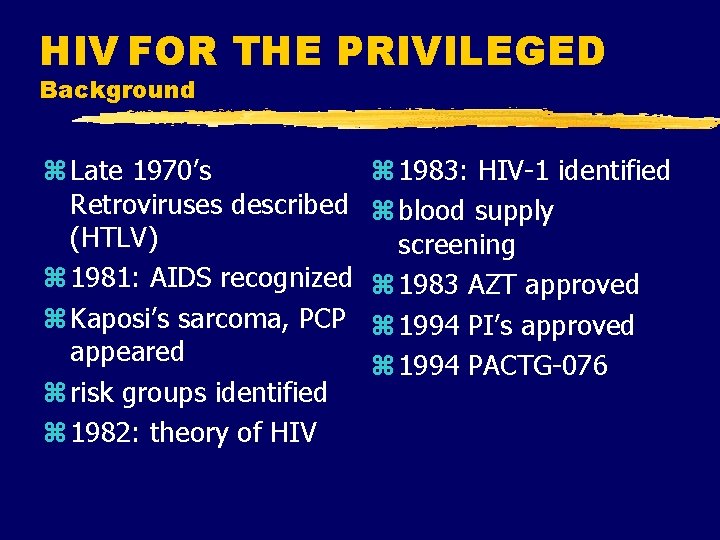
HIV FOR THE PRIVILEGED Background z Late 1970’s Retroviruses described (HTLV) z 1981: AIDS recognized z Kaposi’s sarcoma, PCP appeared z risk groups identified z 1982: theory of HIV z 1983: HIV-1 identified z blood supply screening z 1983 AZT approved z 1994 PI’s approved z 1994 PACTG-076

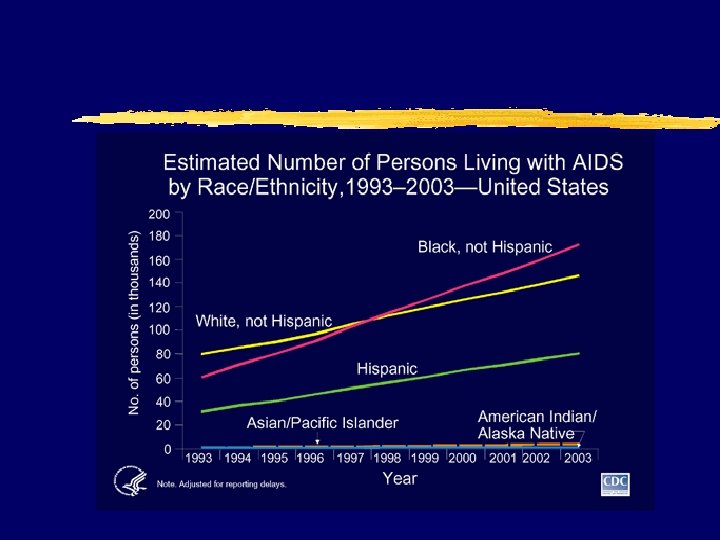
HIV: The Reality z. In the US y. As of December 31, 2000: x 774, 467 persons were reported with AIDS x 448, 060 had died xnumber of persons living with AIDS (322, 865) was the highest ever reported x. Approximately 800, 000 --900, 000 persons in the United States are infected with HIV xapproximately 275, 000 of these persons might not know they are infected

HIV: The Reality Worldwide z In 2002, 14, 000 people/day became infected z At end of 2002 y 42 million people worldwide were living with HIV/AIDS. x 38. 6 million adults x 3. 2 million children younger than 15 years y. Approximately 70 % (29. 4 million) live in Sub-Saharan Africa x 9 % of adults between age 15 and 49 x. In 4 African countries, it’s 30%

Ask the Question Offer CTS-counseling, testing and referral to everyone



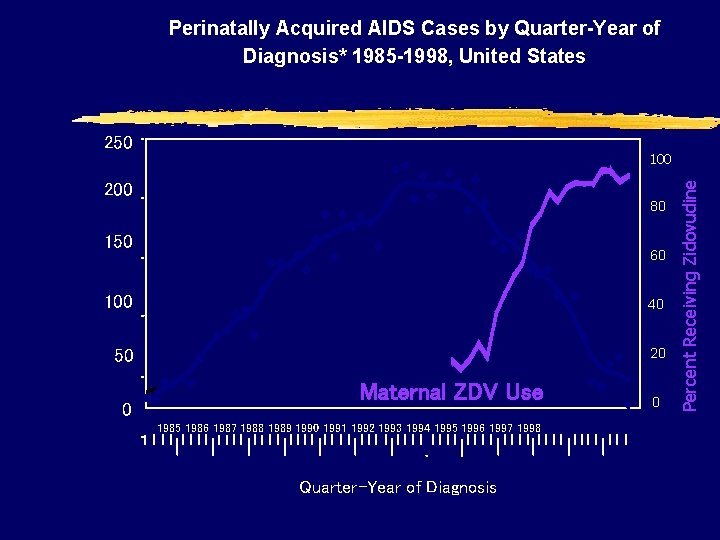
Perinatally Acquired AIDS Cases by Quarter-Year of Diagnosis* 1985 -1998, United States 200 100 Pediatric AIDS Cases 80 150 60 100 40 50 20 0 Maternal ZDV Use 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 Quarter-Year of Diagnosis 0 Percent Receiving Zidovudine Number of Cases 250

The Epidemiology of HIV/AIDS in Women • Women represent the fastest growing • • segment of the HIV epidemic (35% of all new infections in the United States) The CDC estimates that women will account for half of all infections in the United States by the end of 2002 85% of women with HIV are of childbearing age

AIDS Cases in Women 13 Years of Age By Age at Diagnosis, Reported in 1998, United States

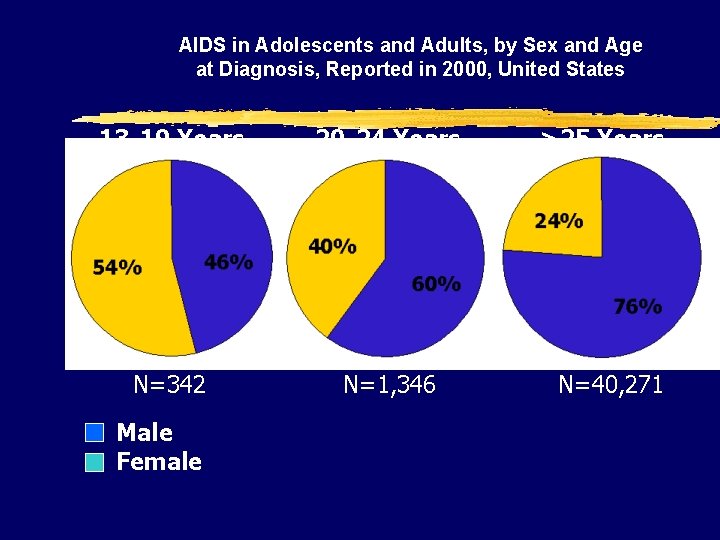
AIDS in Adolescents and Adults, by Sex and Age at Diagnosis, Reported in 2000, United States 13 -19 Years 20 -24 Years N=342 N=1, 346 Male Female >25 Years N=40, 271

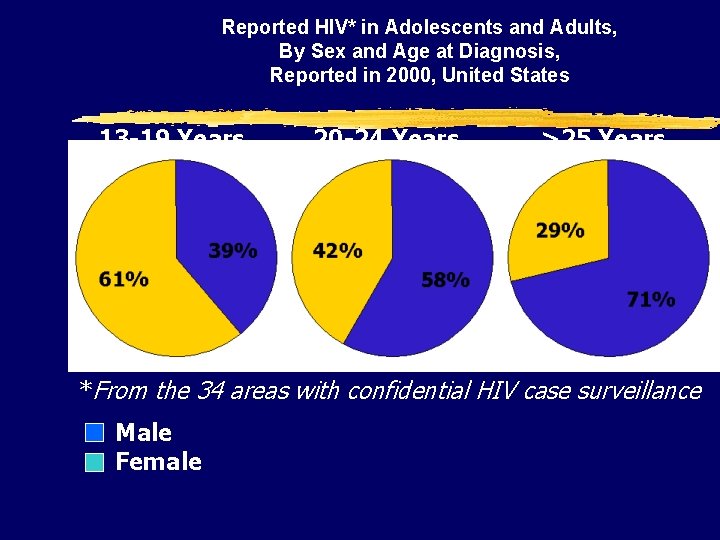
Reported HIV* in Adolescents and Adults, By Sex and Age at Diagnosis, Reported in 2000, United States 13 -19 Years 20 -24 Years >25 Years *From the 34 areas with confidential HIV case surveillance Male Female

Public Health Rationale for Screening • 85% of women with HIV / AIDS are of child-bearing age • It causes significant morbidity and • mortality Tests are acceptable, reliable, and valid

Public Health Rationale for Screening • Interventions are acceptable and of • • proven benefit Benefits of intervention far exceed the cost of screening It upholds women’s right to make informed decisions about testing and to know their HIV status

Guidelines for Screening • Screening should be performed • annually, along with other family planning services Screening should also be performed in response to risk factors, with retesting as needed and agreed to by the patient

Health Care Providers Diagnosing HIV Infection

Who Initiates Testing? In one study …. In this study, 8 providers denied testing to women who specifically asked to be tested

Definition of “Missed Opportunity” Missed Opportunity = An encounter with a health care provider for any reproductive health issue—regardless of risk factors or any contact with any other HCP—by a patient engaging in high-risk behavior where testing was not performed. —Mac. Donald et al. , 1998

Clients should be counseled in Risk Reduction Methods Vaginal and anal sex: • • • Condom use Female condom use Abstinence Reduce # of partners Limit the use of substances prior to sexual activity • Monogamy Oral sex: • Condom use • Dental dam or plastic wrap use • No brushing or flossing prior to performing oral sex • Avoiding ejaculation inside the mouth

Clients should be counseled in Risk Reduction Methods Injection Drug Use • Needle exchange • Cleaning with bleach in absence of new needles • No sharing of works • Don’t inject (other, less risky routes available) Non-Injection Drugs • Reduce intake • Don’t combine drugs • Choose the least risky route • Avoid sexual activity when drunk or high

Contraception and Prevention of HIV 1 Infection z. The only methods which have strong evidence supporting their efficacy to protect against HIV infection are condoms and female condoms z. All clients need to be informed of this fact, and counseled in the consistent and correct usage of these methods

Youth Risk - Understand Youth Risk Behaviors Illinois Female YRBS 1999 38. 1% 26. 4% 16. 9% 42. 9% grades 9 -12 have had intercourse >4 partners sex in past 3 months used alcohol/drugs during most recent episode did not use a condom during the last episode

New drugs

New drugs

New drugs
 Mike liddell
Mike liddell Eric liddell quotes
Eric liddell quotes Edith liddell
Edith liddell Backup and recovery techniques
Backup and recovery techniques Infectious canine hepatitis in dogs
Infectious canine hepatitis in dogs Stages of infection
Stages of infection Hennepin county infectious disease manual
Hennepin county infectious disease manual Infectious disease board review
Infectious disease board review Infectious stunting syndrome
Infectious stunting syndrome Blood smear
Blood smear Infectious nucleic acid
Infectious nucleic acid Chapter 26 infectious disease prevention and control
Chapter 26 infectious disease prevention and control Epidemiological triad
Epidemiological triad Certain infectious and parasitic diseases
Certain infectious and parasitic diseases Infectious waste management
Infectious waste management Infectious disease
Infectious disease Stages of infectious disease
Stages of infectious disease Snc set symbol
Snc set symbol What is the smallest infectious disease agent
What is the smallest infectious disease agent Infectious disease quality controls
Infectious disease quality controls Infectious mononucleosis
Infectious mononucleosis Infectious canine hepatitis in dogs
Infectious canine hepatitis in dogs Noncellular infectious protein particles are called
Noncellular infectious protein particles are called Jerry allison
Jerry allison Allison barfield
Allison barfield Dr lee allison
Dr lee allison Rachel poisonwood bible
Rachel poisonwood bible Takedown book allison van diepen
Takedown book allison van diepen Allison mattingly
Allison mattingly What does pascal’s principle state?
What does pascal’s principle state? Dr allison clarke
Dr allison clarke Holman frenia allison, p.c.
Holman frenia allison, p.c. Allison mankin
Allison mankin The king of all media
The king of all media Allison fansler
Allison fansler Allison
Allison Allison morgan ts
Allison morgan ts Jerry allison
Jerry allison Allison keegan
Allison keegan Jerry allison
Jerry allison Tracy allison mediation
Tracy allison mediation Now allison dean
Now allison dean Allison cowie
Allison cowie Greatest lower bound and least upper bound
Greatest lower bound and least upper bound Allison bentley
Allison bentley Allison corbat
Allison corbat Allison sweeney bio
Allison sweeney bio Graham allison models
Graham allison models Allison gauss
Allison gauss Kirsten norris
Kirsten norris Allison maclaurin
Allison maclaurin Scenario ux example
Scenario ux example Allison horst
Allison horst Allison rossett
Allison rossett Bilali bounama
Bilali bounama Monitor model
Monitor model Allison m. vaillancourt
Allison m. vaillancourt Avatrombopag
Avatrombopag Allison reynolds personalidad
Allison reynolds personalidad Allison
Allison Allison beth quillin
Allison beth quillin Oral surgeon hamlin
Oral surgeon hamlin Kohl's organizational chart
Kohl's organizational chart Garry allison
Garry allison Allison tayler
Allison tayler Allison mumbleau
Allison mumbleau Mount allison university bookstore
Mount allison university bookstore Htc grid mammography
Htc grid mammography Allison zmuda
Allison zmuda Allison lackie
Allison lackie Robert allison sexual assault
Robert allison sexual assault Chuck allison
Chuck allison Allison bloodworth
Allison bloodworth Gmod
Gmod Sab update.com download
Sab update.com download Smart view update
Smart view update Zechariah 2:8-9
Zechariah 2:8-9 Www.gov.ukdbs-update-service
Www.gov.ukdbs-update-service Multiplicative update
Multiplicative update Databze
Databze Bankhead primary school
Bankhead primary school Recovery techniques based on immediate update
Recovery techniques based on immediate update Dhl seafreight
Dhl seafreight Update cmp
Update cmp 811 ticket number
811 ticket number Update windows 7
Update windows 7 Deferred update
Deferred update Position update formula
Position update formula Java game loop
Java game loop Contoh lost update problem
Contoh lost update problem Multiplicative weights update algorithm
Multiplicative weights update algorithm Windws update
Windws update Sql insert update delete query
Sql insert update delete query Section 508 update
Section 508 update Sclm ibm
Sclm ibm Non-updating function module called for updating
Non-updating function module called for updating Microsoft update
Microsoft update Igel management console
Igel management console Mdh situation update
Mdh situation update Iso legislation update
Iso legislation update Eis standards
Eis standards Kb2533623 update for windows 7
Kb2533623 update for windows 7