History of the Atomic Theory and Atomic Structure













- Slides: 25

History of the Atomic Theory and Atomic Structure

Democritus (460 -370 B. C. ) • Greek philosopher • Matter is made of small particles • He named these “ultimate“ particles atoms

Aristotle (384 -322 B. C. ) • Disputed Democritus theory • Always have a smaller piece of matter, no matter the size • Only 4 elements • Theory accepted for 2000 years • Tutor of Alexander

Antoine Lavoisier (1743 -1794) • Law of Conservation of Mass = • When a chemical reaction takes place, matter is not created or destroyed, just changed. • Named oxygen • Beheaded by guillotine

Conservation of Matter • Matter cannot be created or destroyed, just changed. • In a reaction the mass you start with is the mass you end with.

Joseph Proust (1754 -1826) • Law of Definite Proportions = compounds always have the same proportions of elements by mass. • Super radical for his time

John Dalton (1766 -1844) • English schoolteacher and Chemist • Proved that atoms exist • Model a solid sphere • Colorblind (Daltonism)

Dalton also…. • proposed the Law of Multiple Proportions which led directly to the proposal of the Atomic Theory • developed the concept of the mole • proposed a system of symbols to represent atoms of different elements.

Dalton’s Atomic Theory • • • He developed the first atomic theory All matter is made up of atoms Atoms are indestructible and cannot be divided into smaller particles • Atoms of the same element are exactly alike and atoms of different elements are different • In a given compound, the relative number of atoms are constant

J. J. Thomson (1856 -1940) • English Nobel Prize winner • Said atom could be divided into smaller parts • Discovered electrons • “Plum pudding” model of atom • Discovered isotopes of elements

Thomson’s Plum Pudding Model • In this model, the volume of the atom is composed primarily of the more massive (thus larger) positive portion (the plum pudding). The smaller electrons (actually, raisins in the plum pudding ) are dispersed throughout the positive mass to maintain charge neutrality.

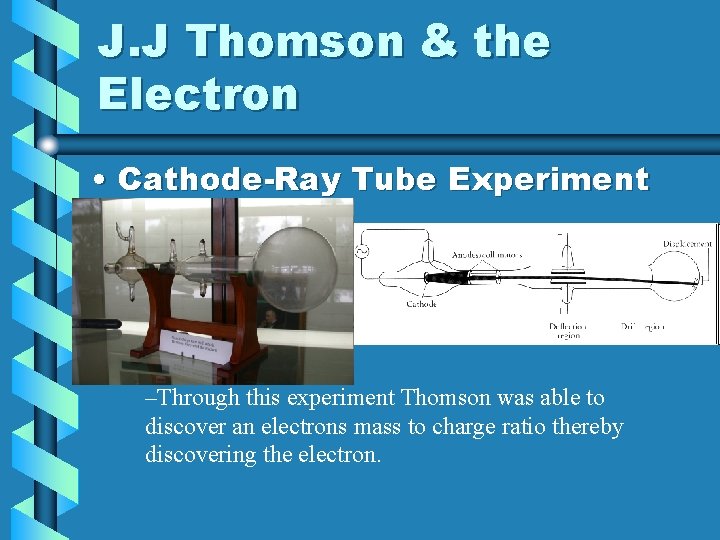
J. J Thomson & the Electron • Cathode-Ray Tube Experiment –Through this experiment Thomson was able to discover an electrons mass to charge ratio thereby discovering the electron.

Eugene Goldstein (1850 -1930) • 1900 • Discovered proton (sometimes gets credit) • Determined protons have a (+) charge • Anode ray experiment • Still supported Thompson’s Plum Pudding model of the atom

Robert Millikan (1868 -1953) • determined the unit charge of the electron with his oil drop experiment • This allowed for the calculation of the mass of the electron and the positively charged atoms.

Ernest Rutherford (1871 -1937) • Worked for Thomson • British physicist • Discovered atom had nucleus • Gold foil experiment

Rutherford’s Gold Foil Experiment • Bombarded thin sheet Au foil w/ positively charged alpha particles. If plum pudding model is right, alpha particles should sail straight through. Some bounced back. He concluded that atom has small, dense, positive core called nucleus. Electrons inside nucleus with lots of empty space between them.

James Chadwick (1892 -1974) • Nucleus also contains particles with no charge called neutrons • discovery of the neutron led directly to the discovery of fission and ultimately to the atomic bomb.

Niels Bohr (1885 -1962) • Danish Nobel Prize winner • Electrons move around nucleus in fixed orbits like planets do around the sun

Francis Aston (1877 -1945) • first person to observe isotopes • His work led Rutherford to predict the existence of the neutron

Louis de. Broglie (1892 -1960) • He was a prince • Won Nobel prize in physics 1929 • Wave-particle dualitly theory of light based upon Planck and Einstein • This created new field of physics – wave mechanics

Werner Heisenberg (1901 -1976) • 1932 Nobel prize Physics for “creation of Quantum mechanics” • Uncertainty principle • “cloud” model (quantum mechanic model)

Max Planck (1858 -1947) • Founder of quantum theory • Nobel prize 1918 • Revolutionized understanding of atomic and subatomic processes • Planck’s constant • Einstein used his work to study photons

Erwin Schrodinger (1887 -1961) • Nobel Prize 1933 • One of founders of quantum mechanics • Schrodinger's equation • Schrodinger’s cat

The Atom l. The smallest particle of an element that retains the same characteristics of that element.

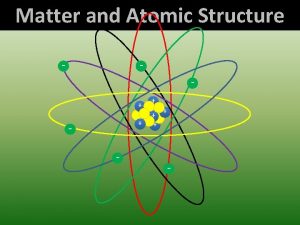
Modern Atomic Model • Electron cloud model • Atom has small positively charged nucleus surrounded by a large negatively charged region of electrons
 1930 modern atom teorisi
1930 modern atom teorisi History of atomic models timeline
History of atomic models timeline Relative formula mass of hcl
Relative formula mass of hcl Mass of oxygen
Mass of oxygen Atomic
Atomic Trends periodic table
Trends periodic table The atomic radius in periodic table
The atomic radius in periodic table Atomic number vs atomic radius
Atomic number vs atomic radius Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry Ap chemistry atomic structure and periodicity
Ap chemistry atomic structure and periodicity Structure of an atom
Structure of an atom Ape man for sodium
Ape man for sodium Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Inter atomic bonding
Inter atomic bonding Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton
Tư thế worm breton Hát lên người ơi
Hát lên người ơi Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực