Flu Mist Influenza Virus Vaccine Live Intranasal for





























- Slides: 65

Flu. Mist™ Influenza Virus Vaccine Live, Intranasal for Use in Healthy Children and Healthy Adults 5 – 64 Years of Age Sponsor Med. Immune Vaccines, Inc. (Formerly Aviron)

Sponsor Presentation James Young, Ph. D President, R&D n Overview, product description and indication Edward Connor, MD Senior Vice President, Clinical Development n n Data supporting efficacy/effectiveness Data supporting safety Transmission and genetic stability Conclusions 2

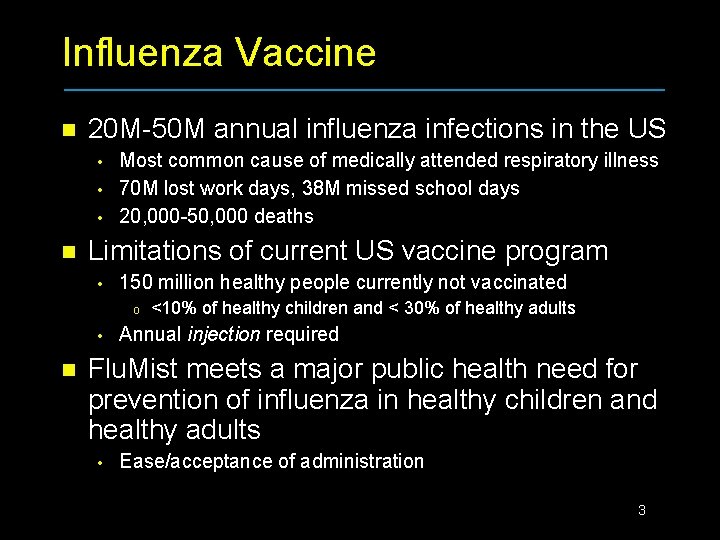
Influenza Vaccine n 20 M-50 M annual influenza infections in the US • • • n Most common cause of medically attended respiratory illness 70 M lost work days, 38 M missed school days 20, 000 -50, 000 deaths Limitations of current US vaccine program • 150 million healthy people currently not vaccinated o • n <10% of healthy children and < 30% of healthy adults Annual injection required Flu. Mist meets a major public health need for prevention of influenza in healthy children and healthy adults • Ease/acceptance of administration 3

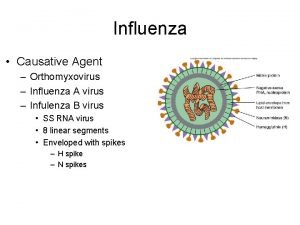
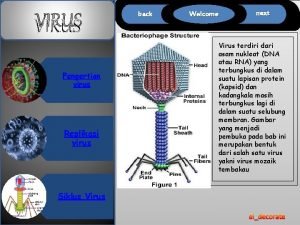
Flu. Mist™ n n Intranasal live, cold-adapted, temperature-sensitive, attenuated influenza virus vaccine Annual vaccine strains derived from type A and type B Master Donor Viruses Vaccine strains express contemporary hemagglutinin and neuraminidase Clinical trials performed with 14 vaccine strains • n 4 A/H 1 N 1, 5 A/H 3 N 2, 5 Type B Multiple mutations in multiple genes • • • At least four mutations in each MDV each confer attenuation in ferrets At least 7 mutations in MDV-A and 8 in MDV-B Phenotypically stable, reversion expected at frequency of < 10 -20 4

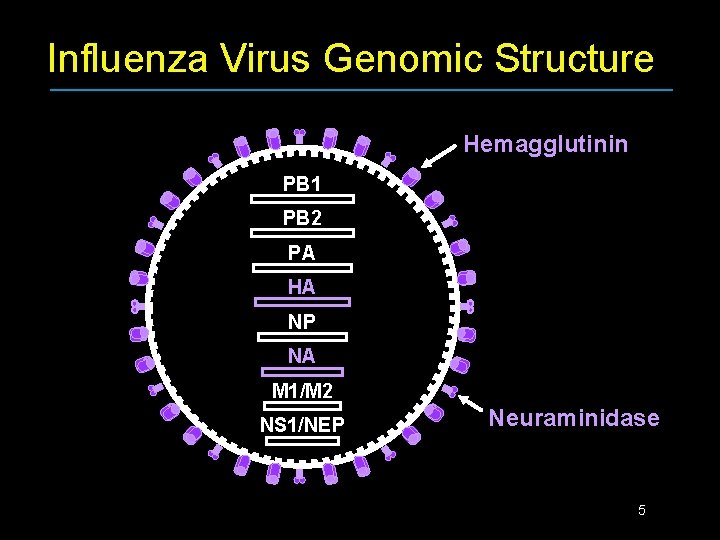
Influenza Virus Genomic Structure Hemagglutinin PB 1 PB 2 PA HA NP NA M 1/M 2 NS 1/NEP Neuraminidase 5

Derivation of New Master Virus Strain Master Donor Virus New Wild Type Strain Co-infect Cells Hemagglutinin and Neuraminidase Genes from Wild Type Confer Relevant Immunity Six genes from MDV Confer Attenuation 6: 2 Vaccine Strain 6

Flu. Mist™ n Manufactured in specific pathogen-free eggs n Comprehensive testing of chickens and vaccine n Safety testing of new vaccine strains in adults n Trivalent (A/H 1 N 1, A/H 3 N 2, B) • 107 TCID 50 of each strain / 0. 5 ml dose n Unit dose, stored frozen, no thimerosal n Administered by nasal sprayer • • • Large droplets (60µm average diameter; 98% >10 µm) Dose = 0. 5 ml (0. 25 ml / nostril) Single annual dose for > 9 years; two doses < 9 years 7

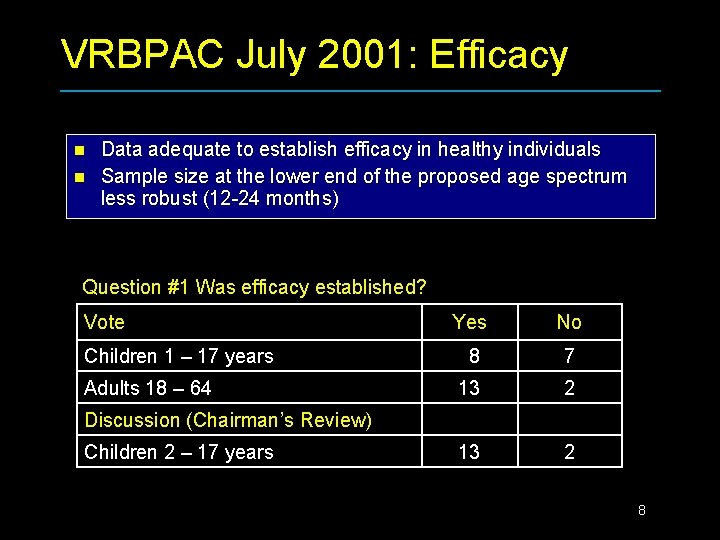
VRBPAC July 2001: Efficacy n n Data adequate to establish efficacy in healthy individuals Sample size at the lower end of the proposed age spectrum less robust (12 -24 months) Question #1 Was efficacy established? Vote Children 1 – 17 years Adults 18 – 64 Yes No 8 7 13 2 Discussion (Chairman’s Review) Children 2 – 17 years 8

VRBPAC July 2001: Safety n n n The final safety data had not been submitted and thus CBER review was ongoing Analysis needed to be completed to resolve open questions particularly those related to pneumonia and asthma Concurrent immunization data needed for children < 24 mos Question #2 Was safety established? Adults and children 1 – 64 years Yes No 4 10 Six of the 10 No votes were qualified as “provisional” until analysis complete 9

Progress Since July 2001 VRBPAC Meeting n n Responded to two Complete Response Letters Established 20 studies as final dataset in BLA • • • n Final analyses • • n 14 placebo-controlled, 6 open-label Representing ~20, 000 subjects receiving ~28, 000 doses of Flu. Mist Submitted final study reports for AV 019, AV 012, and Finnish Daycare Study No signal for pneumonia Possible signal in children < 60 months of age for medically attended asthma/wheezing Concurrent immunization study ongoing 10

Revised Indication “Flu. Mist is indicated for active immunization for the prevention of disease caused by influenza A and B viruses in healthy individuals ages 5 years (60 months) through 64 years of age” 11

Not Proposing Flu. Mist Use In: n Individuals with history of underlying conditions which predispose them to poor outcomes when infected with wild type infuenza virus such as: • • n Asthma or recurrent medically attended wheezing Immunodeficiencies or chronic immunosuppression Other chronic cardiac or pulmonary disorders Chronic metabolic diseases (including diabetes), renal dysfunction, and hemoglobinopathies Concurrent use with other vaccines 12

Clinical Presentation

Overview of Clinical Presentation Review efficacy/effectiveness and safety of Flu. Mist in children and adults n Provide final data for specific open safety questions, including n • • n Asthma and wheezing Pneumonia Provide data on vaccine virus shedding and transmission 14

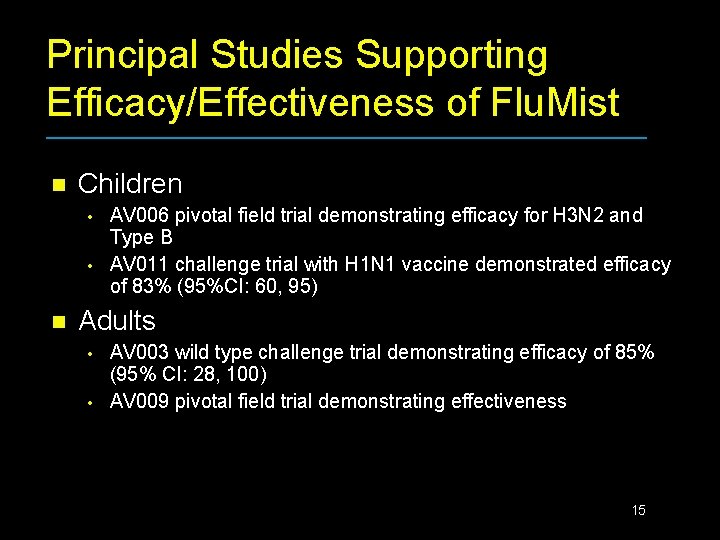
Principal Studies Supporting Efficacy/Effectiveness of Flu. Mist n Children • • n AV 006 pivotal field trial demonstrating efficacy for H 3 N 2 and Type B AV 011 challenge trial with H 1 N 1 vaccine demonstrated efficacy of 83% (95%CI: 60, 95) Adults • • AV 003 wild type challenge trial demonstrating efficacy of 85% (95% CI: 28, 100) AV 009 pivotal field trial demonstrating effectiveness 15

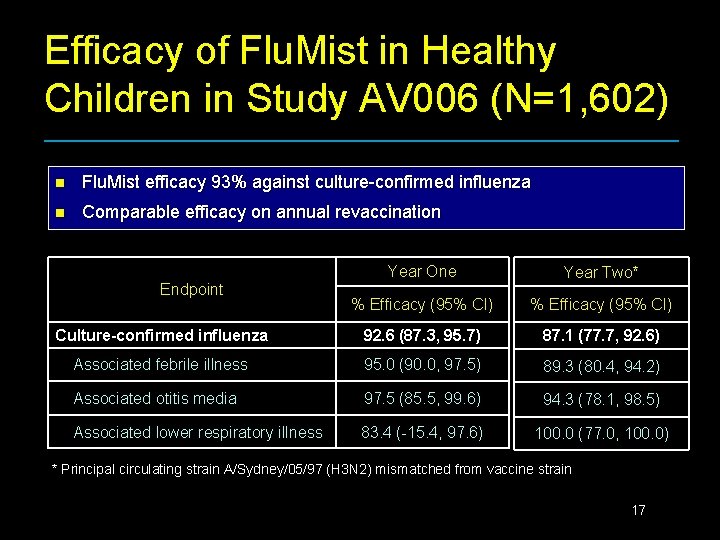
Efficacy Trial Healthy Children Study AV 006 n n n Randomized (2: 1), double-blind, placebo-controlled two year field trial 1, 602 healthy children age 15 -71 months at entry One or two doses in Yr 1 and revaccination dose in Yr 2 Active surveillance with illness cultures Primary endpoint: culture-confirmed influenza Circulating strains • • A/Wuhan/359/95 (H 3 N 2) and B/Beijing/184/93 -like in Year 1 A/Sydney/05/97 (H 3 N 2), a mismatched strain circulated in Year 2 16

Efficacy of Flu. Mist in Healthy Children in Study AV 006 (N=1, 602) n Flu. Mist efficacy 93% against culture-confirmed influenza n Comparable efficacy on annual revaccination Year One Year Two* % Efficacy (95% CI) Culture-confirmed influenza 92. 6 (87. 3, 95. 7) 87. 1 (77. 7, 92. 6) Associated febrile illness 95. 0 (90. 0, 97. 5) 89. 3 (80. 4, 94. 2) Associated otitis media 97. 5 (85. 5, 99. 6) 94. 3 (78. 1, 98. 5) Associated lower respiratory illness 83. 4 (-15. 4, 97. 6) 100. 0 (77. 0, 100. 0) Endpoint * Principal circulating strain A/Sydney/05/97 (H 3 N 2) mismatched from vaccine strain 17

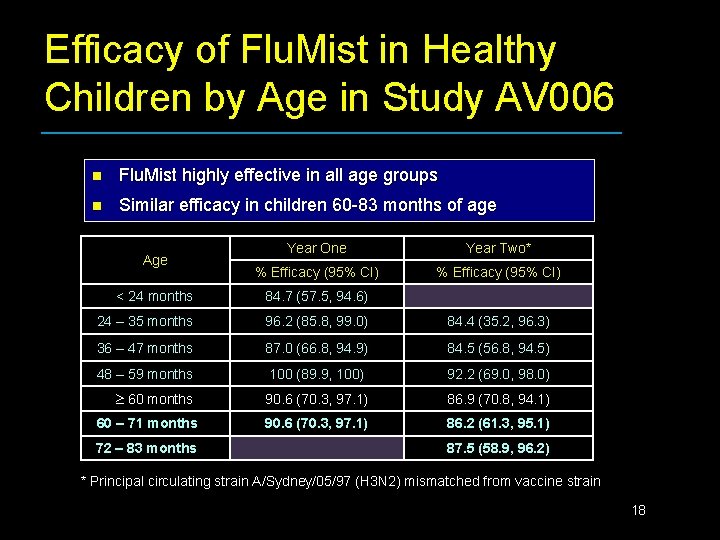
Efficacy of Flu. Mist in Healthy Children by Age in Study AV 006 n Flu. Mist highly effective in all age groups n Similar efficacy in children 60 -83 months of age Age Year One Year Two* % Efficacy (95% CI) < 24 months 84. 7 (57. 5, 94. 6) 24 – 35 months 96. 2 (85. 8, 99. 0) 84. 4 (35. 2, 96. 3) 36 – 47 months 87. 0 (66. 8, 94. 9) 84. 5 (56. 8, 94. 5) 48 – 59 months 100 (89. 9, 100) 92. 2 (69. 0, 98. 0) 60 months 90. 6 (70. 3, 97. 1) 86. 9 (70. 8, 94. 1) 90. 6 (70. 3, 97. 1) 86. 2 (61. 3, 95. 1) 60 – 71 months 72 – 83 months 87. 5 (58. 9, 96. 2) * Principal circulating strain A/Sydney/05/97 (H 3 N 2) mismatched from vaccine strain 18

Effectiveness in Adults Study AV 009 n n n Randomized 2: 1, double-blind, placebo-controlled 4, 561 healthy working adults 18 – 64 years of age Single dose Primary endpoint: incidence of “any febrile illness” Secondary • • n Other illness definitions • • n Associated absenteeism and medically attended illness Number and days of illness Febrile URI, Severe febrile illness (pre-specified) CDC-Influenza-like illness (ILI), DOD-ILI (post-hoc) Circulating strain, A/Sydney/05/97 (H 3 N 2), mismatched 19

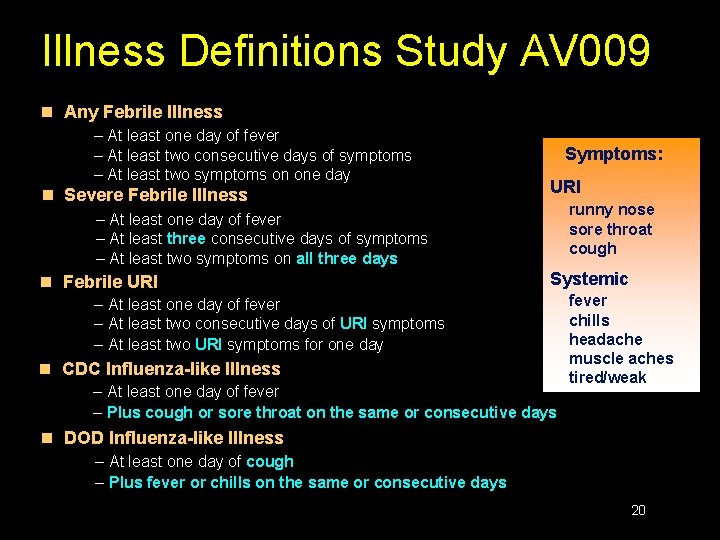
Illness Definitions Study AV 009 n Any Febrile Illness – At least one day of fever – At least two consecutive days of symptoms – At least two symptoms on one day Symptoms: URI n Severe Febrile Illness runny nose – At least one day of fever sore throat – At least three consecutive days of symptoms cough – At least two symptoms on all three days Systemic n Febrile URI fever – At least one day of fever chills – At least two consecutive days of URI symptoms headache – At least two URI symptoms for one day muscle aches n CDC Influenza-like Illness tired/weak – At least one day of fever – Plus cough or sore throat on the same or consecutive days n DOD Influenza-like Illness – At least one day of cough – Plus fever or chills on the same or consecutive days 20

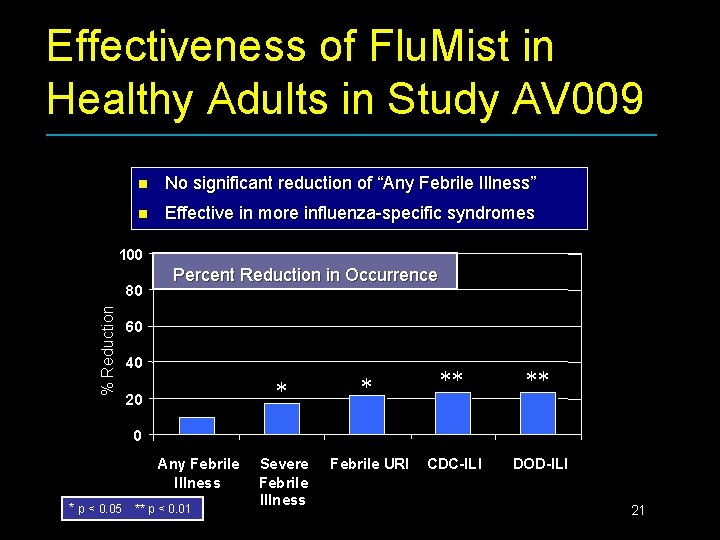
Effectiveness of Flu. Mist in Healthy Adults in Study AV 009 n No significant reduction of “Any Febrile Illness” n Effective in more influenza-specific syndromes 100 % Reduction 80 Percent Reduction in Occurrence 60 40 20 Severe Febrile Illness Febrile URI CDC-ILI DOD-ILI 0 Any Febrile Illness * p < 0. 05 ** p < 0. 01 21

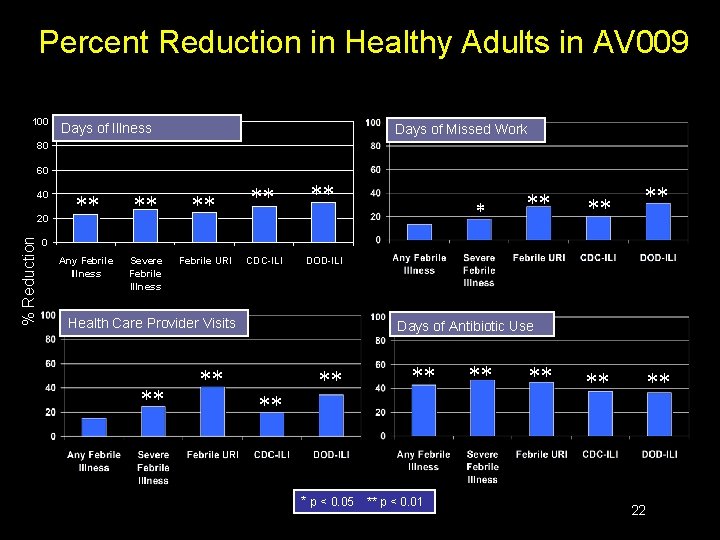
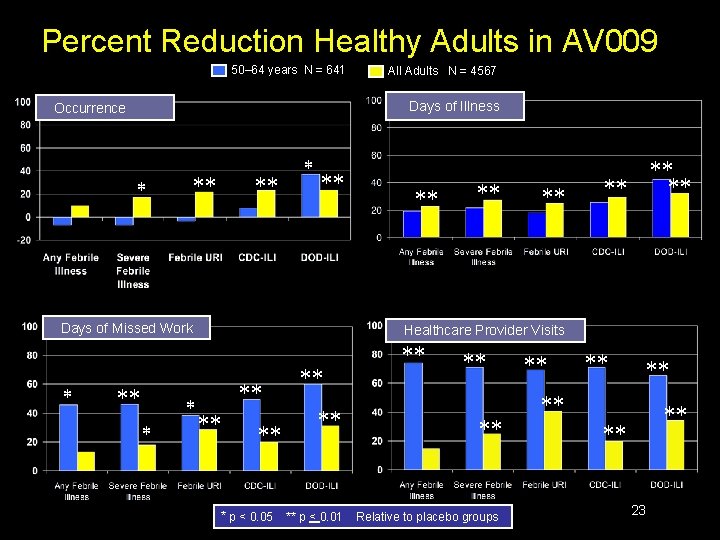
Percent Reduction in Healthy Adults in AV 009 100 Days of Illness Days of Missed Work 80 60 40 % Reduction 20 Any Febrile Illness Severe Febrile Illness Febrile URI CDC-ILI DOD-ILI 0 Health Care Provider Visits Days of Antibiotic Use * p < 0. 05 ** p < 0. 01 22

Percent Reduction Healthy Adults in AV 009 50– 64 years N = 641 Days of Illness Occurrence Days of Missed Work All Adults N = 4567 Healthcare Provider Visits * p < 0. 05 ** p < 0. 01 Relative to placebo groups 23

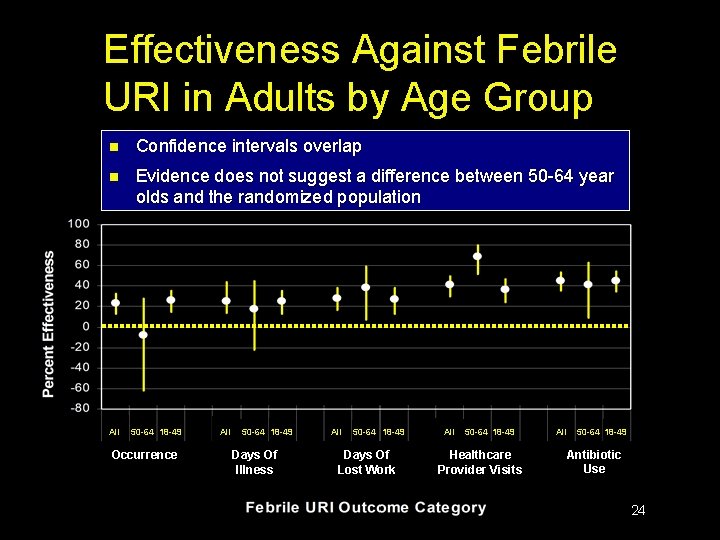
Effectiveness Against Febrile URI in Adults by Age Group n Confidence intervals overlap n Evidence does not suggest a difference between 50 -64 year olds and the randomized population All 50 -64 18 -49 Occurrence All 50 -64 18 -49 Days Of Illness All 50 -64 18 -49 Days Of Lost Work All 50 -64 18 -49 Healthcare Provider Visits All 50 -64 18 -49 Antibiotic Use 24

Efficacy/Effectiveness of Flu. Mist Conclusions n n Flu. Mist was demonstrated to be highly effective in prevention of influenza in healthy children and adults Efficacy/effectiveness observed across age subgroups • • n Field efficacy was established for H 3 N 2 and B strains • • • n Efficacy in children > 60 months of age was similar to the group as a whole and to younger children in AV 006 Effectiveness in 50 -64 year olds was similar to the randomized population in AV 009 H 1 N 1 did not circulate during the study periods Challenge studies in children (vaccine strain) and adults (wild-type strain) with H 1 N 1 support activity Previous field studies with CAIV demonstrated H 1 N 1 efficacy Comparable efficacy after annual revaccination 25

Summary of Data Supporting Flu. Mist Safety n Population studied: • • n General safety measures: • • • n ~16, 000 healthy children, ~3, 000 revaccinated ~ 4, 000 healthy adults SAEs and mortality Reactogenicity Medically attended events Specific issues: • • Asthma/wheezing Pneumonia Conjunctivitis CNS 26

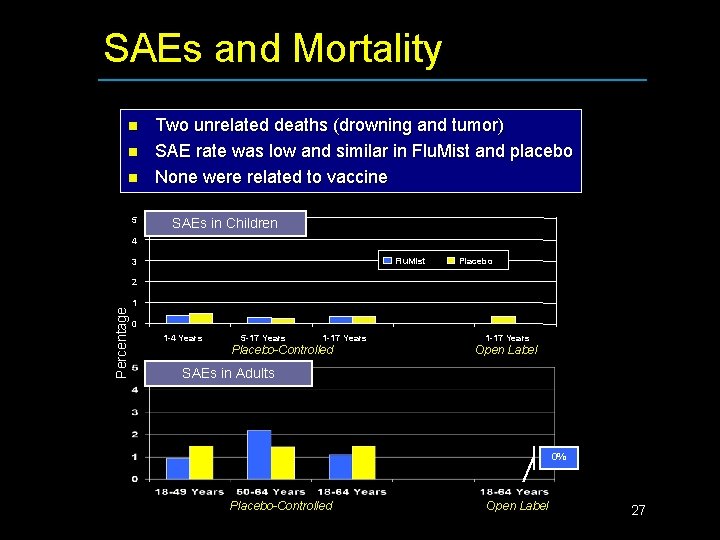
SAEs and Mortality n n n 5 Two unrelated deaths (drowning and tumor) SAE rate was low and similar in Flu. Mist and placebo None were related to vaccine SAEs in Children 4 Flu. Mist 3 Placebo Percentage 2 1 0 1 -4 Years 5 -17 Years 1 -17 Years Placebo-Controlled 1 -17 Years Open Label SAEs in Adults 0% Placebo-Controlled Open Label 27

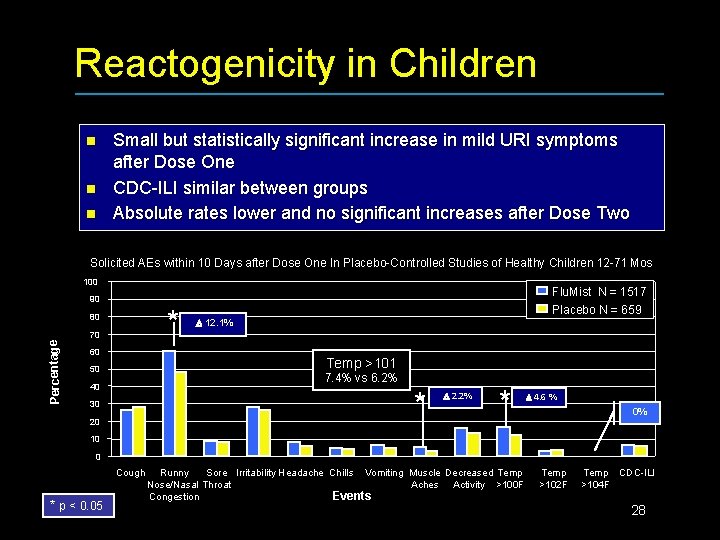
Reactogenicity in Children n Small but statistically significant increase in mild URI symptoms after Dose One CDC-ILI similar between groups Absolute rates lower and no significant increases after Dose Two Solicited AEs within 10 Days after Dose One In Placebo-Controlled Studies of Healthy Children 12 -71 Mos 100 Flu. Mist N = 1517 90 * 80 Percentage 70 60 Placebo N = 659 12. 1% Temp >101 50 7. 4% vs 6. 2% 40 * 30 20 2. 2% * 4. 6 % 0% 10 0 Cough * p < 0. 05 Runny Sore Irritability Headache Chills Vomiting Muscle Decreased Temp Nose/Nasal Throat Aches Activity >100 F Events Congestion Temp >102 F Temp CDC-ILI >104 F 28

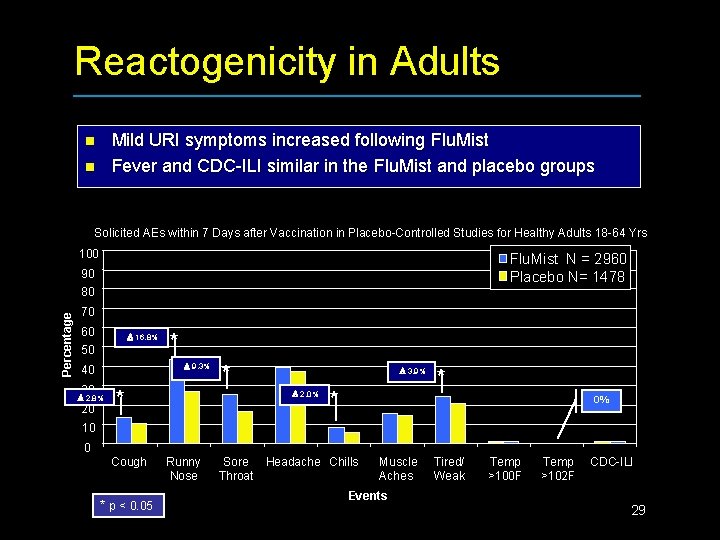
Reactogenicity in Adults Mild URI symptoms increased following Flu. Mist Fever and CDC-ILI similar in the Flu. Mist and placebo groups n n Solicited AEs within 7 Days after Vaccination in Placebo-Controlled Studies for Healthy Adults 18 -64 Yrs 100 Flu. Mist N = 2960 Placebo N= 1478 90 Percentage 80 70 60 16. 8% 50 9. 3% 40 30 2. 8% 20 * * * 3. 9% 2. 0% * * 0% 10 0 Cough * p < 0. 05 Runny Nose Sore Throat Headache Chills Muscle Aches Events Tired/ Weak Temp >100 F Temp >102 F CDC-ILI 29

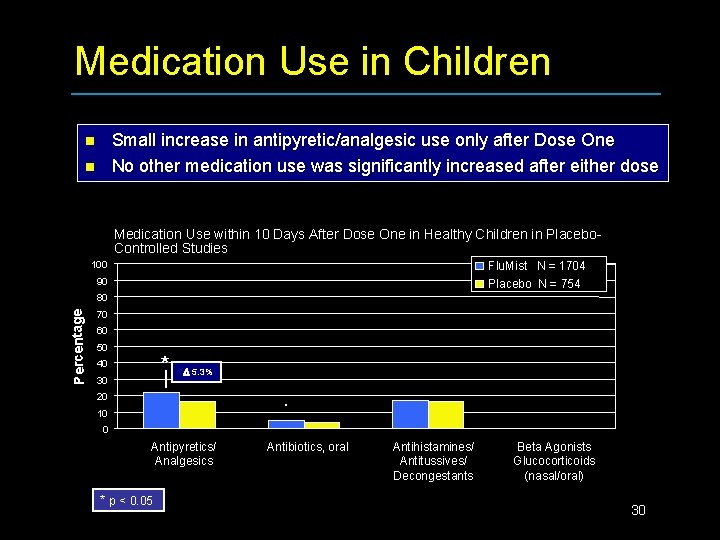
Medication Use in Children Small increase in antipyretic/analgesic use only after Dose One No other medication use was significantly increased after either dose n n Medication Use within 10 Days After Dose One in Healthy Children in Placebo. Controlled Studies 100 Flu. Mist N = 1704 Placebo N = 754 90 Percentage 80 70 60 50 * 40 30 5. 3% 20 10 0 Antipyretics/ Analgesics * p < 0. 05 Antibiotics, oral Antihistamines/ Antitussives/ Decongestants Beta Agonists Glucocorticoids (nasal/oral) 30

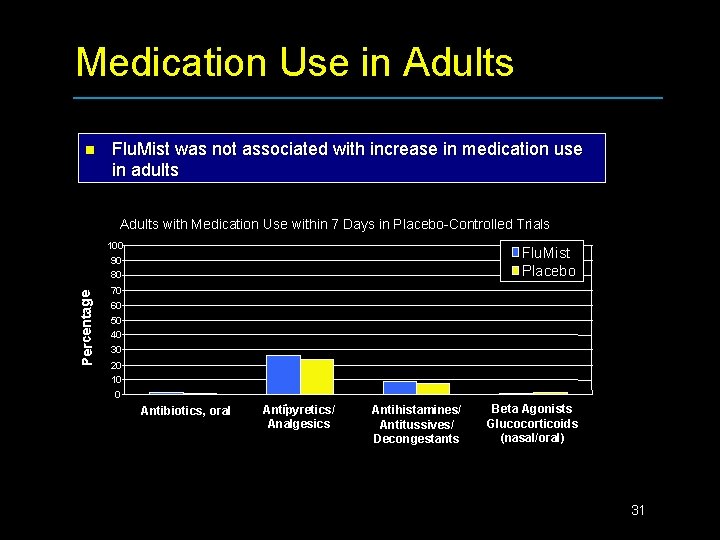
Medication Use in Adults n Flu. Mist was not associated with increase in medication use in adults Adults with Medication Use within 7 Days in Placebo-Controlled Trials Percentage 100 90 80 Flu. Mist Placebo 70 60 50 40 30 20 10 0 Antibiotics, oral Antipyretics/ Analgesics Antihistamines/ Antitussives/ Decongestants Beta Agonists Glucocorticoids (nasal/oral) 31

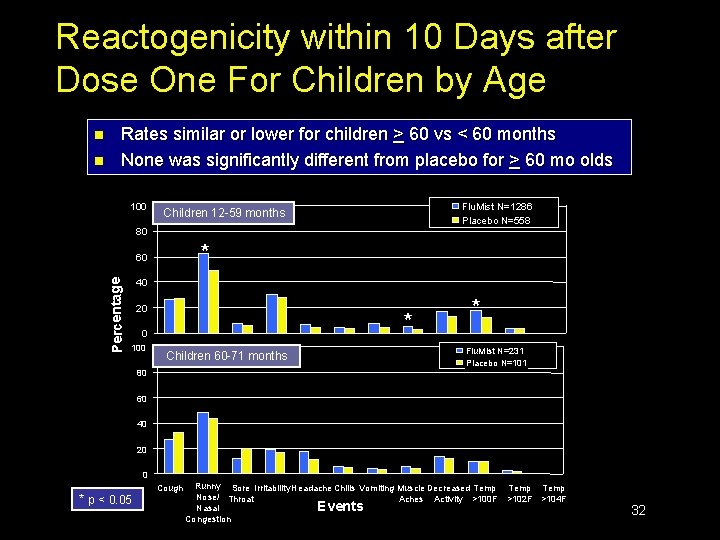
Reactogenicity within 10 Days after Dose One For Children by Age n n Rates similar or lower for children > 60 vs < 60 months None was significantly different from placebo for > 60 mo olds 100 Flu. Mist N=1286 Placebo N=558 Children 12 -59 months 80 * Percentage 60 40 20 * 0 100 Children 60 -71 months 80 * Flu. Mist N=231 Placebo N=101 60 40 20 0 * p < 0. 05 Cough Runny Sore Irritability Headache Chills Vomiting Muscle Decreased Temp Nose/ Throat Aches Activity >100 F Nasal Events Congestion Temp >102 F >104 F 32

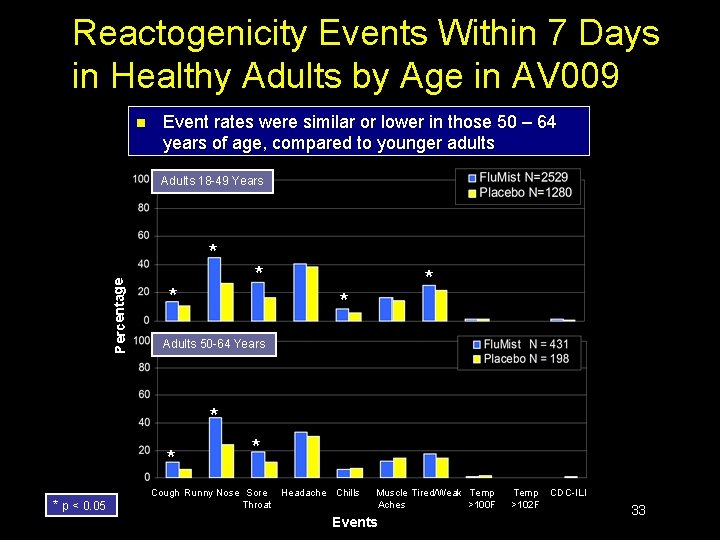
Reactogenicity Events Within 7 Days in Healthy Adults by Age in AV 009 n Event rates were similar or lower in those 50 – 64 years of age, compared to younger adults Adults 18 -49 Years Percentage * * * Adults 50 -64 Years * * * p < 0. 05 * Cough Runny Nose Sore Headache Chills Throat Muscle Tired/Weak Temp Aches >100 F Events Temp >102 F CDC-ILI 33

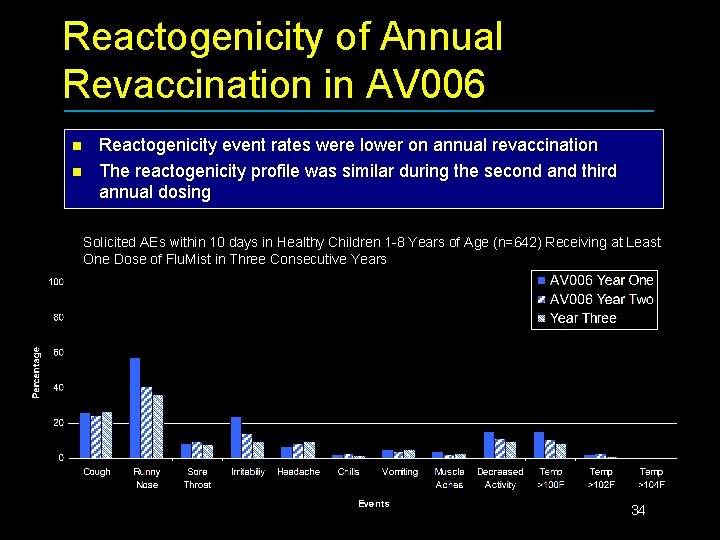
Reactogenicity of Annual Revaccination in AV 006 n n Reactogenicity event rates were lower on annual revaccination The reactogenicity profile was similar during the second and third annual dosing Solicited AEs within 10 days in Healthy Children 1 -8 Years of Age (n=642) Receiving at Least One Dose of Flu. Mist in Three Consecutive Years 34

Reactogenicity Summary n n n Flu. Mist associated with mild URI symptoms in children and adults No significant increase in acute influenza-like illness and no significant difference between the groups for fever >101 o. F following Flu. Mist administration Reactogenicity rates lower following annual revaccination 35

Medically Attended Events in Children and Adolescents Study AV 019 (Kaiser) n n Randomized 2: 1, double-blind, placebo-controlled Safety of Flu. Mist in 9, 689 children Two doses 1 -8 yrs of age, One dose 9 -17 yrs of age Outcomes ascertained from diagnosis in HMO database • n Medically attended events (MAEs) and SAEs within 42 days >1, 500 comparisons made using setting, dose, age group and diagnosis without statistical adjustment • • • Emergency Department (ED), Clinic, Hospital, and Combined settings Dose One, Dose Two, and combined doses 1 -17 years, 1 -8 years, 9 -17 years, 18 -35 months, 12 -17 months 36

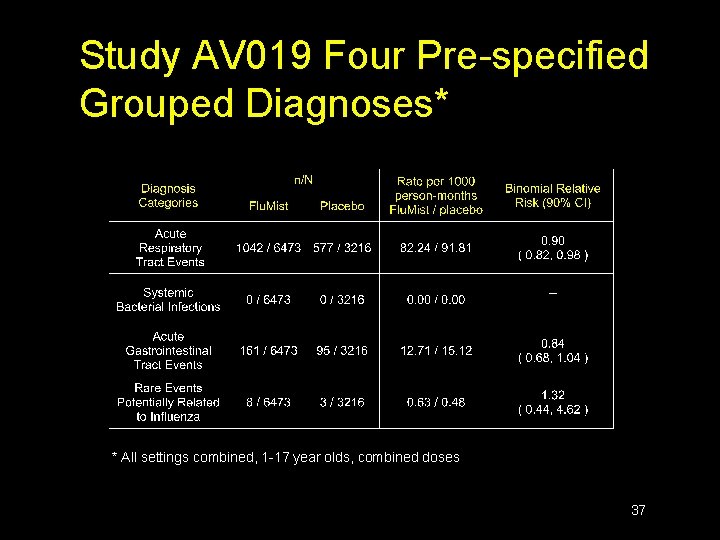
Study AV 019 Four Pre-specified Grouped Diagnoses* * All settings combined, 1 -17 year olds, combined doses 37

Study AV 019 Individual MAEs n n 14 MAEs were significantly increased and 21 decreased in Flu. Mist recipients Rate significantly increased in Flu. Mist group and a cause-and-effect relationship could not be excluded for: ü URI ü Musculoskeletal pain ü Asthma 38

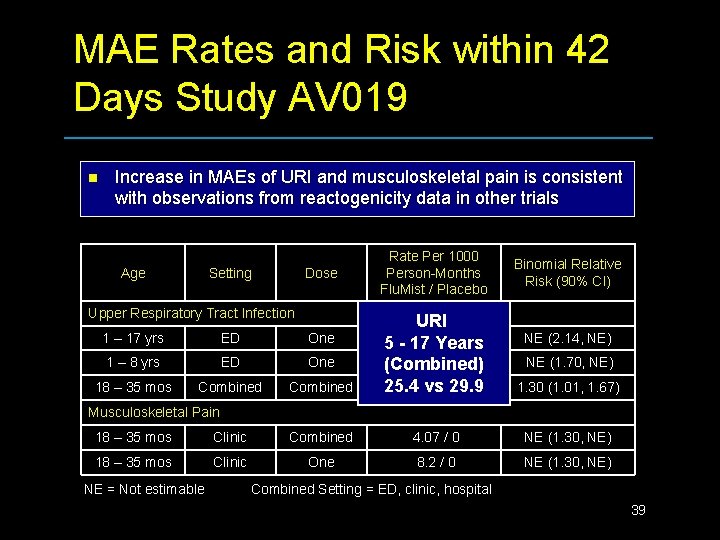
MAE Rates and Risk within 42 Days Study AV 019 n Increase in MAEs of URI and musculoskeletal pain is consistent with observations from reactogenicity data in other trials Age Setting Dose Upper Respiratory Tract Infection Rate Per 1000 Person-Months Flu. Mist / Placebo 1 – 17 yrs ED One 1 – 8 yrs ED One 18 – 35 mos Combined URI 1. 35 /0 5 - 17 Years 2. 05 / 0 (Combined) 25. 488. 9 vs/ 29. 9 68. 6 Binomial Relative Risk (90% CI) NE (2. 14, NE) NE (1. 70, NE) 1. 30 (1. 01, 1. 67) Musculoskeletal Pain 18 – 35 mos Clinic Combined 4. 07 / 0 NE (1. 30, NE) 18 – 35 mos Clinic One 8. 2 / 0 NE (1. 30, NE) NE = Not estimable Combined Setting = ED, clinic, hospital 39

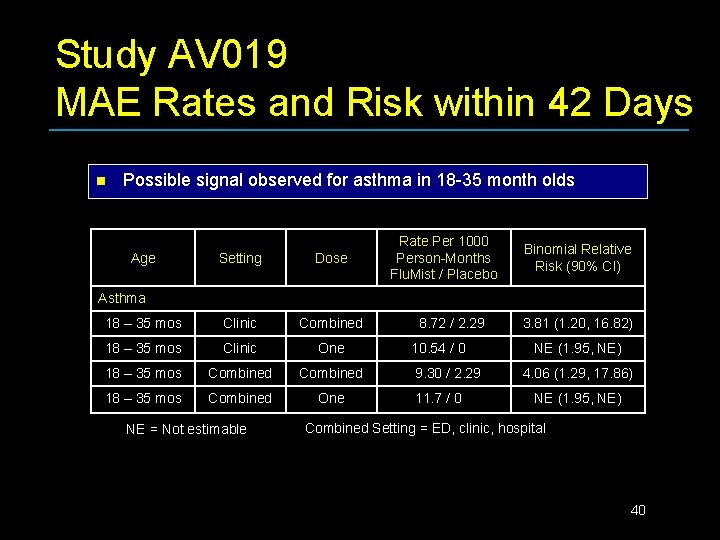
Study AV 019 MAE Rates and Risk within 42 Days n Possible signal observed for asthma in 18 -35 month olds Age Setting Dose 18 – 35 mos Clinic Combined 18 – 35 mos Clinic One 18 – 35 mos Combined One Rate Per 1000 Person-Months Flu. Mist / Placebo Binomial Relative Risk (90% CI) Asthma NE = Not estimable 8. 72 / 2. 29 10. 54 / 0 9. 30 / 2. 29 11. 7 / 0 3. 81 (1. 20, 16. 82) NE (1. 95, NE) 4. 06 (1. 29, 17. 86) NE (1. 95, NE) Combined Setting = ED, clinic, hospital 40

Asthma and Wheezing Study AV 019 n Database searched and medical records reviewed • • • n Assess use of terms to describe asthma and wheezing events Assess the circumstances of the medically attended event Identify history of asthma and wheezing in participants Additional analyses using: • • “Asthma” or “Asthma + Wheezing” outcomes Cumulative 6 month age groups Dose One, Dose Two History positive and history negative 41

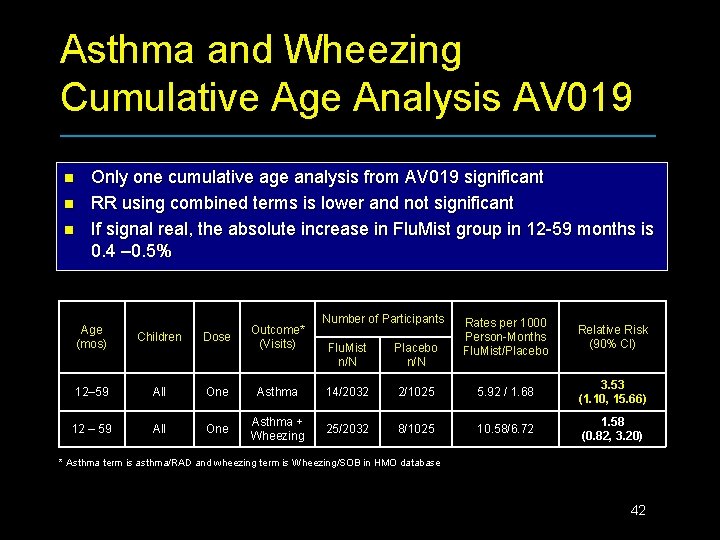
Asthma and Wheezing Cumulative Age Analysis AV 019 n n n Only one cumulative age analysis from AV 019 significant RR using combined terms is lower and not significant If signal real, the absolute increase in Flu. Mist group in 12 -59 months is 0. 4 – 0. 5% Age (mos) Children Dose Outcome* (Visits) 12– 59 All One 12 – 59 All One Number of Participants Rates per 1000 Person-Months Flu. Mist/Placebo Relative Risk (90% CI) Flu. Mist n/N Placebo n/N Asthma 14/2032 2/1025 5. 92 / 1. 68 3. 53 (1. 10, 15. 66) Asthma + Wheezing 25/2032 8/1025 10. 58/6. 72 1. 58 (0. 82, 3. 20) * Asthma term is asthma/RAD and wheezing term is Wheezing/SOB in HMO database 42

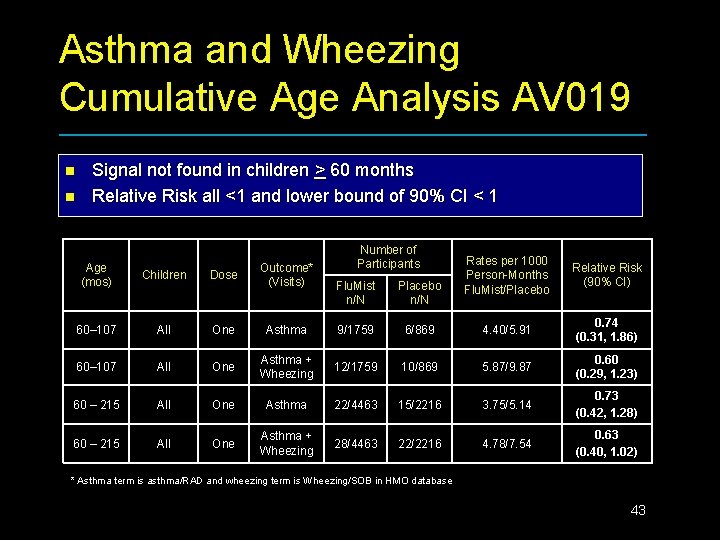
Asthma and Wheezing Cumulative Age Analysis AV 019 n n Signal not found in children > 60 months Relative Risk all <1 and lower bound of 90% CI < 1 Age (mos) Children Dose Outcome* (Visits) 60– 107 All One 60– 107 All 60 – 215 Number of Participants Rates per 1000 Person-Months Flu. Mist/Placebo Relative Risk (90% CI) Flu. Mist n/N Placebo n/N Asthma 9/1759 6/869 4. 40/5. 91 0. 74 (0. 31, 1. 86) One Asthma + Wheezing 12/1759 10/869 5. 87/9. 87 0. 60 (0. 29, 1. 23) All One Asthma 22/4463 15/2216 3. 75/5. 14 0. 73 (0. 42, 1. 28) All One Asthma + Wheezing 28/4463 22/2216 4. 78/7. 54 0. 63 (0. 40, 1. 02) * Asthma term is asthma/RAD and wheezing term is Wheezing/SOB in HMO database 43

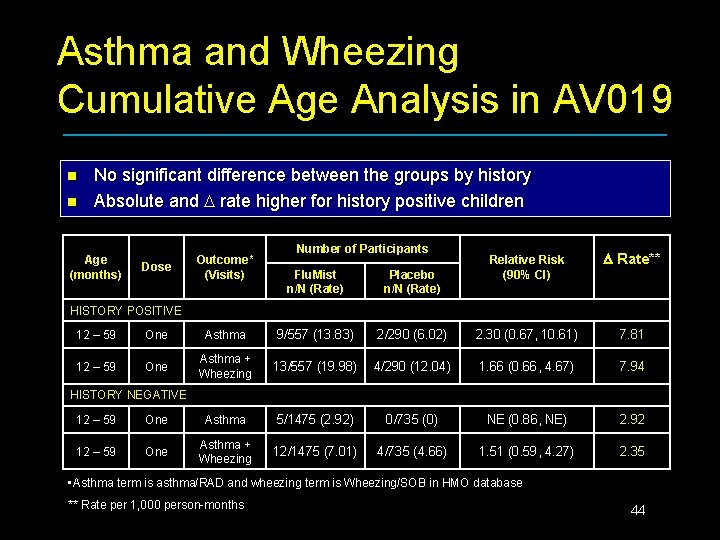
Asthma and Wheezing Cumulative Age Analysis in AV 019 n n No significant difference between the groups by history Absolute and rate higher for history positive children Age (months) Dose Outcome* (Visits) Number of Participants Flu. Mist n/N (Rate) Placebo n/N (Rate) Relative Risk (90% CI) Rate** HISTORY POSITIVE 12 – 59 One Asthma 9/557 (13. 83) 2/290 (6. 02) 2. 30 (0. 67, 10. 61) 7. 81 12 – 59 One Asthma + Wheezing 13/557 (19. 98) 4/290 (12. 04) 1. 66 (0. 66, 4. 67) 7. 94 HISTORY NEGATIVE 12 – 59 One Asthma 5/1475 (2. 92) 0/735 (0) NE (0. 86, NE) 2. 92 12 – 59 One Asthma + Wheezing 12/1475 (7. 01) 4/735 (4. 66) 1. 51 (0. 59, 4. 27) 2. 35 • Asthma term is asthma/RAD and wheezing term is Wheezing/SOB in HMO database ** Rate per 1, 000 person-months 44

Asthma and Wheezing Data from Other Large Safety Studies n n In AV 006 no significant increase in risk for asthma or wheezing in cumulative age analysis In AV 012 the event rate was not inconsistent with rates in AV 019 in younger children • No placebo group thus interpretation of comparison between the pre and post vaccination periods is complicated 45

Asthma and Wheezing Severity n Two children hospitalized for asthma/wheezing • • • n Flu. Mist (AV 006) – for 1 day 8 days after dose in Year 2 Placebo (AV 002) – 2 x for 1 day (4, 33 days post-dosing) Both had a history of asthma No hospitalizations in AV 019 for asthma • Events were outpatient/ED visits and most were associated with medical treatment 46

Summary of Asthma/Wheezing n Among all analyses of large placebo-controlled trials in children, a statistical difference was only observed in AV 019 • • n Children 12 - 59 mos, RR 3. 53 (1. 10, 15. 66) for “Asthma” Rate is higher for history positive children No signal in those 60 months of age 47

Other Open Issues Conjunctivitis n Pneumonia n CNS events n Concurrent immunization n 48

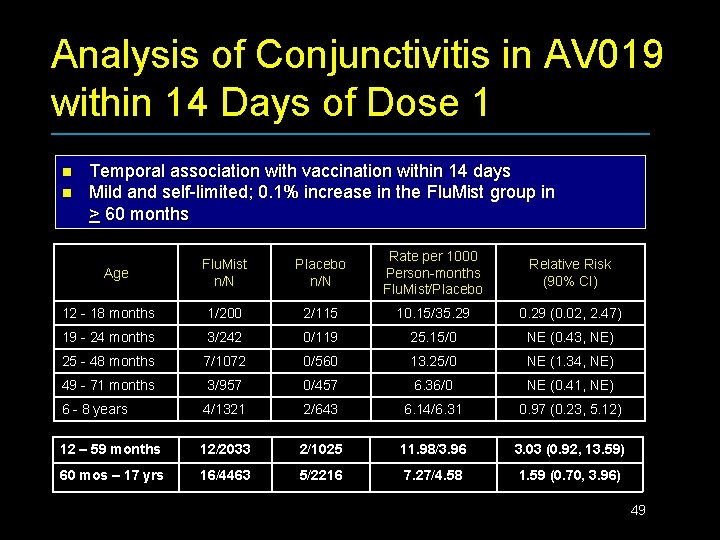
Analysis of Conjunctivitis in AV 019 within 14 Days of Dose 1 n n Temporal association with vaccination within 14 days Mild and self-limited; 0. 1% increase in the Flu. Mist group in > 60 months Flu. Mist n/N Placebo n/N Rate per 1000 Person-months Flu. Mist/Placebo Relative Risk (90% CI) 12 - 18 months 1/200 2/115 10. 15/35. 29 0. 29 (0. 02, 2. 47) 19 - 24 months 3/242 0/119 25. 15/0 NE (0. 43, NE) 25 - 48 months 7/1072 0/560 13. 25/0 NE (1. 34, NE) 49 - 71 months 3/957 0/457 6. 36/0 NE (0. 41, NE) 6 - 8 years 4/1321 2/643 6. 14/6. 31 0. 97 (0. 23, 5. 12) 12 – 59 months 12/2033 2/1025 11. 98/3. 96 3. 03 (0. 92, 13. 59) 60 mos – 17 yrs 16/4463 5/2216 7. 27/4. 58 1. 59 (0. 70, 3. 96) Age 49

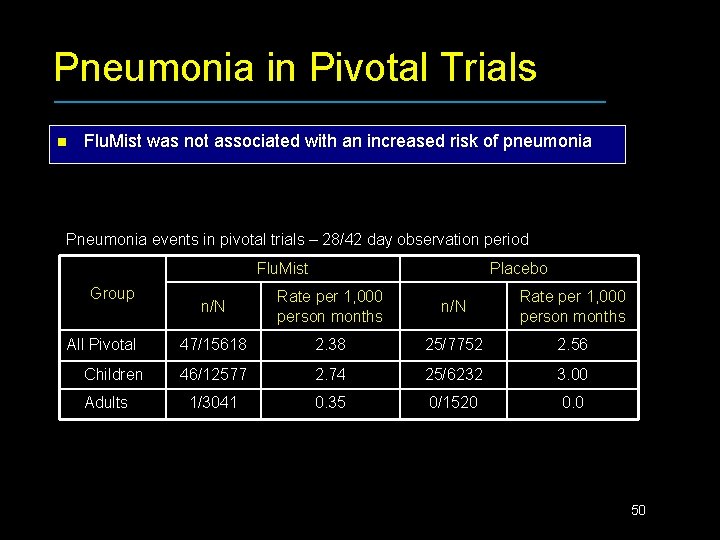
Pneumonia in Pivotal Trials n Flu. Mist was not associated with an increased risk of pneumonia Pneumonia events in pivotal trials – 28/42 day observation period Flu. Mist Group All Pivotal Children Adults Placebo n/N Rate per 1, 000 person months 47/15618 2. 38 25/7752 2. 56 46/12577 2. 74 25/6232 3. 00 1/3041 0. 35 0/1520 0. 0 50

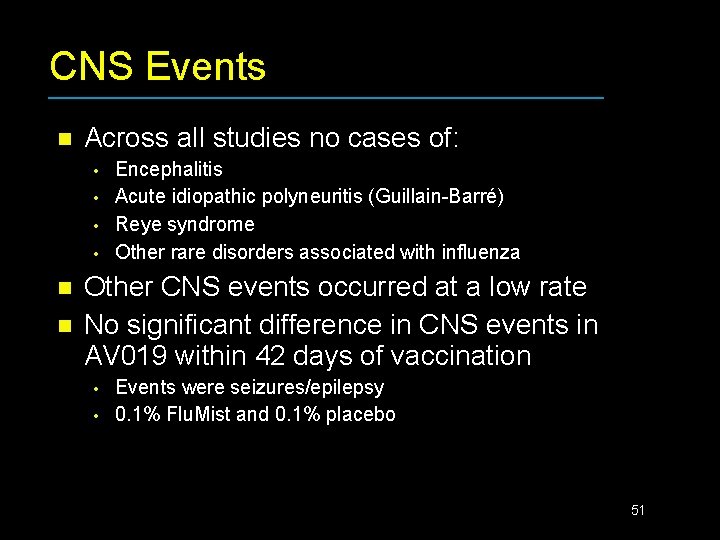
CNS Events n Across all studies no cases of: • • n n Encephalitis Acute idiopathic polyneuritis (Guillain-Barré) Reye syndrome Other rare disorders associated with influenza Other CNS events occurred at a low rate No significant difference in CNS events in AV 019 within 42 days of vaccination • • Events were seizures/epilepsy 0. 1% Flu. Mist and 0. 1% placebo 51

Concurrent Immunization n n Excluded from proposed label Trial ongoing of MMRII® and VARIVAX® • n Fully enrolled (N=1, 251) Trials planned and in discussion with CBER for other childhood vaccines 52

Characterization of Shed Vaccine Virus and Vaccine Virus Transmission

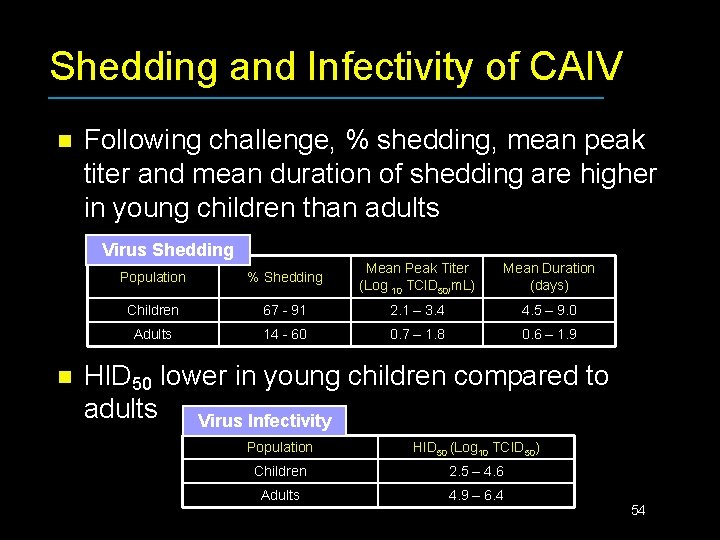
Shedding and Infectivity of CAIV n Following challenge, % shedding, mean peak titer and mean duration of shedding are higher in young children than adults Virus Shedding n Population % Shedding Mean Peak Titer (Log 10 TCID 50/m. L) Mean Duration (days) Children 67 - 91 2. 1 – 3. 4 4. 5 – 9. 0 Adults 14 - 60 0. 7 – 1. 8 0. 6 – 1. 9 HID 50 lower in young children compared to adults Virus Infectivity Population HID 50 (Log 10 TCID 50) Children 2. 5 – 4. 6 Adults 4. 9 – 6. 4 54

Finnish Daycare Trial n n Randomized (1: 1), double-blind, placebocontrolled 197 children age 8 months - 36 months • • n 98 Flu. Mist 99 placebo (93 in playgroups with vaccinees) 51 playgroups in two cities in Finland • • • 45 had both vaccine and placebo children All but 2 were in separate buildings with little chance of comingling of participants and staff among playgroups Average of 4. 1 study children per playgroup Children attended daycare > 3 days/week and > 4 hours/day Each placebo child exposed to an average of 1. 9 vaccinees 55

Finnish Daycare Trial n n One of the largest and most comprehensive vaccine virus transmission studies Nasal cultures on the first two days after dosing and at least 3 x/week for 3 weeks • • n n Over 2, 000 cultures: ~10/child Extensive genotyping and phenotyping of isolates Designed to maximize the chance of vaccine virus transmission Statistical methodology • Estimation of probability of transmission using Reed-Frost model which takes into account the number of vaccine and placebo interactions 56

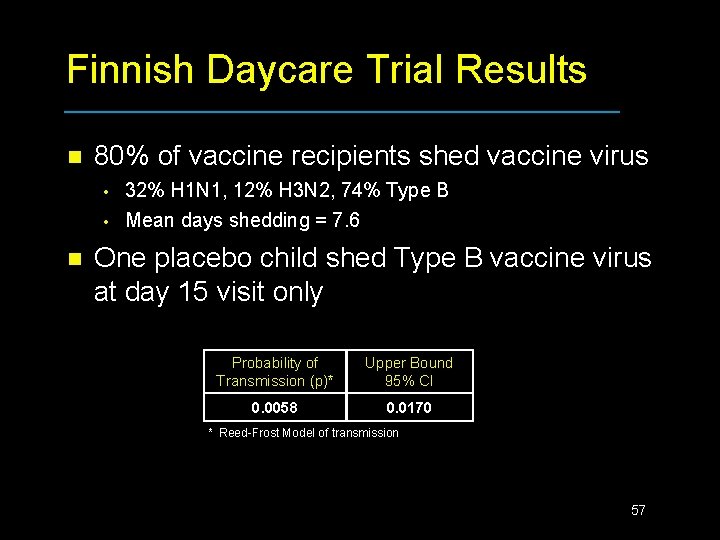
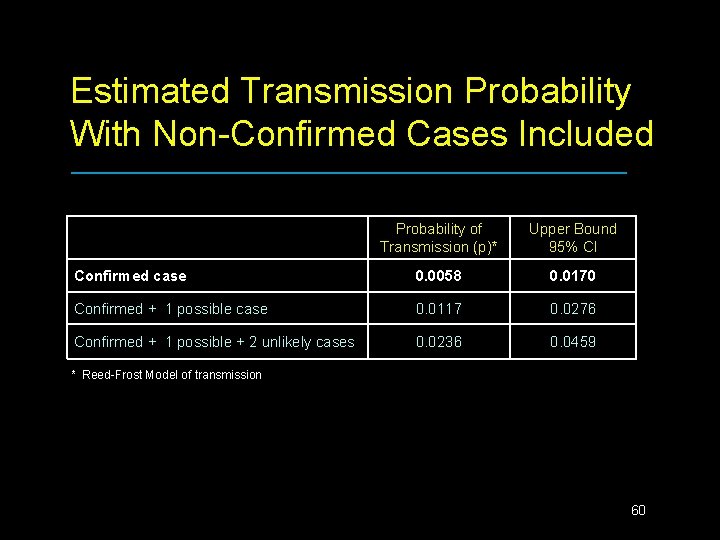
Finnish Daycare Trial Results n 80% of vaccine recipients shed vaccine virus • • n 32% H 1 N 1, 12% H 3 N 2, 74% Type B Mean days shedding = 7. 6 One placebo child shed Type B vaccine virus at day 15 visit only Probability of Transmission (p)* Upper Bound 95% CI 0. 0058 0. 0170 * Reed-Frost Model of transmission 57

Finnish Daycare Trial Results n n Wild type A/H 3 N 2 circulating in community Six additional placebo children shed Type A influenza virus • • Two shed wild type A strains Four shed type A virus that could not be re-isolated and thus could not be confirmed as wild-type or vaccine virus 58

Placebo Participants Shedding Unidentified Influenza A Virus #1 (Possible Case) • Shed on one day in a playgroup where one vaccinee shed vaccine virus seven and nine days earlier #2 (Unlikely Case) • Shed on two occasions. The first was before any vaccination occurred in the playgroup; the second was one day after the vaccination in the playgroup #3 (Unlikely Case) • Shed on one occasion. No other participant within the playgroup shed A virus, one shed B five days earlier #4 (Not Possible) • Shed two days before vaccine introduced into playgroup 59

Estimated Transmission Probability With Non-Confirmed Cases Included Probability of Transmission (p)* Upper Bound 95% CI Confirmed case 0. 0058 0. 0170 Confirmed + 1 possible case 0. 0117 0. 0276 Confirmed + 1 possible + 2 unlikely cases 0. 0236 0. 0459 * Reed-Frost Model of transmission 60

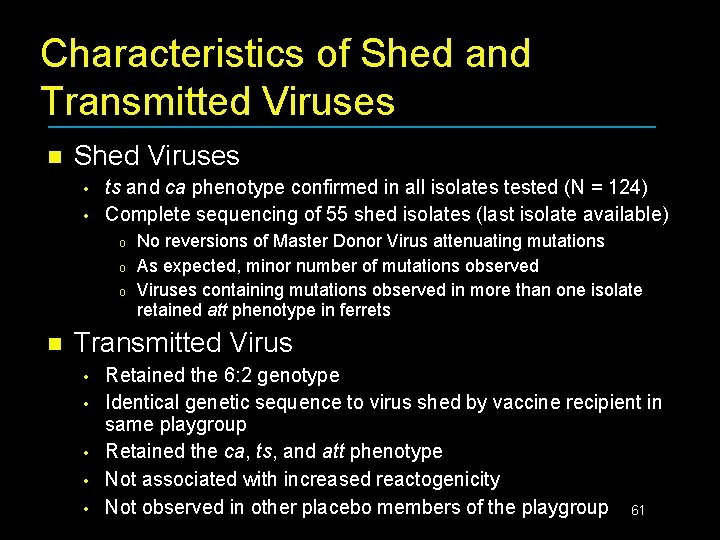
Characteristics of Shed and Transmitted Viruses n Shed Viruses • • ts and ca phenotype confirmed in all isolates tested (N = 124) Complete sequencing of 55 shed isolates (last isolate available) o o o n No reversions of Master Donor Virus attenuating mutations As expected, minor number of mutations observed Viruses containing mutations observed in more than one isolate retained att phenotype in ferrets Transmitted Virus • • • Retained the 6: 2 genotype Identical genetic sequence to virus shed by vaccine recipient in same playgroup Retained the ca, ts, and att phenotype Not associated with increased reactogenicity Not observed in other placebo members of the playgroup 61

Finnish Daycare Study Summary n n In the day care setting the probability of transmission was estimated to be 0. 0058 – 0. 0236. No phenotypic or genotypic reversion observed in shed or transmitted viruses 62

Overall Conclusions n Efficacy of Flu. Mist demonstrated in preventing culture confirmed influenza in children • • n Effectiveness of Flu. Mist demonstrated in adults • • n Efficacy consistent across age subgroups studied Efficacy in children > 60 months was similar to the group as a whole Effectiveness consistent across age subgroups No evidence 50 – 64 year olds differed from population as a whole Efficacy comparable on annual revaccination 63

Overall Conclusions n Flu. Mist is safe in healthy children > 60 months through 17 yrs and healthy adults 18 yrs through 64 yrs of age • • • n n n Additional information needed to assess risk/benefit in children < 60 months of age Safety profile similar and event rates lower on annual revaccination Risk of vaccine virus transmission is low • • n Increase in mild, self-limited URI symptoms No increase in fever > 101 o. F or CDC-ILI following administration In adults safety profile consistent across age subgroups, including 5064 year olds Transmission probability of 0. 006 - 0. 024 in daycare Rates in older children and adults will be lower Genetic and phenotypic reversion not observed 64

Proposed Indication “Flu. Mist is indicated for active immunization for the prevention of disease caused by influenza A and B viruses in healthy individuals ages 5 years (60 months) through 64 years of age” 65
 Stomach flu vs influenza
Stomach flu vs influenza Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Caldwell luc operation indications
Caldwell luc operation indications Virus de la influenza
Virus de la influenza Influenza virus replication
Influenza virus replication Suppose a student carrying a flu virus
Suppose a student carrying a flu virus Influenza
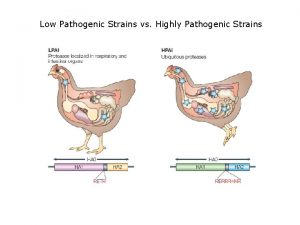
Influenza Low pathogenic avian influenza
Low pathogenic avian influenza Metodo dei cerchi di heuvers
Metodo dei cerchi di heuvers Influenza
Influenza Infulenza
Infulenza Is influenza a airborne disease
Is influenza a airborne disease The great influenza rhetorical analysis essay
The great influenza rhetorical analysis essay Influenza ww1
Influenza ww1 Flu watch canada
Flu watch canada Homophone of mourning
Homophone of mourning Diane gould
Diane gould Decoding the flu case study answers
Decoding the flu case study answers The european economy
The european economy Actividades con pronombres demostrativos
Actividades con pronombres demostrativos Flu root word definition
Flu root word definition Flu timeline
Flu timeline Texto con fla fle fli flo flu
Texto con fla fle fli flo flu Spanish flu
Spanish flu Oraciones con fla fle fli flo flu
Oraciones con fla fle fli flo flu Flu produit chimique
Flu produit chimique Spanish flu
Spanish flu Mec flu
Mec flu Flu definition
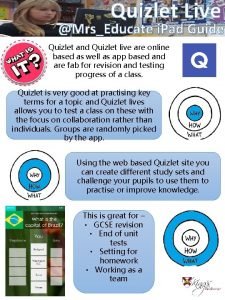
Flu definition Www.quizlet/live
Www.quizlet/live Live happy live healthy
Live happy live healthy Mist 2090
Mist 2090 Messtechnik
Messtechnik Mist-331
Mist-331 Dry mist disinfection machine
Dry mist disinfection machine Mist algorithm
Mist algorithm Water mist hydraulic calculations
Water mist hydraulic calculations Técnica mist surfactante
Técnica mist surfactante Teorie centrálních míst
Teorie centrálních míst Mis diuretic syrup
Mis diuretic syrup Mist surfactante
Mist surfactante Pollock's lavender mist is an example of _____.
Pollock's lavender mist is an example of _____. Sulfuric acid mist
Sulfuric acid mist 9 line
9 line Carminative mixture formula
Carminative mixture formula Cara kerja mist blower
Cara kerja mist blower Zitate ernährung hippokrates
Zitate ernährung hippokrates Plant propagation algorithm
Plant propagation algorithm Homophones for leek
Homophones for leek Rosalind mist
Rosalind mist Mistdemon
Mistdemon Mist algorithm
Mist algorithm Fire suppression
Fire suppression Lågenergihus nyproduktion
Lågenergihus nyproduktion Karttecken höjdkurva
Karttecken höjdkurva Meios steg för steg
Meios steg för steg Rbk-mätning
Rbk-mätning Formel för lufttryck
Formel för lufttryck Elektronik för barn
Elektronik för barn Särskild löneskatt för pensionskostnader
Särskild löneskatt för pensionskostnader Densitet vatten
Densitet vatten Tack för att ni har lyssnat
Tack för att ni har lyssnat Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Trög för kemist
Trög för kemist Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Mjälthilus
Mjälthilus