FLU The underestimated threat Influenza Virus Types Type





























- Slides: 39

FLU The underestimated threat.

Influenza Virus Types • Type A – humans and other animals – all age groups – moderate to severe illness • Type B – milder epidemics – humans only – primarily affects children • Type C - uncommon strain, no epidemic

Increased Risk • • Age 65 and older Any age with chronic medical conditions Pregnant women Children 6 -23 months

How the Flu Spreads

Spread of Flu • Droplet Spread – from a person’s cough or sneeze – person touches respiratory droplets on another person or object and then touches their own mouth or nose • Incubation period = 1 -4 days

Symptoms • Adults- shed virus 1 day before developing symptoms to 7 days after getting sick • Young children- can shed virus for longer than 7 days

Hospitalization from Influenza • Highest rate among young children and persons >65 yrs • 114, 000 hospitalizations/yr with 57% occurring in ages < 65 yrs • Highest # caused by type A (H 3 N 2) viruses

Death rates from influenza-associated pulmonary and circulation deaths/100, 000 persons • • 0 -44 yr: 0. 4 - 0. 6 50 -64 yr: 7. 5 65 yrs: 98. 3 Reasons: – more older people has inc. – Influenza A associated with higher mortality – Influenza A predominates in 90% of seasons from 1990 -99 compared w/57% of seasons 1976 -90

Preventing the Flu • Good Health Habits • Vaccination • Antiviral Medications

Good Health Habits • Avoid close contact • Stay home when you are sick • Cover your mouth • Clean your hand • Avoid touching your eyes, nose or mouth • Get plenty of rest • Drink plenty of liquids • The simplest way to avoid the flu is to avoid crowds. Can’t keep you kids cooped up? Frequent hand washing is the next best thing

Vaccination

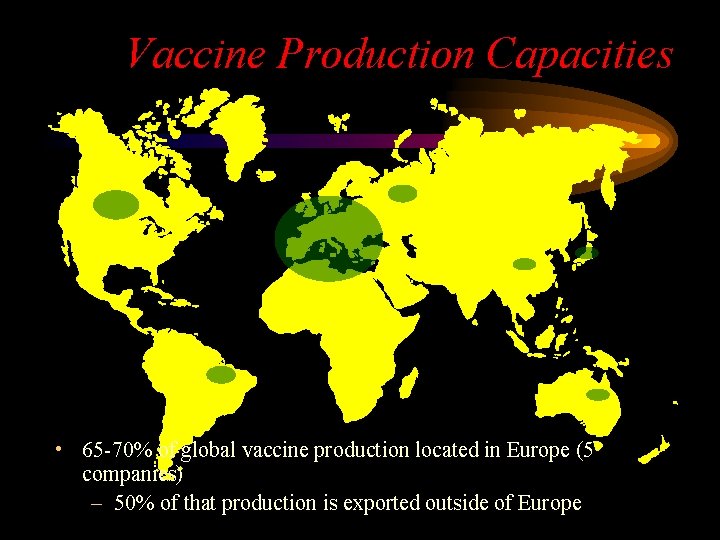
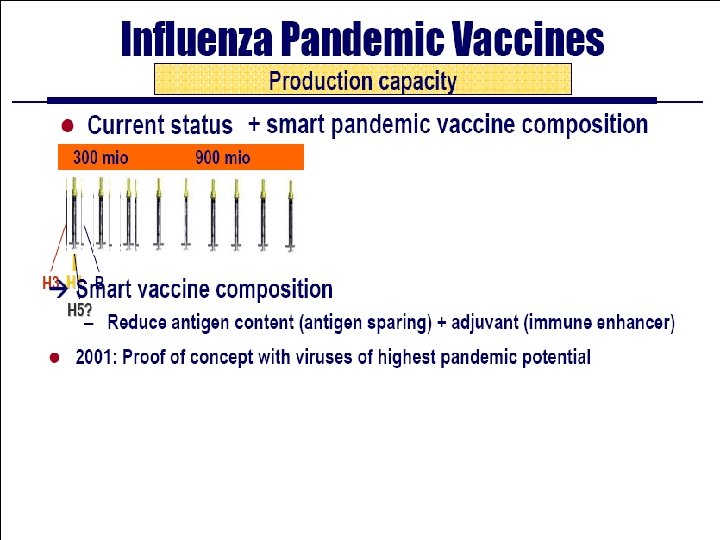
Vaccine Production Capacities • 65 -70% of global vaccine production located in Europe (5 companies) – 50% of that production is exported outside of Europe




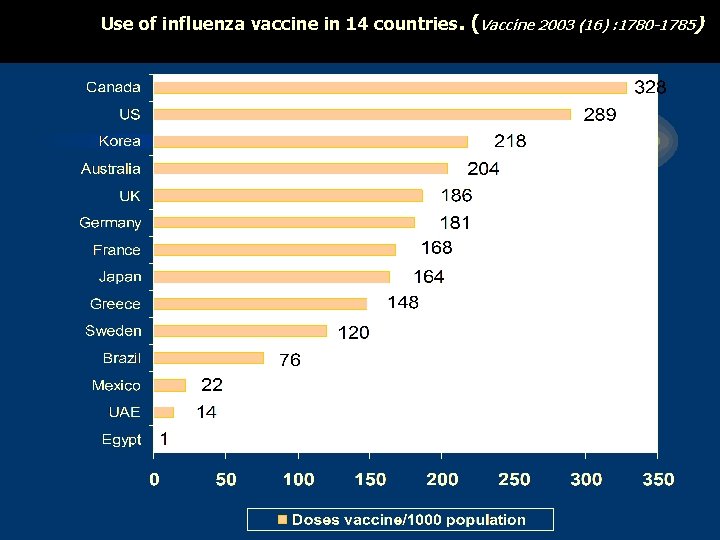
Use of influenza vaccine in 14 countries. (Vaccine 2003 (16) : 1780 -1785)

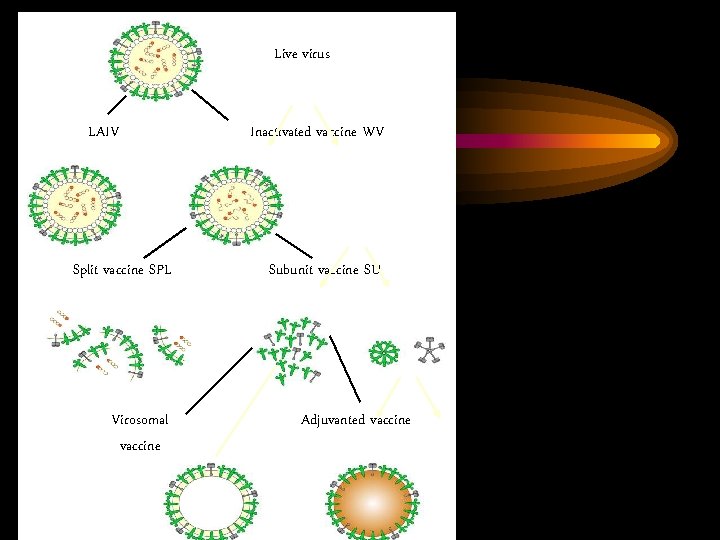
Live virus LAIV Split vaccine SPL Inactivated vaccine WV Subunit vaccine SU rosettes Virosomal vaccine Adjuvanted vaccine

Vaccination • Best way to prevent flu • Selection of virus for manufactured vaccine made in Feb and April each year • Get vaccinated each fall • People at high risk should get vaccinated • 2 kinds of vaccines – inactivated – live attenuates (LAIV) (for ages 5 - 49)

Who Should Not Get Vaccine • Have severe allergy to hen’s eggs (anaphylactic allergic rxn) • People who previously developed Guillian. Barre syndrome (GBS) w/in 6 weeks after getting a flu shot

Influenza Vaccination Strategy • Yearly vaccination of high risk persons is the most effective means of reducing the effect of influenza – persons with increased risk – close contacts and care-givers of persons with increased risk

Persons at High Risk • All persons 50 years of age or older • Persons >6 months of age with chronic illness • Residents of long-term care facilities • Pregnant women (2 nd and 3 rd trimesters) • Children 6 months to 18 years receiving chronic aspirin therapy • Children 6 -23 months of age

Chronic Medical Conditions • • • Pulmonary (e. g. COPD, asthma) Cardiovascular (e. g. CHF) Metabolic (e. g. diabetes) Renal (e. g. chronic renal failure) Hemoglobinopathies (e. g. sickle cell) Immunosuppression (e. g. HIV)

HIV Infection • Persons with HIV at higher risk for complications of influenza • Vaccine induces protective antibody titers in many HIV-infected persons • Transient increase in HIV replication reported • Vaccine will benefit many HIV-infected persons

Pregnancy and Inactivated Influenza Vaccine • Risk of hospitalization 4 times higher than nonpregnant women • Risk of complications comparable to nonpregnant women with high-risk medical conditions • Vaccination recommended if pregnant during influenza season

Contacts of High-Risk Persons • Household members and caregivers of high-risk persons (including children 0 -23 months) • Health care providers, including home care • Employees of long-term care facilities

Other Groups • Providers of essential community services • Foreign travelers • Students • Anyone who wishes to reduce the likelihood of becoming ill from influenza

Composition of the 2003 -2004 Influenza Vaccine • A/Moscow/10/99 (H 3 N 2) (A/Panama/2007/99) • A/New Caledonia/20/99 (H 1 N 1) • B/Hong Kong/330/2001

Composition of the 2004 -2005 Influenza Vaccine* • A/Fujian/411/2002 (H 3 N 2) (A/Wyoming/3/2003) • A/New Caledonia/20/99 (H 1 N 1) • B/Shanghai/361/2002 (B/Jilin/20/2003 or B/Jiangsu/10/2003) *strains in (parenthesis) are antigenically identical to the selected strains and may be used in the vaccines

Live Attentuated Intranasal Influenza (LAIV) • Contains weakened live influenza vs killed viruses • Administered by nasal spray • Contains 3 different live (but weakened) viruses, which stimulate body to make antibodies

Dosage-LAIV • 0. 5 m. L of vaccine: 0. 25 m. L for each nostril • Children aged 5 -8 previously unvaccinated: receive 2 doses separated by 6 -10 weeks • Children aged 5 -8 previously vaccinated: receive 1 dose (do not require a 2 nd dose) • Persons aged 9 -49: receive 1 dose

Efficacy & Effectiveness of LAIVadults • • 85% overall efficiency Fewer days of illness 15 -42% fewer health care provider visits 43 -47% less use of antibiotics

LIAV Side Effects • Children – – – runny nose headache vomiting muscle aches fever • Adults – – – runny nose headache sore throat cough fever

Inactivated Influenza Vaccine • Contains two type A and one type B • Made from purified, egg grown viruses that have been inactivated or killed • Antibiotics can be added to prevent bacterial contamination • Vaccinated people develop high postvaccination hemagglutination inhibition antibody titers

Effectiveness of Inactivated Vaccine- Children • 77% - 91% effective against influenza respiratory illness

Effectiveness of Inactivated Vaccine-Adults • Aged < 65 yrs old: – 70 -90% efficient – work absenteeism, health-care resources • Aged > 65 yrs old: – 50 -60% effective in preventing hospitalization for pneumonia and influenza – 80% effective in preventing death

Side Effects to Inactivated Vaccine • Soreness at vaccination site • Fever, malaise, myalgia • Guillain Barre Syndrome: 1 additional case per 1 million people – Body's immune system attacks part of the nervous system and results in weakness or tingling sensations in the legs that can spread to the arms and upper body. – Can result in paralysis

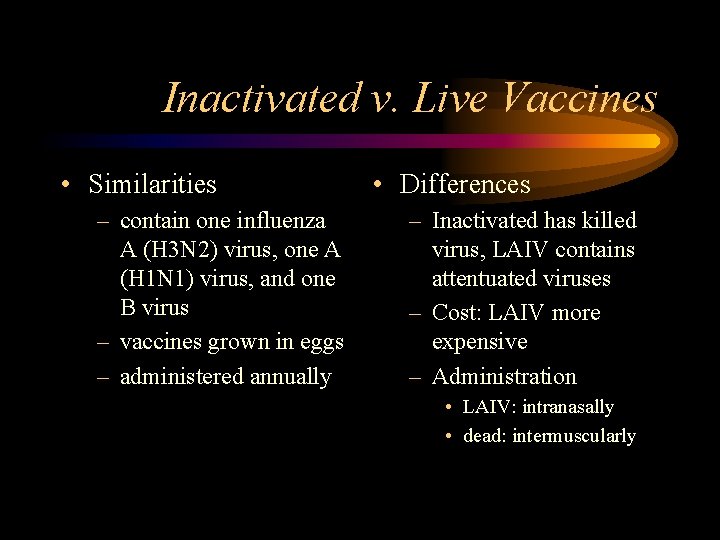
Inactivated v. Live Vaccines • Similarities – contain one influenza A (H 3 N 2) virus, one A (H 1 N 1) virus, and one B virus – vaccines grown in eggs – administered annually • Differences – Inactivated has killed virus, LAIV contains attentuated viruses – Cost: LAIV more expensive – Administration • LAIV: intranasally • dead: intermuscularly

