Influenza Programme 201920 What is flu Flu Report
























- Slides: 32

Influenza Programme 2019/20

What is flu?

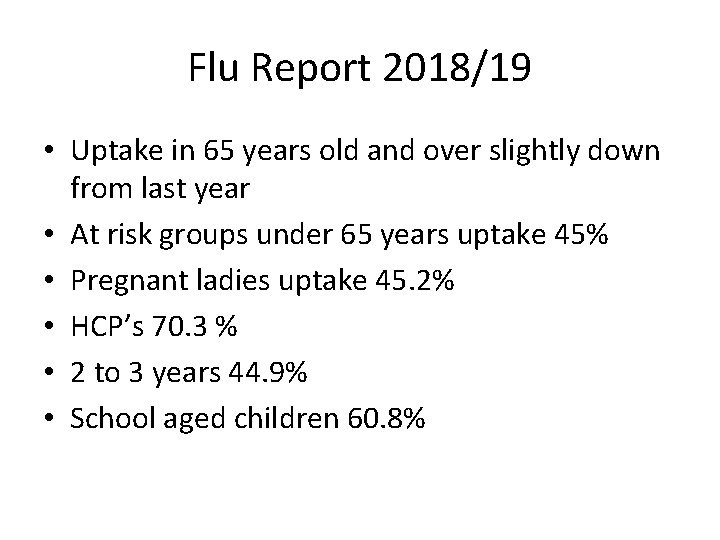
Flu Report 2018/19 • Uptake in 65 years old and over slightly down from last year • At risk groups under 65 years uptake 45% • Pregnant ladies uptake 45. 2% • HCP’s 70. 3 % • 2 to 3 years 44. 9% • School aged children 60. 8%

Flu programme 2019/20 • Extension to an additional cohort children, those in school year 6 • All children aged 2 to 10 years on 31 st August 2019 • 2 to 3 year olds DOB 1/9/2015 to 31/8/2017 • 4 to 10 year olds DOB 1/9/2008 to 31/8/2015

Other groups who should receive flu vaccine The list of clinical risk groups is not exhaustive Healthcare practitioners should apply clinical judgement to take into account the risk of flu exacerbating any underlying disease as well as the risk of serious illness from flu itself Flu vaccine should be offered to such patients even if the individual is not in the clinical risk groups specified in the risk groups list 5 The national flu immunisation programme 2015/16

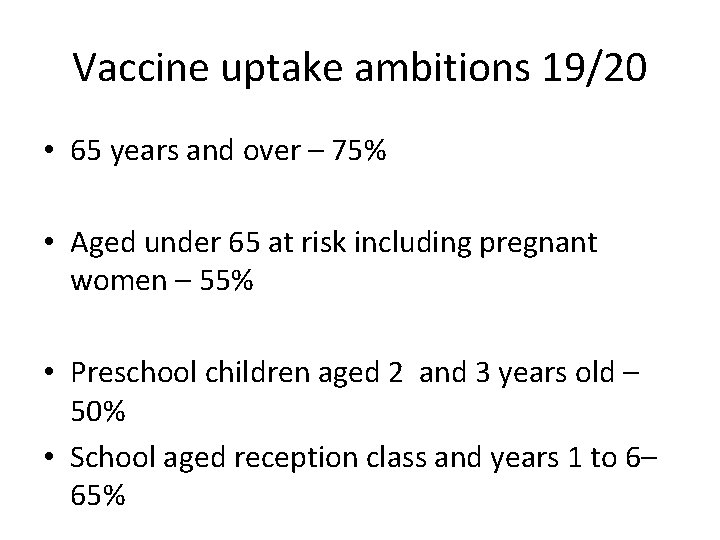
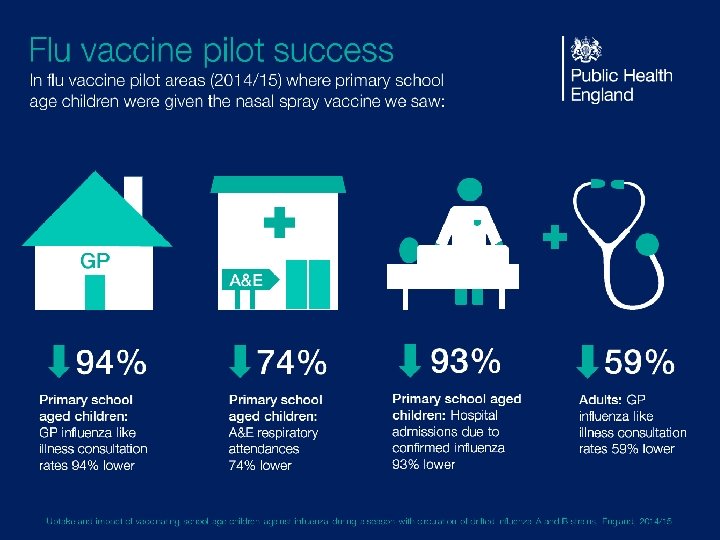
Vaccine uptake ambitions 19/20 • 65 years and over – 75% • Aged under 65 at risk including pregnant women – 55% • Preschool children aged 2 and 3 years old – 50% • School aged reception class and years 1 to 6– 65%

Flu strains for 2019/20 • A/Brisbane/02/2018 (H 1 N 1)pdm 09 -like virus; • A/Kansas/14/2017 (H 3 N 2)-like virus; • B/Colorado/06/2017 -like virus (B/Victoria/2/87 lineage) • Quadrivalent inactivated vaccines • B/Phuket/3073/2013 -like virus (B/Yamagata/16/88 lineage).

Vaccine Effectiveness • Vaccine effectiveness (VE) varies from one season to the next. Overall effectiveness has been estimated at between 30 -60% for adults aged 18 to 65 years for flu infection in primary care » In 2018/19, the introduction of Fluad (a. T. I. V. ) 8 over Aged 65 and

How do we improve vaccine effectiveness in those aged 65 and over? 9 over Factor Solution Immunosenescence (age related reduction in immune response) May be addressed by the use of an adjuvanted or higher dose vaccine to boost response in those over 65 years of age against all vaccine strains Genetic drift of the circulating strain Genetic drift creates a mismatch between the vaccine strains and the circulating wild type strains. For the 2018/19 season there was a change in the WHO recommended A (H 3 N 2) and B vaccine strains with the aim that this would be a better match to strains that circulated during the season Egg adaptation can be addressed using cell based vaccines which do not require the use of eggs in the manufacturing process Aged 65 and

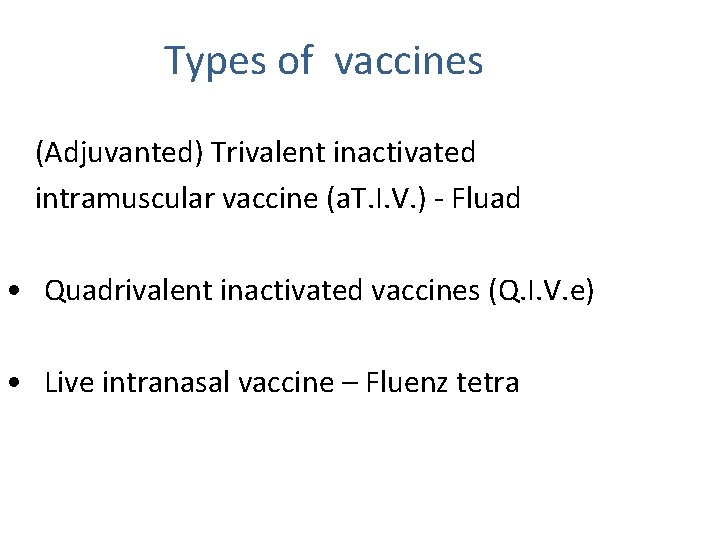
Types of vaccines (Adjuvanted) Trivalent inactivated intramuscular vaccine (a. T. I. V. ) - Fluad • Quadrivalent inactivated vaccines (Q. I. V. e) • Live intranasal vaccine – Fluenz tetra

New vaccine for 2019/20 • Quadrivalent influenza cell culture vaccine (QIVc) • Flucelvax Tetra – recommended for flu programme 18 years and over

Quadrivalent influenza cell culture vaccine (QIVc) • QIVc has a similar safety profile to other flu vaccines. More than 36 million doses of cell-based vaccine have been administered with no serious safety concerns 12 over Aged 65 and

Quadrivalent influenza cell culture vaccine (QIVc) • This manufacturing process eliminates the risk of egg-adaptation and may result in the vaccine containing virus that is a closer match to wild-type circulating flu viruses (though cell-adaptation can still occur) 13 over Aged 65 and

Quadrivalent vaccines (QIV) • Patients in clinical at risk groups aged 18 years to under 65 years including pregnant ladies will be offered the Q. I. V vaccine • Can be Q. I. Ve or Q. I. Vc vaccines

Caution • Vaccines with similar names but have different content • Flu. CELvax Tetra - New Q. I. V. c - Egg free vaccine • Fluarix Tetra - Q. I. V. e has a low egg content

Vaccinating pregnant ladies Vaccinate any trimester Passive immunity for the first few months of life Maternity services are involved with the programme

What is the risk to my baby? • May be increase risk with premature birth • Smaller birth weight

Risk to women • Pregnant women are at greater risk of hospitalisation and mortality rate is several times higher than that for non pregnant women in the same age group • All pregnant ladies have the inactivated vaccine

2019/20 flu vaccine recommendations for those aged 65 years and over • Two of these vaccines are eligible for reimbursement on the NHS Adjuvanted trivalent inactivated influenza vaccine (a. TIV) introduced in 2018/19 Quadrivalent influenza cell culture vaccine (QIVc) (licenced in December 2018) 19 Aged 65 and over

Administration of vaccination • Preferably given by intramuscular injection • Individuals on stable anticoagulation therapy can receive intramuscular vaccination • Individuals with bleeding disorders may be vaccinated intramuscularly under guidance of GP

Children aged 6/12 to less than 2 years – QIV Children aged 2 up to 17 years Fluenz Tetra (LAVI) Those 2 years or above in whom LAVI is contraindicated PHE has purchased a suitable QIV


Severe asthma or active wheezing • live flu vaccine is not recommended for children and adolescents with severe asthma or active wheezing, eg those who are currently taking or have been prescribed oral steroids for respiratory disease in the last 14 days • children currently taking a high dose inhaled steroid – Budesonide >800 mcg/day or equivalent (eg Fluticasone >500 mcg/day) should only be given live flu vaccine on the advice of their specialist 23 The national childhood flu immunisation programme 2017/18

Contraindications to flu vaccines • The live attenuated flu vaccine should not be given to children who are: • Severely immunodeficient due to conditions or immunosuppressive therapy: § Acute and chronic leukaemias § Lymphoma § HIV positive patient not on highly active antiretroviral therapy § Cellular immune deficiencies § High dose steroids • Receiving salicylate therapy • Known to be pregnant

Contraindications to flu vaccines • Confirmed anaphylactic reaction to a previous dose of flu vaccine or any component of the vaccine • Other than an ovalbumin anaphylaxis , further guidance in the ‘Green Boo. K’

• JCVI has advised (JCVI, 2015) that children with an egg allergy – including those with previous anaphylaxis to egg – can be safely vaccinated with LAIV in any setting (including primary care and schools). As long as there are no other contraindications • Reference: The Green Book – flu chapter April 2019

• The only exception is for children who have required admission to intensive care for a previous severe anaphylaxis to egg, for whom no data are available; such children are best given LAIV in the hospital setting. LAIV remains the preferred vaccine for this group and the intranasal route is less likely to cause systemic reactions

Offer the egg free vaccine • The egg free vaccine is licensed from 9 years old • Can be offered to children with a egg allergy 9 years and over

NHS Specialist Pharmacy Services SPS • During the 2018/19 flu season the NHS Specialist Pharmacy Service (SPS) became aware of issues being encountered by health and social care organisations in offering employees the seasonal flu vaccine, including peer to peer vaccination, within the Patient Group Direction (PGD) legislation for occupational health purposes.

SPS • Provided a written instruction template for seasonal influenza vaccination. The written instruction can be adopted by organisations following the signed authorisation by an appropriate doctor • Vaccine Update June 2019

Any Questions? • Any questions?

References • https: //www. gov. uk/government/collections/i mmunisation • http: //vk. ovg. ox. ac. uk/vk/influenza-flu