Avian Flu Detection Research and Vaccine Production Influenza




- Slides: 21

Avian Flu: Detection, Research and Vaccine Production

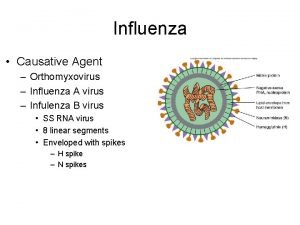
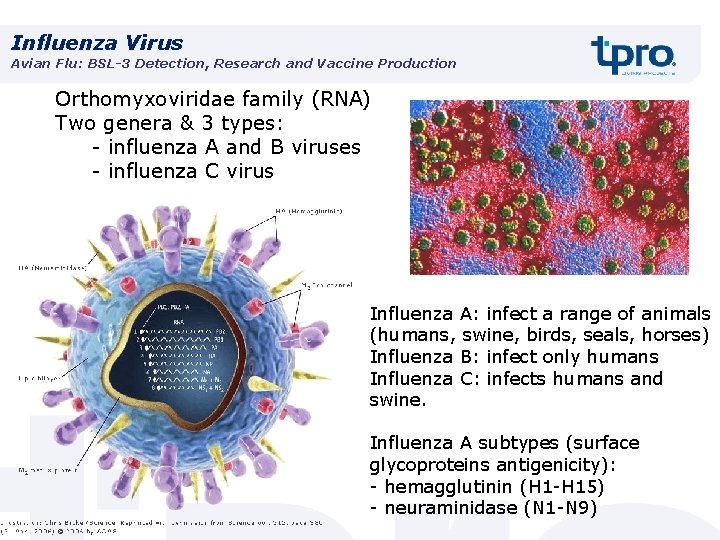
Influenza Virus Avian Flu: BSL-3 Detection, Research and Vaccine Production Orthomyxoviridae family (RNA) Two genera & 3 types: - influenza A and B viruses - influenza C virus Influenza A: infect a range of animals (humans, swine, birds, seals, horses) Influenza B: infect only humans Influenza C: infects humans and swine. Influenza A subtypes (surface glycoproteins antigenicity): - hemagglutinin (H 1 -H 15) - neuraminidase (N 1 -N 9)

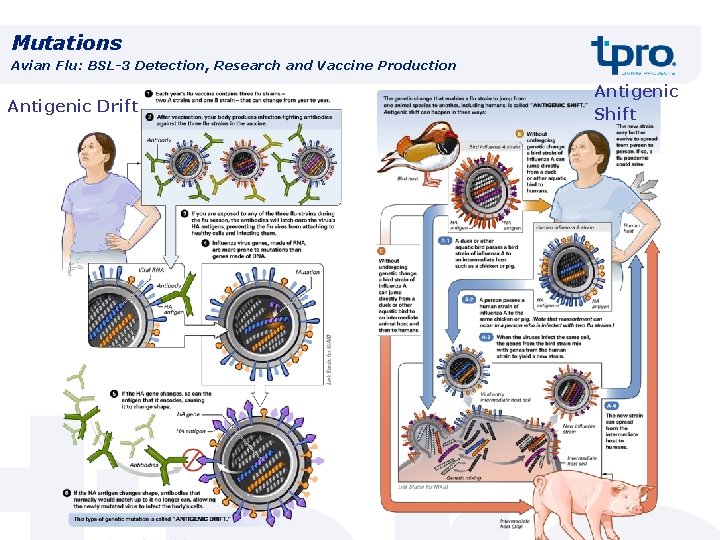
Mutations Avian Flu: BSL-3 Detection, Research and Vaccine Production Antigenic Drift Antigenic Shift

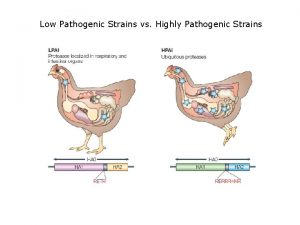
Avian Influenza Viruses Avian Flu: BSL-3 Detection, Research and Vaccine Production Avian strains of influenza A viruses Wild water birds (natural reservoir) infection avirulent, little/no symptoms. To poultry species (chickens, turkeys) mild clinical symptoms Transmision: direct physical contact, through contact with surfaces, water or feed contaminated with virus. LPAIV: low pathogenic avian influenza virus HPAIV: high pathogenic avian influenza virus

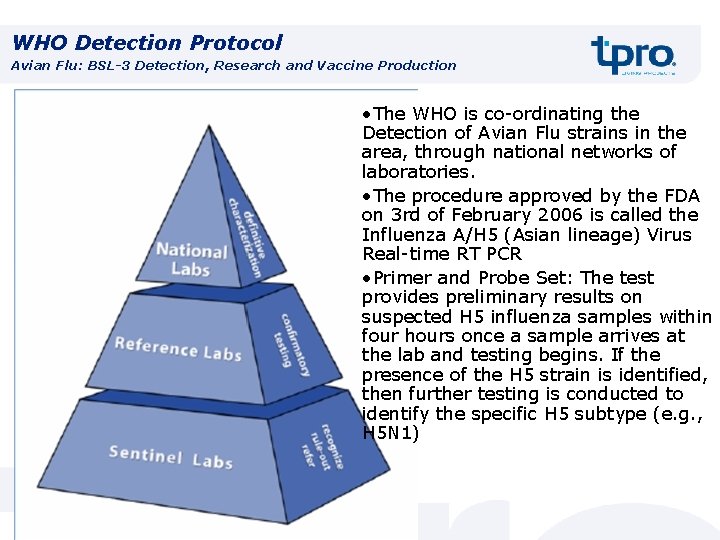
WHO Detection Protocol Avian Flu: BSL-3 Detection, Research and Vaccine Production • The WHO is co-ordinating the Detection of Avian Flu strains in the area, through national networks of laboratories. • The procedure approved by the FDA on 3 rd of February 2006 is called the Influenza A/H 5 (Asian lineage) Virus Real-time RT PCR • Primer and Probe Set: The test provides preliminary results on suspected H 5 influenza samples within four hours once a sample arrives at the lab and testing begins. If the presence of the H 5 strain is identified, then further testing is conducted to identify the specific H 5 subtype (e. g. , H 5 N 1)

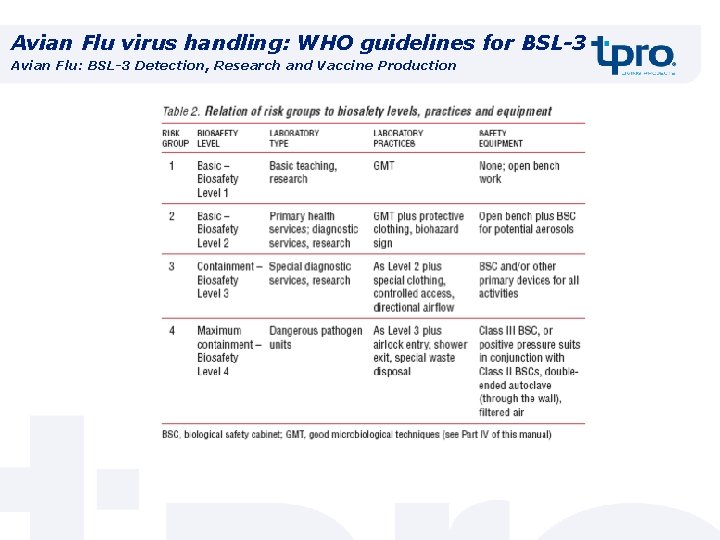
Avian Flu virus handling: WHO guidelines for BSL-3 Avian Flu: BSL-3 Detection, Research and Vaccine Production

Avian Flu virus handling: WHO guidelines for BSL-3 Avian Flu: BSL-3 Detection, Research and Vaccine Production

Avian Flu virus handling: WHO guidelines for BSL-3 Avian Flu: BSL-3 Detection, Research and Vaccine Production

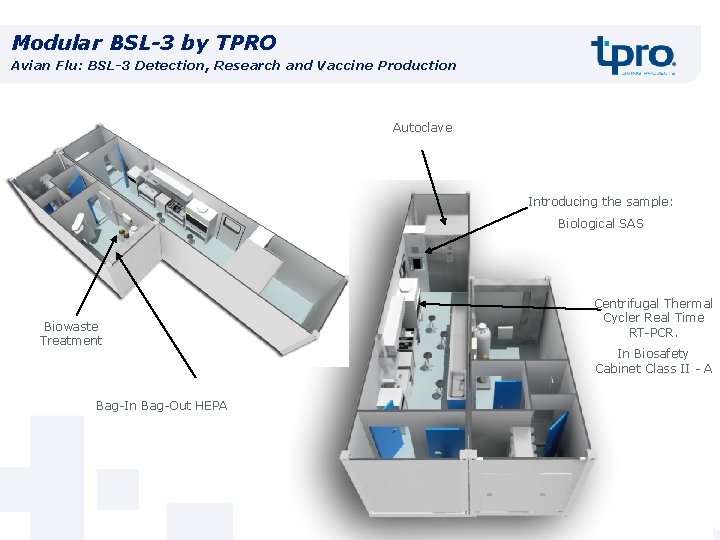
Modular BSL-3 by TPRO Avian Flu: BSL-3 Detection, Research and Vaccine Production Autoclave Introducing the sample: Biological SAS Biowaste Treatment Bag-In Bag-Out HEPA Centrifugal Thermal Cycler Real Time RT-PCR. In Biosafety Cabinet Class II - A

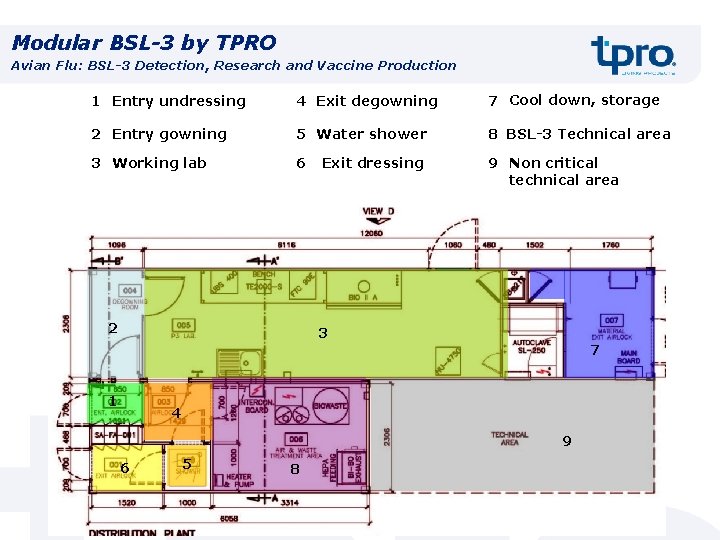
Modular BSL-3 by TPRO Avian Flu: BSL-3 Detection, Research and Vaccine Production 1 Entry undressing 4 Exit degowning 7 Cool down, storage 2 Entry gowning 5 Water shower 8 BSL-3 Technical area 3 Working lab 6 9 Non critical technical area 2 Exit dressing 3 7 1 4 9 6 5 8

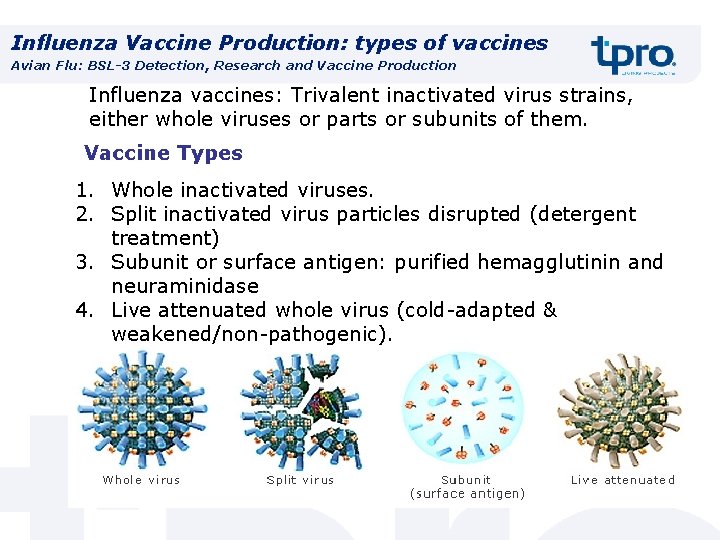
Influenza Vaccine Production: types of vaccines Avian Flu: BSL-3 Detection, Research and Vaccine Production Influenza vaccines: Trivalent inactivated virus strains, either whole viruses or parts or subunits of them. Vaccine Types 1. Whole inactivated viruses. 2. Split inactivated virus particles disrupted (detergent treatment) 3. Subunit or surface antigen: purified hemagglutinin and neuraminidase 4. Live attenuated whole virus (cold-adapted & weakened/non-pathogenic).

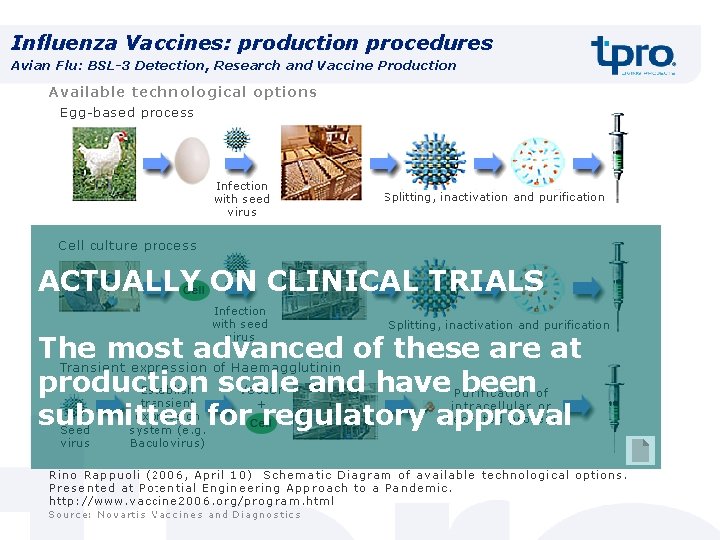
Influenza Vaccines: production procedures Avian Flu: BSL-3 Detection, Research and Vaccine Production ACTUALLY ON CLINICAL TRIALS The most advanced of these are at production scale and have been submitted for regulatory approval

Avian Flu vaccine: production procedures Avian Flu: BSL-3 Detection, Research and Vaccine Production Vaccines produced in fertilized chicken eggs Eggs 11 days after fertilization Each strain is grown separately Embryo infected virus multiplication Harvest, purification, inactivation and production 1 -2 eggs to produce 1 dose of vaccine Entire production process lasts at least six months

Avian Flu vaccine: production procedures Avian Flu: BSL-3 Detection, Research and Vaccine Production Flu vaccine based on cell or tissue cultures Mammalian kidney cells are preferably used for these cell cultures. Virus is injected into these cells, multiply before cells’ outer walls are removed Harvest, purification, inactivation and production Process similar to biotechnological fermentation, in which you move from small liter jars to huge fermenters during production. Described in the mid-nineties and is still in its experimental stage Sanofi Pasteur Cell Culture-Based Seasonal Influenza Vaccine Enters First Clinical Trial (27 Sep 2006) PER. C 6® cell line in 20, 000 liters bioreactor.

Avian Flu vaccine: production procedures Avian Flu: BSL-3 Detection, Research and Vaccine Production EGG-BASED PRODUCTION Advantages: ü Well established and cost-effective ü Lower cost • Disadvantages: Extensive planning: long timeline for million eggs procurement • Limited flexibility in case of exponentially increasing demand (pandemic not contained & defeated): • production takes too long • eggs don’t grow on demand • Potential impurities in eggs (antibiotics, other viruses) may cause sterility problems • Risk of allergies against egg albumin • Growth of epidemic viruses in eggs result in variants that are antigenically distinct from the original viruses • Emerging endemic viruses sometimes do not grow at all •

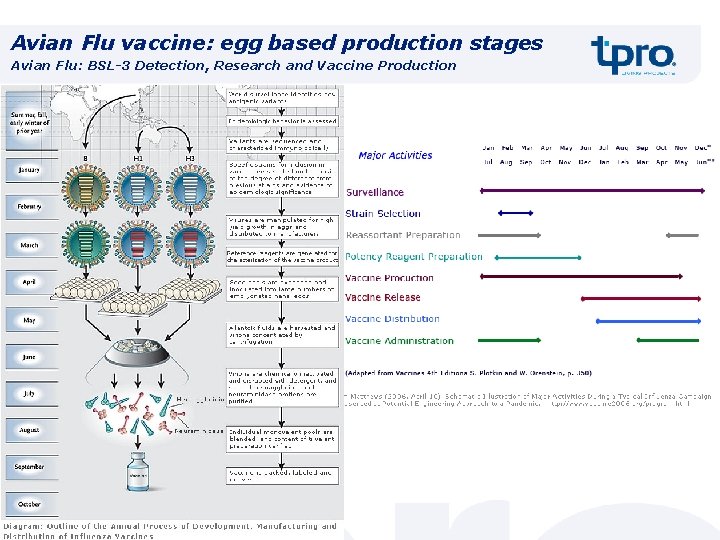
Avian Flu vaccine: egg based production stages Avian Flu: BSL-3 Detection, Research and Vaccine Production

Avian Flu vaccine: egg based production Avian Flu: BSL-3 Detection, Research and Vaccine Production Reassortment To maximize virus yield from eggs, reassortants of influenza A strains are often utilized

Avian Flu vaccine: egg based production Avian Flu: BSL-3 Detection, Research and Vaccine Production Large scale not viable to use SPF eggs High quality “production” eggs are obtained from accredited flocks certified to rigorous quality control standards. Eggs are incubated for 10 -11 days before inoculation Inoculation of Working Seed Virus (manually/automated machinery) into the allantoic cavity of the egg Virus cultivation: Eggs incubation at 35 -37 o. C at a relative humidity of around 65% for 48 to 72 hours to allow the virus to multiply

Avian Flu vaccine: production procedures Avian Flu: BSL-3 Detection, Research and Vaccine Production Harvest: Allantoic fluid. Manual or automated) vacuum systems Then usually clarified by centrifugation to remove cellular debris. Production process will vary from manufacturer to manufacturer, but the next stages normally include: - chemical inactivation: formaldehyde and betapropiolactone - concentration - purification: centrifugation on a density gradient or by column chromatography

Avian Flu vaccine: production facility design Avian Flu: BSL-3 Detection, Research and Vaccine Production process: manually vs automatic machines Aseptic manufacturing IM vials suspension BSL-2 Pharmaceutical critical utilities ü HVAC & Clean Room ü Pharmaceutical waters: PW & WFI ü Pure Steam ü CIP/SIP system Virus contaminated waste treatment ü Plastics, glass, paper Autoclave (121ºC; 30 min) ü Liquids in container Autoclave (121ºC; 30 min) ü Liquid effluent (drains) BW (chemical) ü Solids – eggs waste products Pasteurization (90 secs, >70ºC)

www. tpro. es
 Low pathogenic avian influenza
Low pathogenic avian influenza Stomach flu vs influenza
Stomach flu vs influenza Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Produksi multimedia
Produksi multimedia Polygastric examples
Polygastric examples Avian taxonomy
Avian taxonomy Porter novelli healthstyles survey
Porter novelli healthstyles survey Paraphrasing proverbs
Paraphrasing proverbs Ahas birds
Ahas birds Bash ahas
Bash ahas Bird digestive system functions
Bird digestive system functions Dispersal definition
Dispersal definition Chordata aves
Chordata aves Enantiornithes
Enantiornithes Metodo dei cerchi di heuvers
Metodo dei cerchi di heuvers Flu causative agent
Flu causative agent The great influenza rhetorical analysis
The great influenza rhetorical analysis Influenza
Influenza Influenza
Influenza Is influenza a airborne disease
Is influenza a airborne disease Rimantidina
Rimantidina Influenza ww1
Influenza ww1