ERT 1083 PHYSICAL CHEMISTRY SECOND LAW OF THERMODYNAMICS

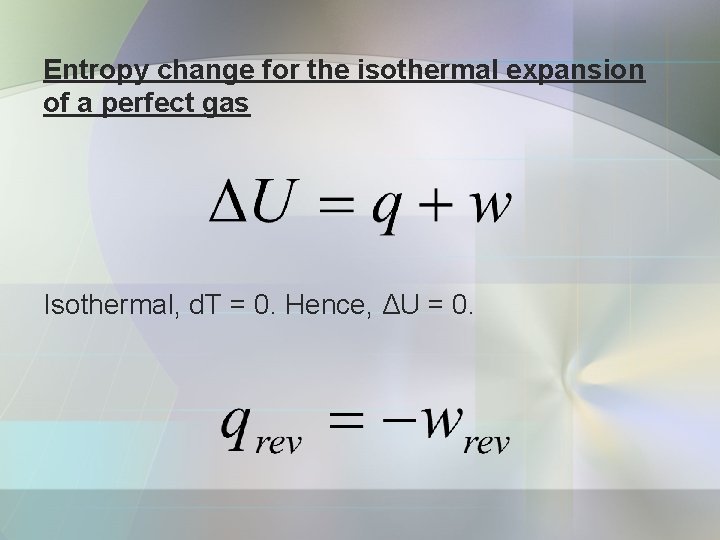





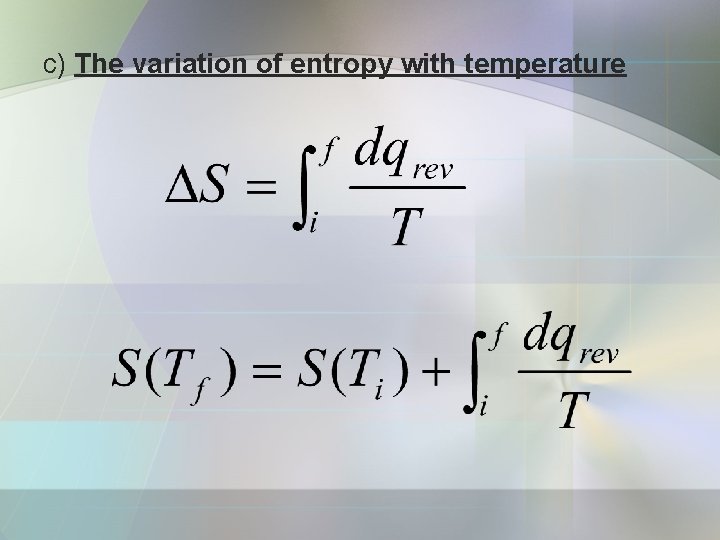




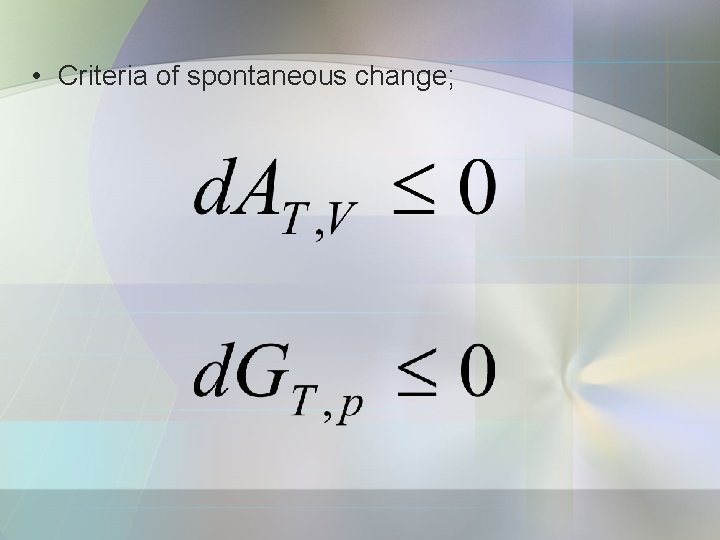



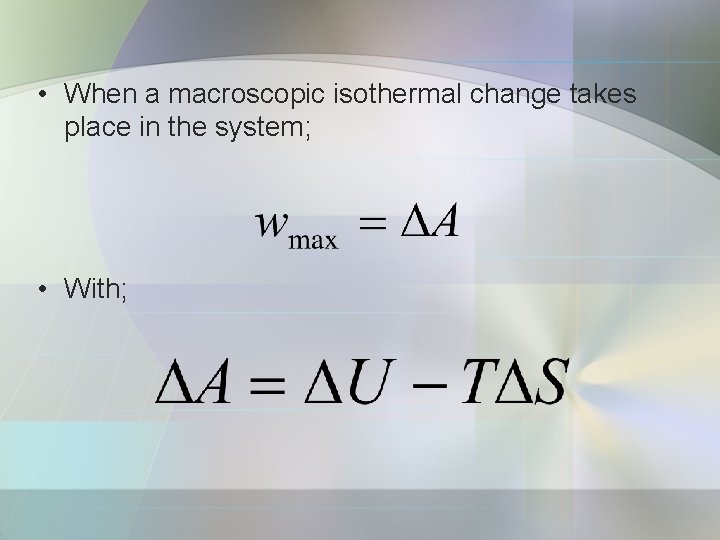



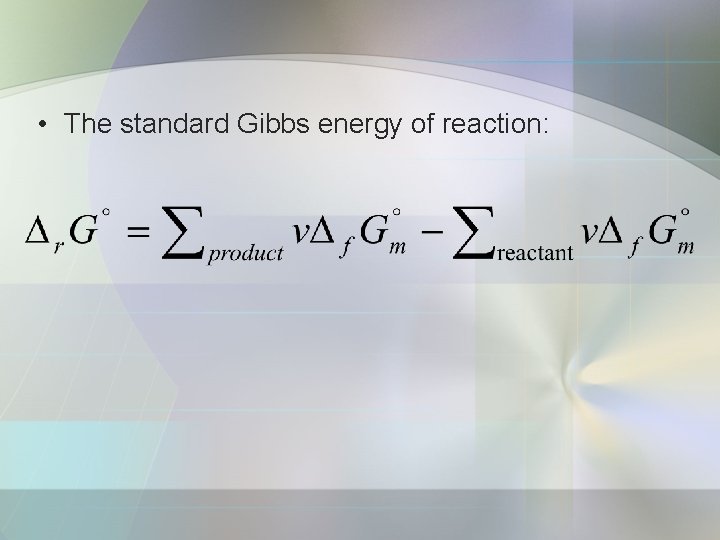
- Slides: 36

ERT 108/3 PHYSICAL CHEMISTRY SECOND LAW OF THERMODYNAMICS Prepared by: Pn. Hairul Nazirah Abdul Halim

About This Chapter • To explain the origin of the spontaneity of physical and chemical change. • • Heat engines Entropy Calculation of entropy changes Helmholtz and Gibbs energies

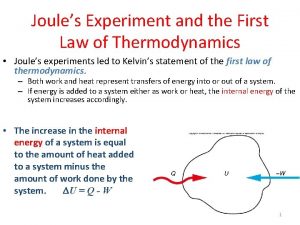
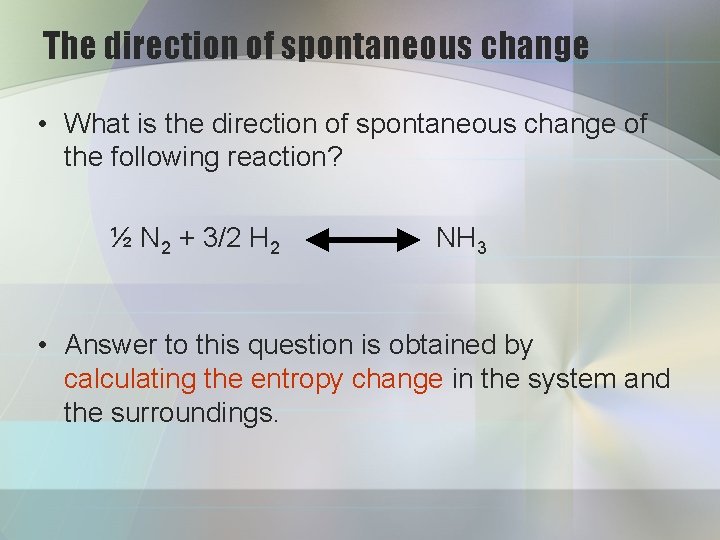
The direction of spontaneous change • What is the direction of spontaneous change of the following reaction? ½ N 2 + 3/2 H 2 NH 3 • Answer to this question is obtained by calculating the entropy change in the system and the surroundings.

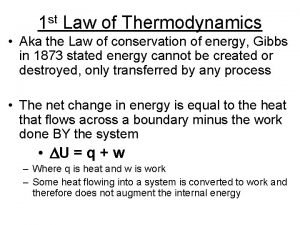
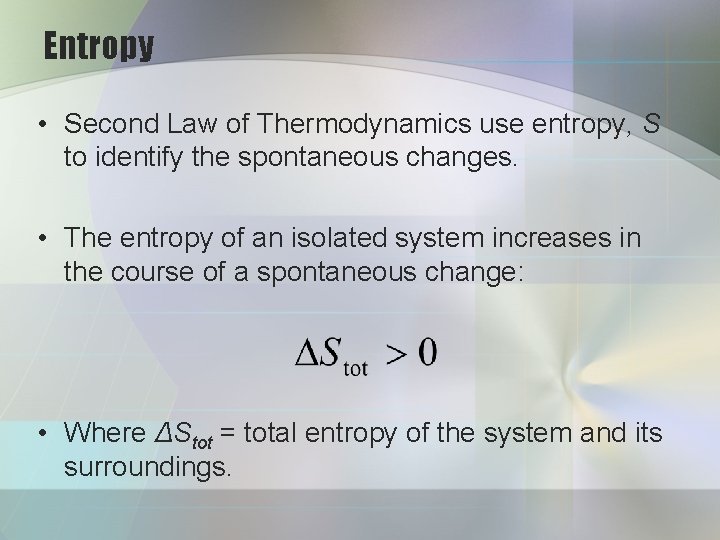
Entropy • Second Law of Thermodynamics use entropy, S to identify the spontaneous changes. • The entropy of an isolated system increases in the course of a spontaneous change: • Where ΔStot = total entropy of the system and its surroundings.

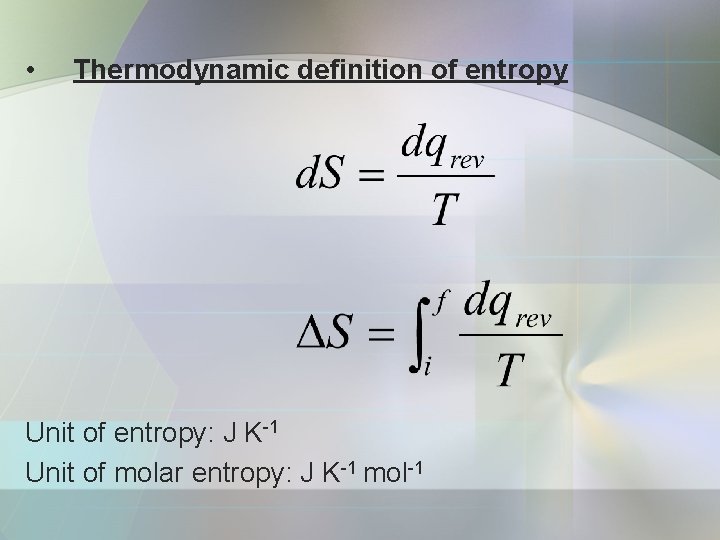
• Thermodynamic definition of entropy Unit of entropy: J K-1 Unit of molar entropy: J K-1 mol-1
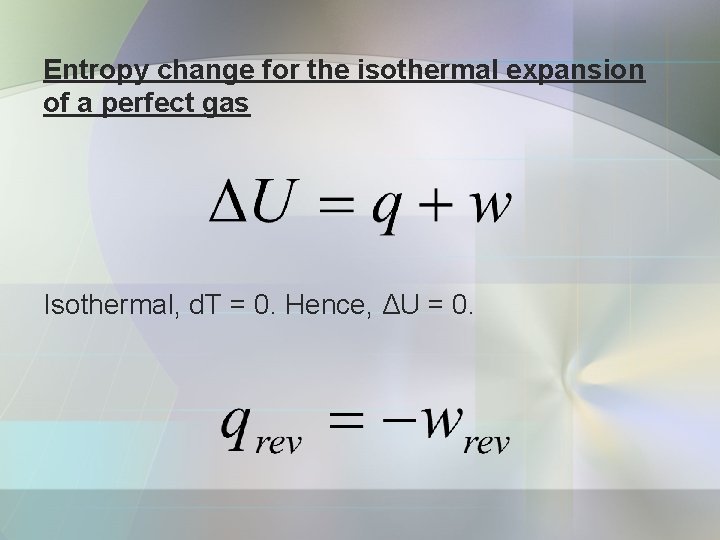
Entropy change for the isothermal expansion of a perfect gas Isothermal, d. T = 0. Hence, ΔU = 0.

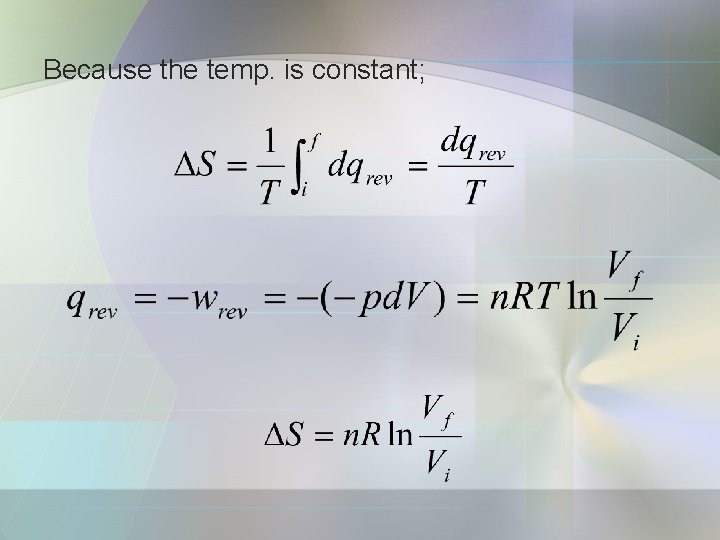
Because the temp. is constant;

Heat Engines Automobile engine • Automobile engine operates in a cyclic process of fuel intake, compression, ignition and expansion, and exhaust. • Occurs several thousand times per minutes. • Is used to perform work on the surroundings. • The system consists of piston & cylinder assembly with diathermal walls. • The expansion and contraction of the gas caused by changes in its temperature drives the piston in or out of cylinder.

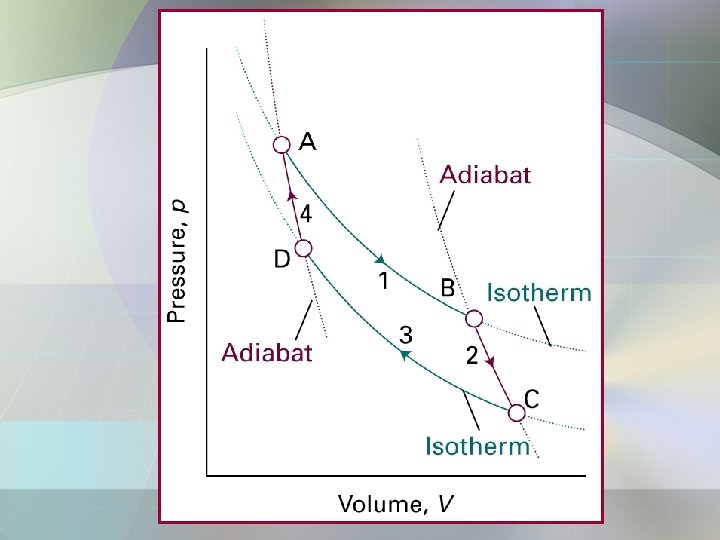
A reversible Carnot Cycle consist of 4 reversible stages: 1. Reversible isothermal expansion from A to B. d. S = qh/Th. 2. Reversible adiabatic expansion from B to C. d. S = 0. 3. Reversible isothermal compression from C to D. d. S = qc/Tc 4. Reversible adiabatic compression from D to A. d. S = 0.


• The total change in entropy around the cycle is;

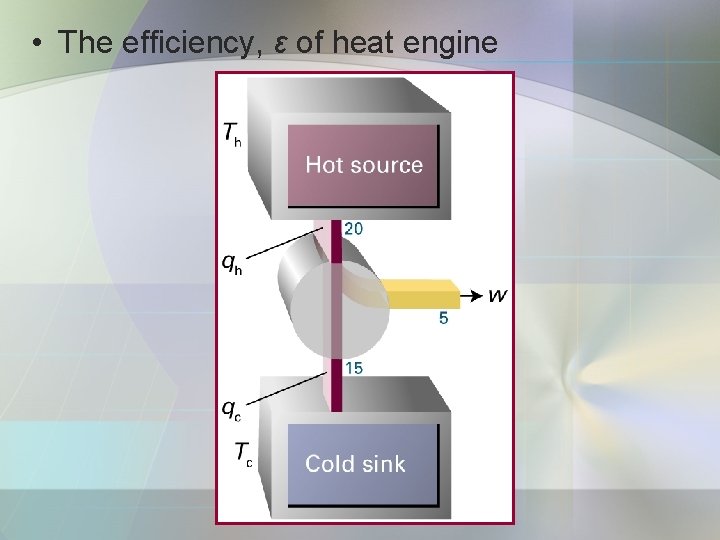
• The efficiency, ε of heat engine

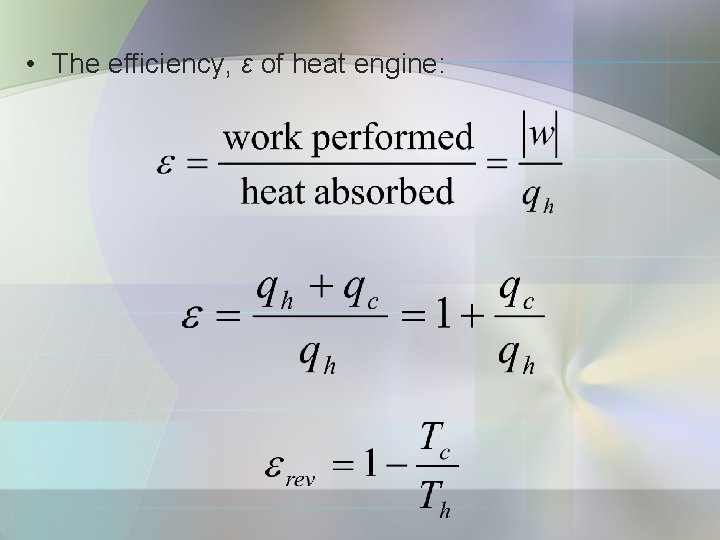
• The efficiency, ε of heat engine:

c) Thermodynamic Temperature Thermodynamic temperature scale is defined as;

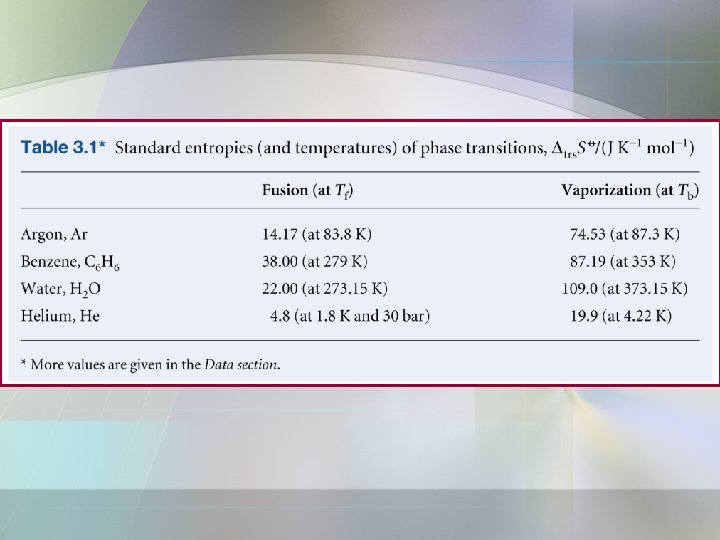
Entropy Changes a) Entropy change of phase transition at the transition temp. • • Phase transition such as solid melts to liquid, liquid phase turns into a gas. At constant pressure; • The change in molar entropy of the system;



b) The expansion of a perfect gas The change in entropy of a perfect gas that expands isothermally from Vi to Vf is;
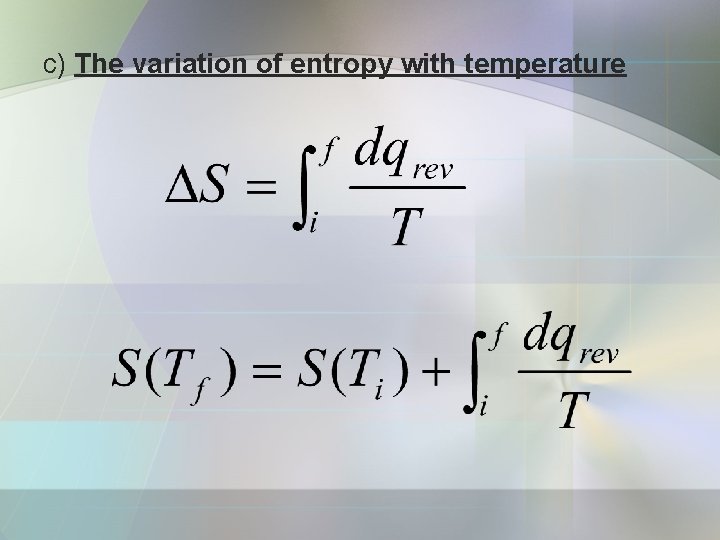
c) The variation of entropy with temperature

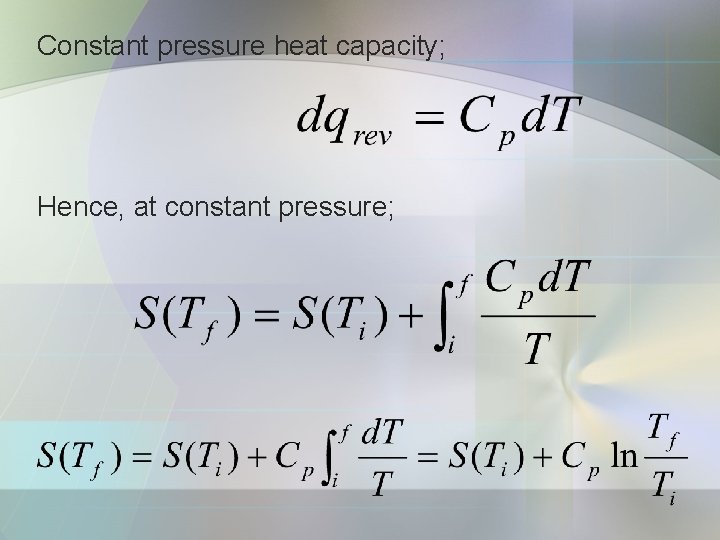
Constant pressure heat capacity; Hence, at constant pressure;

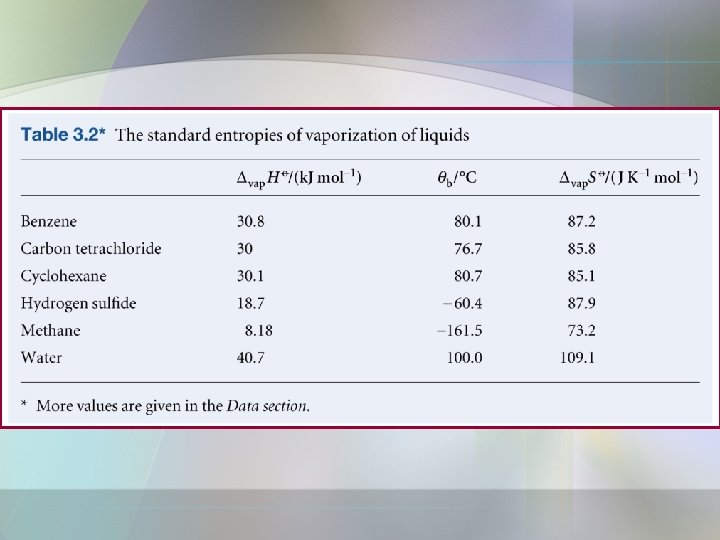
d) The measurement of entropy If a substance melts at Tf and boils at Tb, then its entropy above its boiling temperature is;

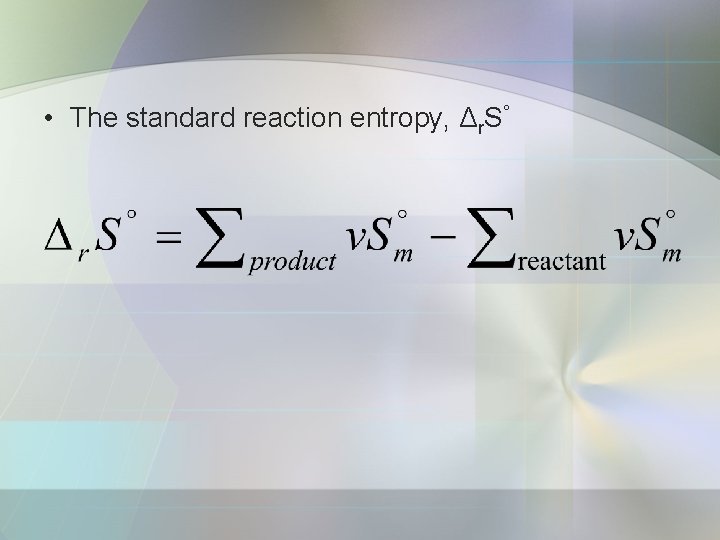
• The standard reaction entropy, Δr. S°

The Helmholtz and Gibss Energies • Consider a system in thermal equilibrium with its surroundings at a temperature T. • When a change in the system occurs and there is a transfer of energy as heat between system and its surrounding;

• Criteria for spontaneity Consider heat transfer at constant volume, dqv = d. U; At constant V and no additional work;

• When heat is transferred at constant pressure, dqp = d. H;

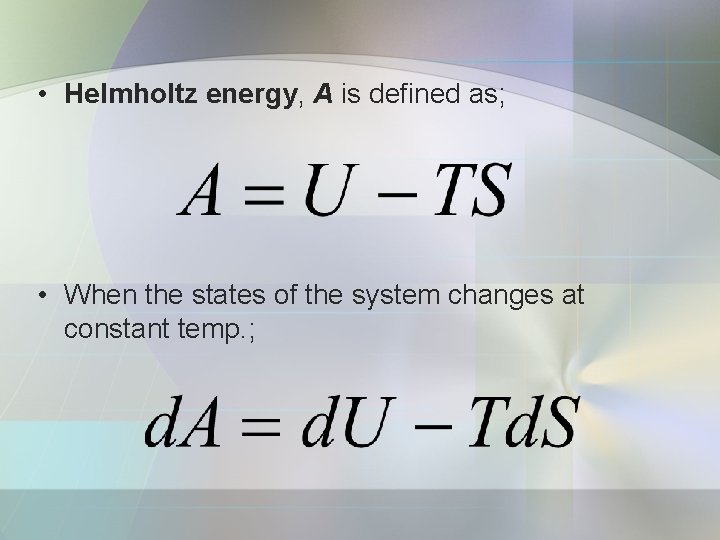
• Helmholtz energy, A is defined as; • When the states of the system changes at constant temp. ;

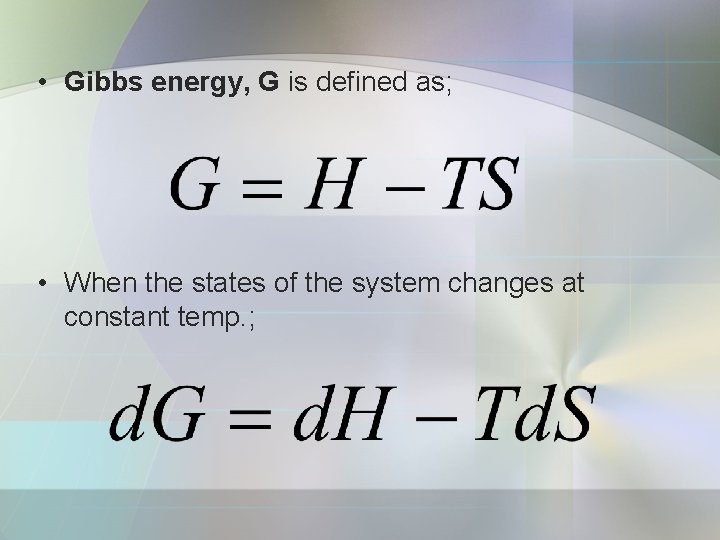
• Gibbs energy, G is defined as; • When the states of the system changes at constant temp. ;
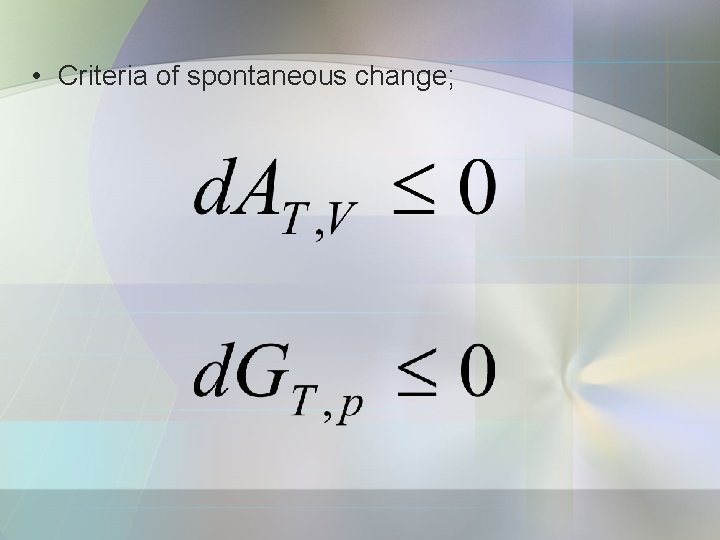
• Criteria of spontaneous change;

b) Some remarks on the Helmholz energy • A change in system at constant temp. and volume is spontaneous if. • The criterion of equilibrium,

& Both of the above equations are interpreted as follows: • –ve value of d. A is favoured by –ve value of d. U and +ve value of Td. S.

c) Maximum work The change in the Helmholtz function is equal to the maximum work accompanying a process; A sometimes called the ‘maximum work function’ or ‘work function’.
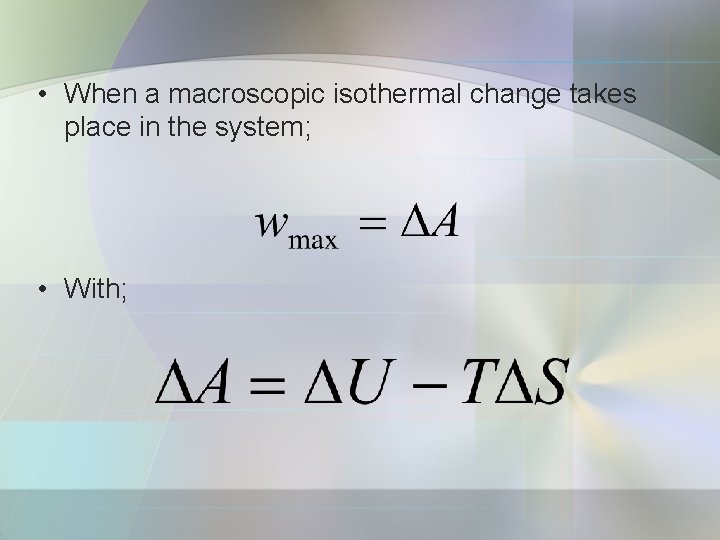
• When a macroscopic isothermal change takes place in the system; • With;

c) Some remarks on the Gibbs energy • • Gibbs energy = free energy At constant pressure and temperature, chemical reactions are spontaneous in the direction of decreasing Gibbs energy. If G decreases as the reaction proceeds: spontaneous tendency to convert reactants to products. If G increases, the reverse reaction is spontaneous.

e) Maximum additional (non-expansion) work Wad, max = Maximum additional (non-expansion) work, is given by the change in Gibbs energy;

Standard Molar Gibbs Energies • The standard entropies and enthalpies of reaction can be combined to obtain the standard Gibbs energy of reaction, Δr. G°;
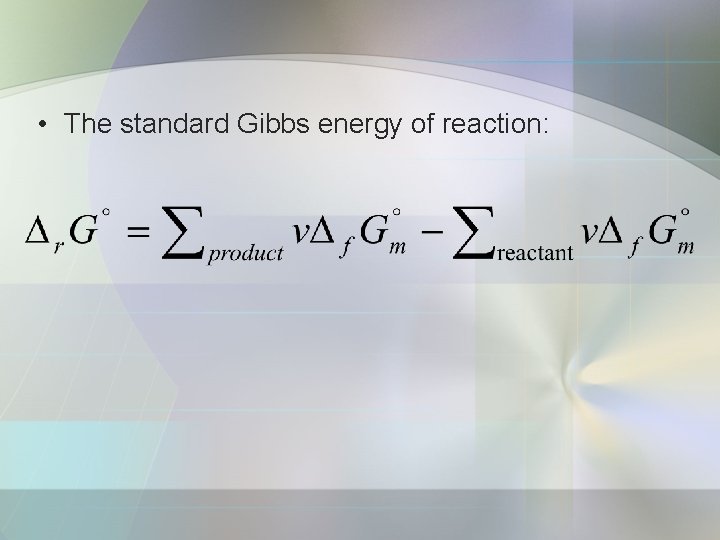
• The standard Gibbs energy of reaction:
 Kelvin planck statement
Kelvin planck statement State second law of thermodynamics
State second law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion Law of thermodynamics in chemistry
Law of thermodynamics in chemistry Pt = p.ert merupakan rumus dari pertumbuhan penduduk
Pt = p.ert merupakan rumus dari pertumbuhan penduduk Ert controller
Ert controller Ert tool
Ert tool Ert diagram
Ert diagram Ert erp definition
Ert erp definition Ert erp definition
Ert erp definition Ert 1 programa
Ert 1 programa Ert products malaysia
Ert products malaysia Intermediate beam design
Intermediate beam design Statistical thermodynamics in chemistry
Statistical thermodynamics in chemistry 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Ap chemistry thermodynamics
Ap chemistry thermodynamics Ap chemistry thermochemistry
Ap chemistry thermochemistry 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 186 282 miles per second into meters per second
186 282 miles per second into meters per second Energy balance thermodynamics
Energy balance thermodynamics 0th law of thermodynamics definition
0th law of thermodynamics definition Zeroth law of thermodynamics examples
Zeroth law of thermodynamics examples First law of thermodynamics
First law of thermodynamics 1th law of thermodynamics
1th law of thermodynamics Joules experiment
Joules experiment Example of adiabatic process
Example of adiabatic process In a flow process the work transfer may be of which type
In a flow process the work transfer may be of which type What are the laws of thermodynamics
What are the laws of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt