ERT 2064 Thermodynamics CHAPTER 2 The First Law







- Slides: 31

ERT 206/4 Thermodynamics CHAPTER 2 The First Law and Other Basic Concepts Miss. Rahimah Bt. Othman Email: rahimah@unimap. edu. my

COURSE OUTCOME 1 CO 1) 1. Chapter 1: Introduction to Thermodynamics 2. Chapter 2: The First Law and Other Basic Concepts Define, discuss, apply and analyze internal energy, first law, energy balance-closed system, thermodynamic state and state function, equilibrium, the Phase Rule, reversible process, constant-V and constant-P processes, enthalpy and heat capacity. 3. Chapter 3: Volumetric properties of pure fluids 4. Chapter 4: Heat effects 5. Chapter 5: Second law of thermodynamics 6. Chapter 6: Thermodynamics properties of fluids

Fundamental Laws of Thermodynamics • First law of thermodynamics – energy can be converted from one form to another, but cannot be created or destroyed and the total quantity of energy is constant. (Eg: A stone is falling down from the highest place) - Potential energy convert to kinetic energy. • Second law of thermodynamics – energy consist of quality and quantity where the real processes normally occurred within the decreasing quality of energy. (Eg: A cup of hot coffee become a cool coffee after a few minutes) – Energy with low temperature.


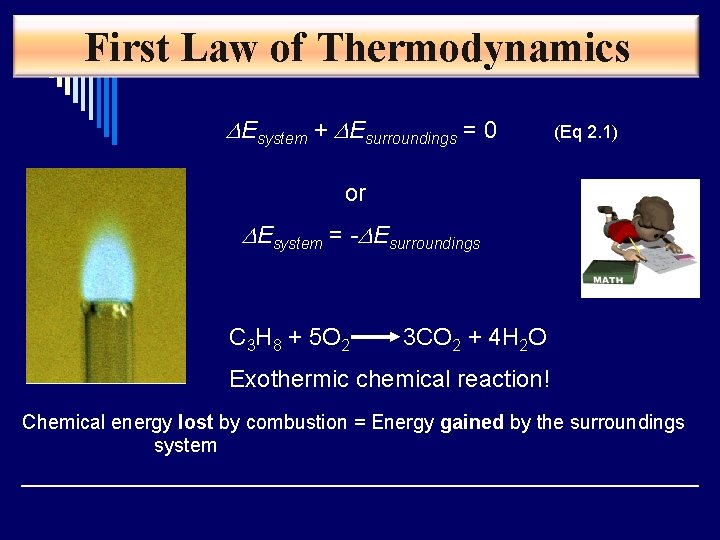
First Law of Thermodynamics DEsystem + DEsurroundings = 0 (Eq 2. 1) or DEsystem = -DEsurroundings C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O Exothermic chemical reaction! Chemical energy lost by combustion = Energy gained by the surroundings system

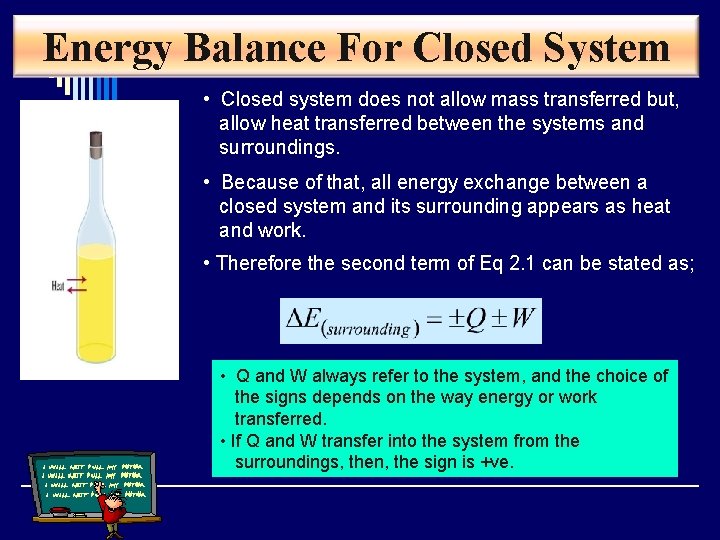
Energy Balance For Closed System • Closed system does not allow mass transferred but, allow heat transferred between the systems and surroundings. • Because of that, all energy exchange between a closed system and its surrounding appears as heat and work. • Therefore the second term of Eq 2. 1 can be stated as; • Q and W always refer to the system, and the choice of the signs depends on the way energy or work transferred. • If Q and W transfer into the system from the surroundings, then, the sign is +ve.

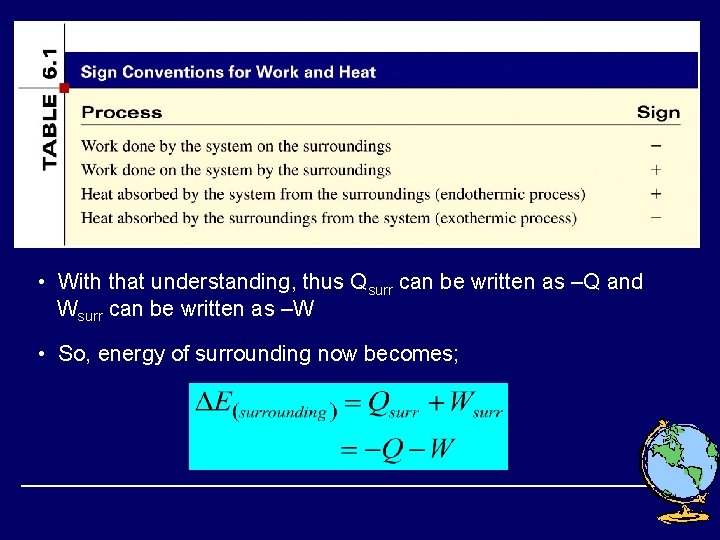
• With that understanding, thus Qsurr can be written as –Q and Wsurr can be written as –W • So, energy of surrounding now becomes;

• Equation 2. 1 now becomes; (2. 2) • This can be reduced to Eq 2. 3 where Ut is total Internal Energy of the system; (2. 3) • Equation 2. 3 only applies to finite change, where as, for differential change, it becomes as; (2. 4)

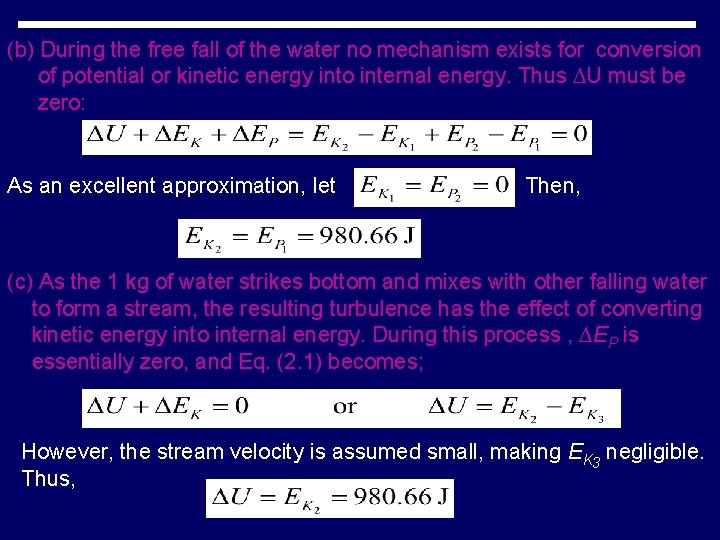
Water flows over a waterfall 100 mm in height. Take 1 kg of the water as the system, and assume that it does not exchange energy with it surroundings. (a) What is the potential energy of the water at the top of the falls with respect to the base of the falls? (b) What is the kinetic energy of the water just before it strikes bottom? (c) After the 1 kg of water enters the stream below the falls, what change has occurred in its state? Solution The 1 kg of water exchanges no energy with the surroundings. Thus, for each part of the process Eq. (2. 1) reduces to; (a) From Eq. (1. 7) with g equal to its standard value.

(b) During the free fall of the water no mechanism exists for conversion of potential or kinetic energy into internal energy. Thus ∆U must be zero: As an excellent approximation, let Then, (c) As the 1 kg of water strikes bottom and mixes with other falling water to form a stream, the resulting turbulence has the effect of converting kinetic energy into internal energy. During this process , ∆EP is essentially zero, and Eq. (2. 1) becomes; However, the stream velocity is assumed small, making EK 3 negligible. Thus,

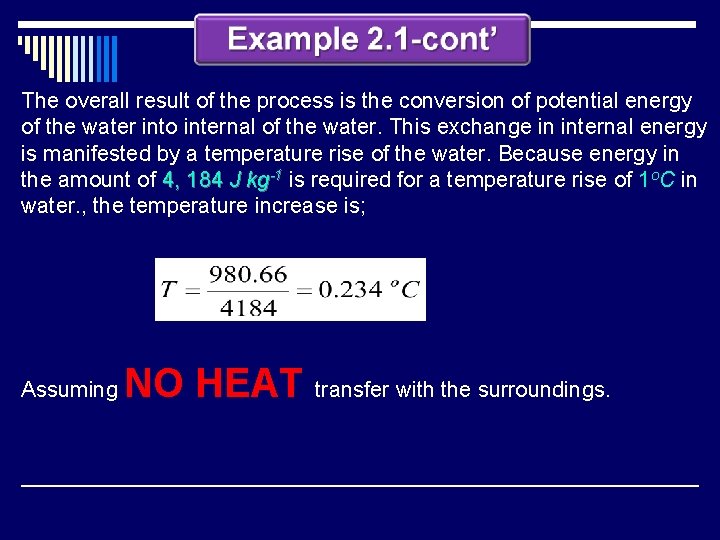
The overall result of the process is the conversion of potential energy of the water into internal of the water. This exchange in internal energy is manifested by a temperature rise of the water. Because energy in the amount of 4, 184 J kg-1 is required for a temperature rise of 1 o. C in water. , the temperature increase is; Assuming NO HEAT transfer with the surroundings.

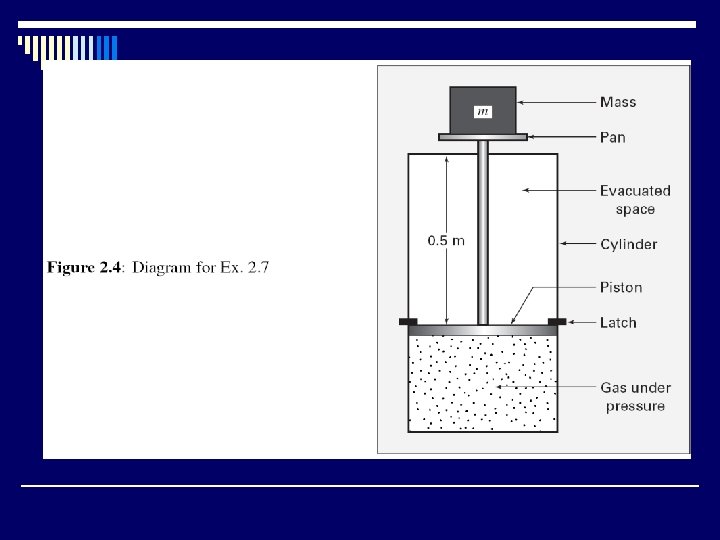
A gas is confined in a cylinder by a piston. The initial pressure of the gas is 7 bar and the volume is 0. 10 m 3. The piston is held in place by latches in the cylinder wall. The whole apparatus is placed in a total vacuum. What is the energy change of the apparatus if the restraining latches are removed so that the gas suddenly expands to double its initial volume, the piston striking other latches at the end of the process? Solution Because the question concerns the entire apparatus, the system is taken as the gas, piston, and cylinder. No work is done during the process, because no force internal to the system moves, and no heat is transferred through the vacuum surrounding the apparatus. Hence Q and W are zero, and the total energy of the system does not change. Without further information we can say nothing about the distribution of energy among the parts of the system. This may well be different than the initial distribution.


If the process described in Eg. 2. 2 is repeated, not in a vacuum but in air at atmospheric pressure of 101. 3 k. Pa, what is the energy change of the apparatus? Assume the rate of heat exchange between the apparatus and the surrounding air is slow compared with the rate at which the process occurs. Solution 1 The system is chosen as before, but here work is done by the system in pushing back the atmosphere. It is evaluated as the product of the force of atmospheric pressure on the back side of the piston F = Patm A and the displacement of the piston ∆l = ∆Vt/A. Here, A is the area of the piston and ∆Vt is the volume change of the gas. This is work done by the system on the surroundings, and is a negative quantity; thus,

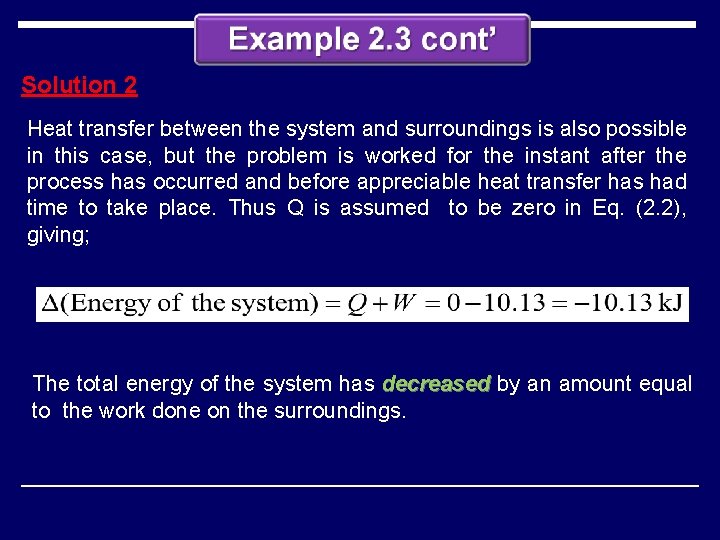
Solution 2 Heat transfer between the system and surroundings is also possible in this case, but the problem is worked for the instant after the process has occurred and before appreciable heat transfer has had time to take place. Thus Q is assumed to be zero in Eq. (2. 2), giving; The total energy of the system has decreased by an amount equal to the work done on the surroundings.

When a system is taken from a state a to state b as in the Fig 2. 1 along path acb, acb 100 J of heat flows into the system and the system does 40 J of work (a) How much heat flows into the system along path aeb if the work done by the system is 20 J (b) The system returns from b to a along bda. If the work done on the system is 30 J, does the system absorb or liberate heat? How much?

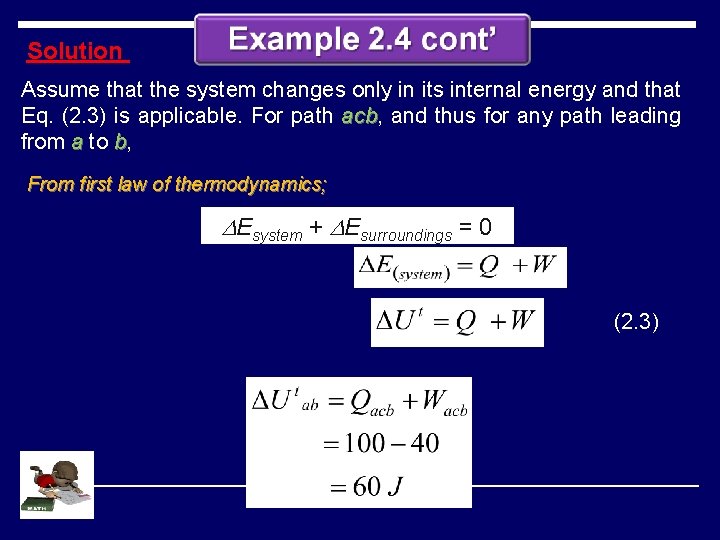
Solution Assume that the system changes only in its internal energy and that Eq. (2. 3) is applicable. For path acb, acb and thus for any path leading from a to b, From first law of thermodynamics; DEsystem + DEsurroundings = 0 (2. 3)

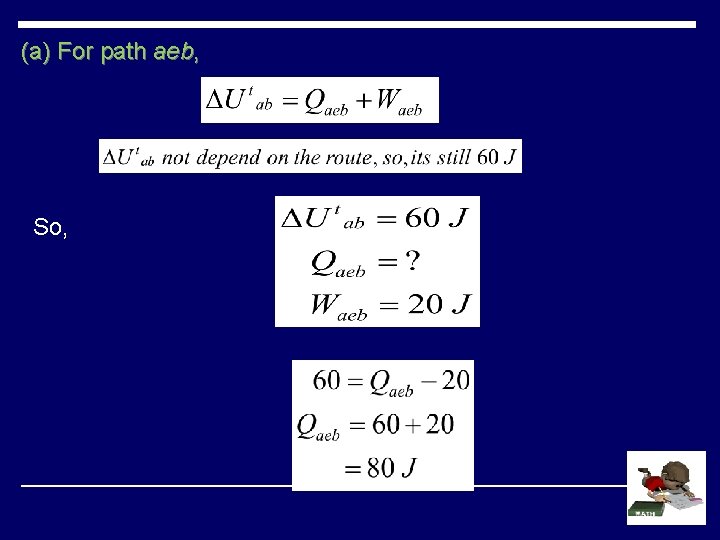
(a) For path aeb, So,

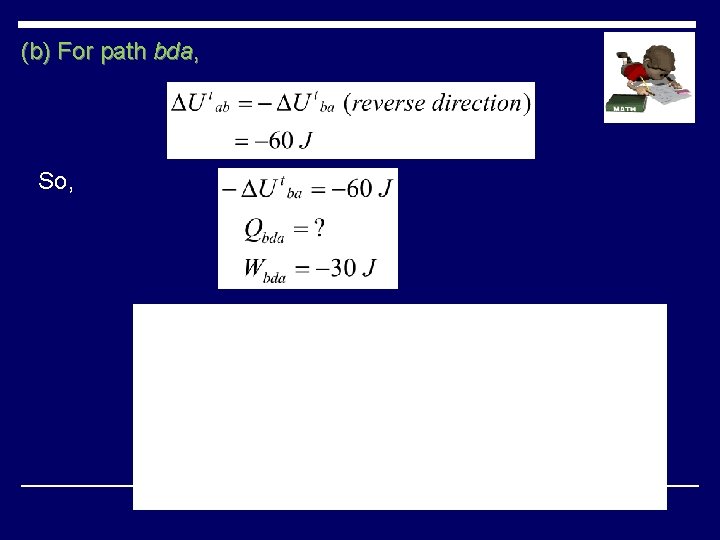
(b) For path bda, So,

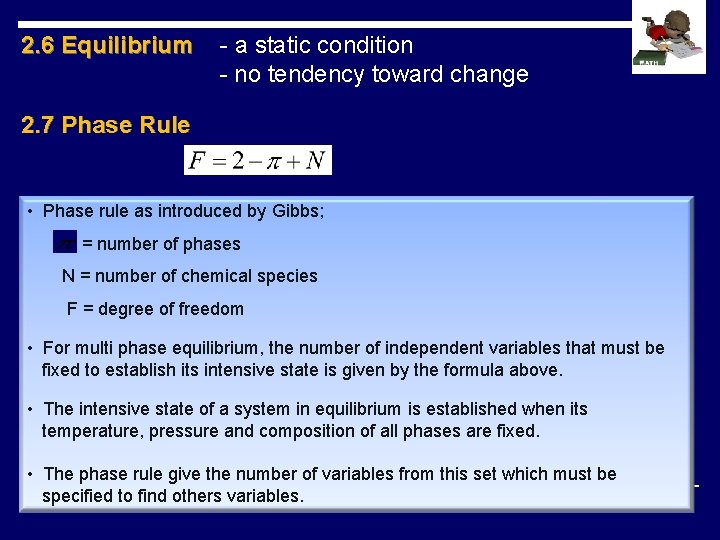
2. 6 Equilibrium - a static condition - no tendency toward change 2. 7 Phase Rule • Phase rule as introduced by Gibbs; = number of phases N = number of chemical species F = degree of freedom • For multi phase equilibrium, the number of independent variables that must be fixed to establish its intensive state is given by the formula above. • The intensive state of a system in equilibrium is established when its temperature, pressure and composition of all phases are fixed. • The phase rule give the number of variables from this set which must be specified to find others variables.

How many degrees of systems? freedom has each of the following (a) Liquid water in equilibrium with its vapor (b) Liquid water in equilibrium with a mixture of water vapor and nitrogen (c) A liquid solution of alcohol in water in equilibrium with its vapor Solution (a) The system contains a single chemical species existing as two phases (one liquid and one vapor). Thus, This result is in agreement with the fact that for a given pressure water has but one boiling point. Temperature or pressure, but not both, may be specified for a system comprised of water in equilibrium with its vapor.

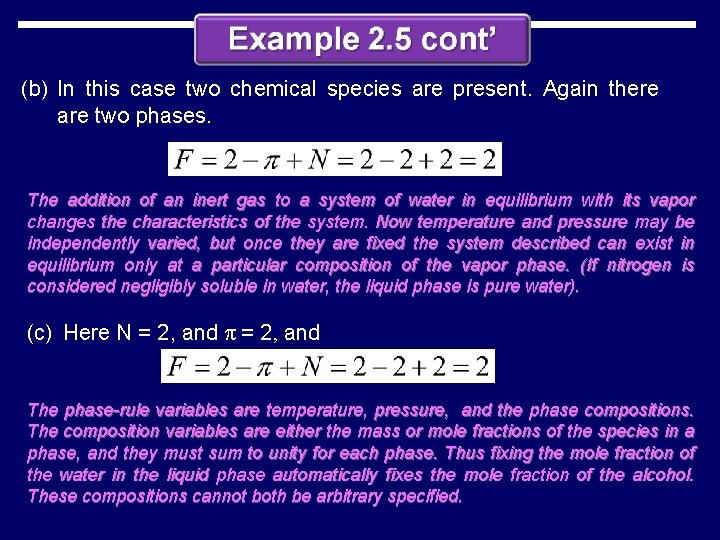
(b) In this case two chemical species are present. Again there are two phases. The addition of an inert gas to a system of water in equilibrium with its vapor changes the characteristics of the system. Now temperature and pressure may be independently varied, but once they are fixed the system described can exist in equilibrium only at a particular composition of the vapor phase. (If nitrogen is considered negligibly soluble in water, the liquid phase is pure water). (c) Here N = 2, and π = 2, and The phase-rule variables are temperature, pressure, and the phase compositions. The composition variables are either the mass or mole fractions of the species in a phase, and they must sum to unity for each phase. Thus fixing the mole fraction of the water in the liquid phase automatically fixes the mole fraction of the alcohol. These compositions cannot both be arbitrary specified.

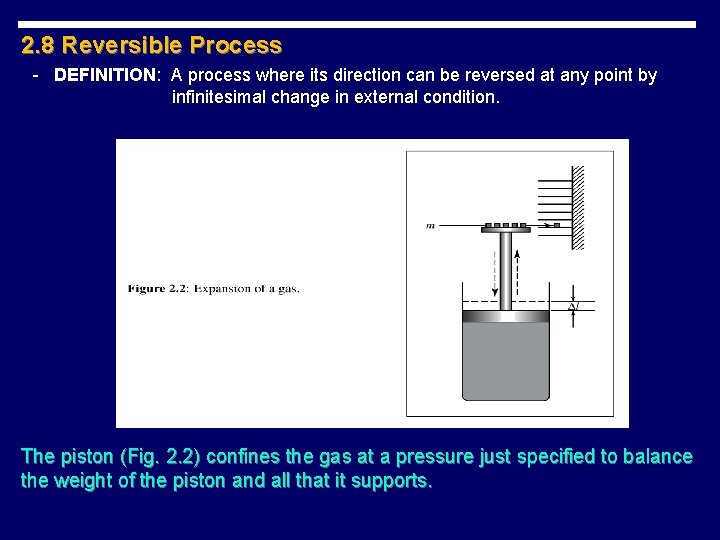
2. 8 Reversible Process - DEFINITION: A process where its direction can be reversed at any point by infinitesimal change in external condition. The piston (Fig. 2. 2) confines the gas at a pressure just specified to balance the weight of the piston and all that it supports.

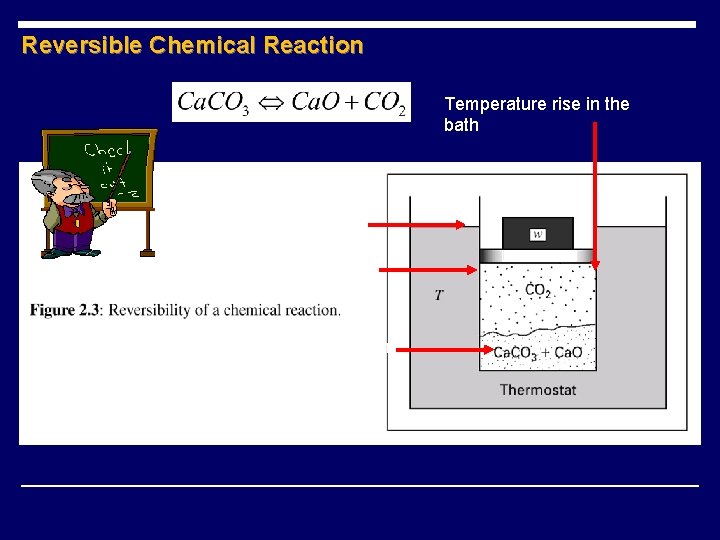
Reversible Chemical Reaction Temperature rise in the bath Increased the weight CO 2 pressure rises CO 2 combines with Ca. O to form Ca. CO 3 (exothermic process)

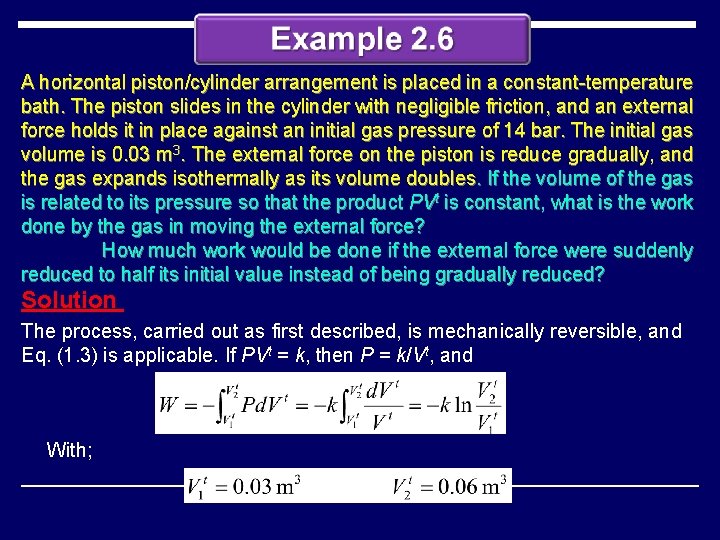
A horizontal piston/cylinder arrangement is placed in a constant-temperature bath. The piston slides in the cylinder with negligible friction, and an external force holds it in place against an initial gas pressure of 14 bar. The initial gas volume is 0. 03 m 3. The external force on the piston is reduce gradually, and the gas expands isothermally as its volume doubles. If the volume of the gas is related to its pressure so that the product PVt is constant, what is the work done by the gas in moving the external force? How much work would be done if the external force were suddenly reduced to half its initial value instead of being gradually reduced? Solution The process, carried out as first described, is mechanically reversible, and Eq. (1. 3) is applicable. If PVt = k, then P = k/Vt, and With;

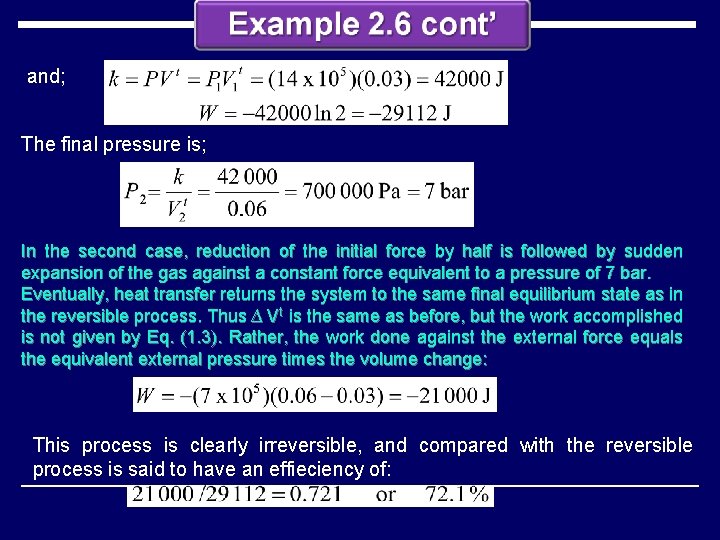
and; The final pressure is; In the second case, reduction of the initial force by half is followed by sudden expansion of the gas against a constant force equivalent to a pressure of 7 bar. Eventually, heat transfer returns the system to the same final equilibrium state as in the reversible process. Thus ∆ Vt is the same as before, but the work accomplished is not given by Eq. (1. 3). Rather, the work done against the external force equals the equivalent external pressure times the volume change: This process is clearly irreversible, and compared with the reversible process is said to have an effieciency of:

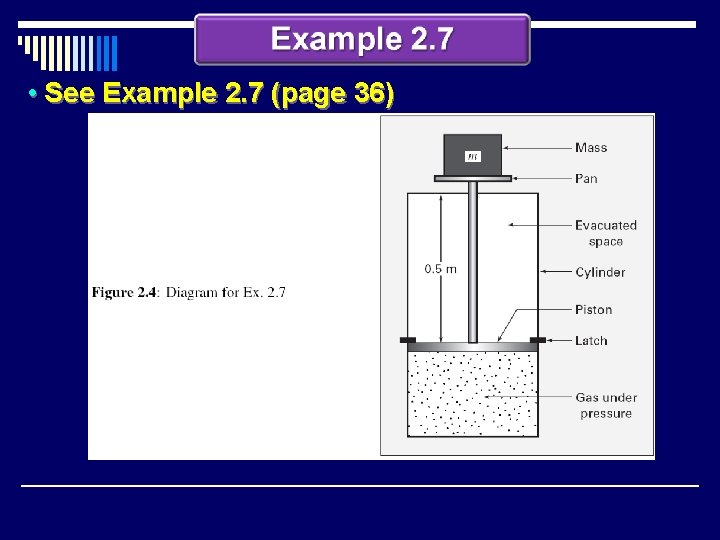
• See Example 2. 7 (page 36)

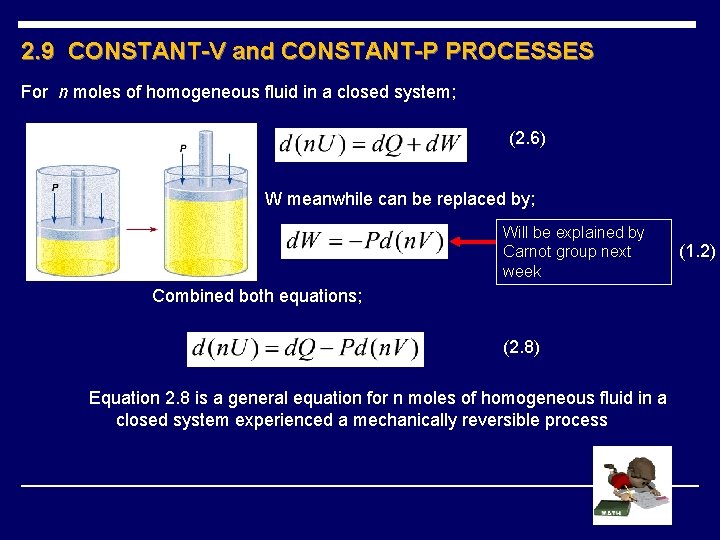
2. 9 CONSTANT-V and CONSTANT-P PROCESSES For n moles of homogeneous fluid in a closed system; (2. 6) W meanwhile can be replaced by; Will be explained by Carnot group next week Combined both equations; (2. 8) Equation 2. 8 is a general equation for n moles of homogeneous fluid in a closed system experienced a mechanically reversible process (1. 2)

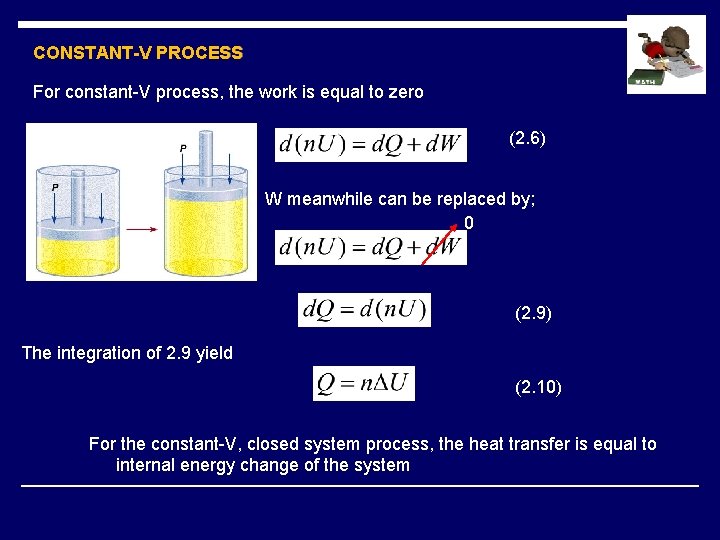
CONSTANT-V PROCESS For constant-V process, the work is equal to zero (2. 6) W meanwhile can be replaced by; 0 (2. 9) The integration of 2. 9 yield (2. 10) For the constant-V, closed system process, the heat transfer is equal to internal energy change of the system

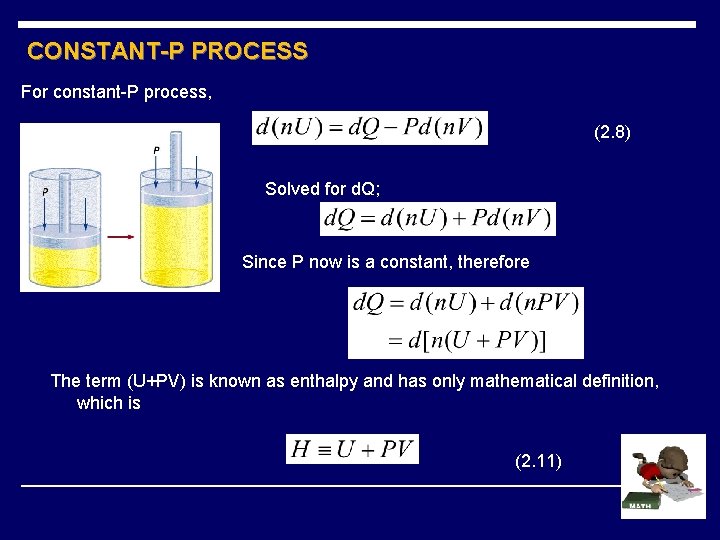
CONSTANT-P PROCESS For constant-P process, (2. 8) Solved for d. Q; Since P now is a constant, therefore The term (U+PV) is known as enthalpy and has only mathematical definition, which is (2. 11)

To be continue… THANK YOU