ERT 108 Physical Chemistry The Second Law of



















- Slides: 40

ERT 108 Physical Chemistry The Second Law of Thermodynamics by Miss Anis Atikah binti Ahmad anisatikah@unimap. edu. my

Outline • • The Second Law of Thermodynamics Heat Engines Entropy Calculation of entropy changes Entropy, Reversibility and Irreversibility The thermodynamics temperature scale What is entropy?

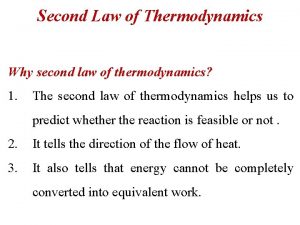
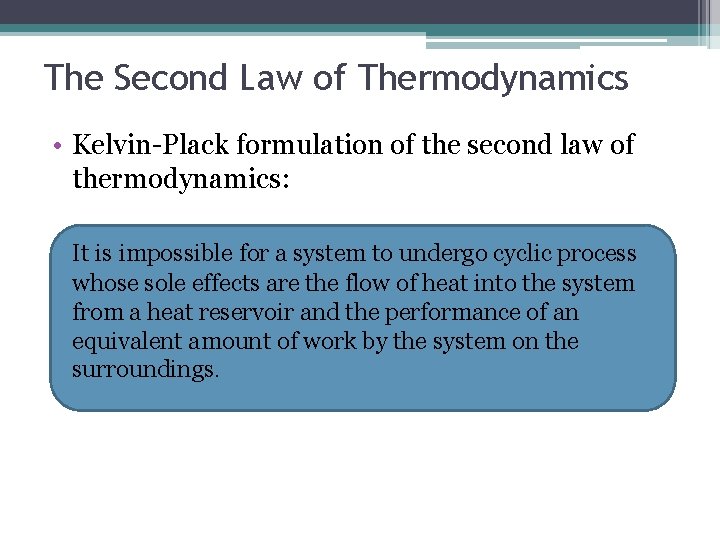
The Second Law of Thermodynamics • Kelvin-Plack formulation of the second law of thermodynamics: It is impossible for a system to undergo cyclic process whose sole effects are the flow of heat into the system from a heat reservoir and the performance of an equivalent amount of work by the system on the surroundings.

The Second Law of Thermodynamics Does this system violate the first law? It is impossible to build a cyclic machine that converts 100% heat into work.

The Second Law of Thermodynamics Any heat engine must eject heat into the cold reservoir

Heat Engines • Heat engine: a device that operates in a thermodynamic cycle and does a certain amount of net positive work as a result of heat transfer from a high-temperature body to a lowtemperature body. (eg: the internal-combustion engine and the gas turbine)

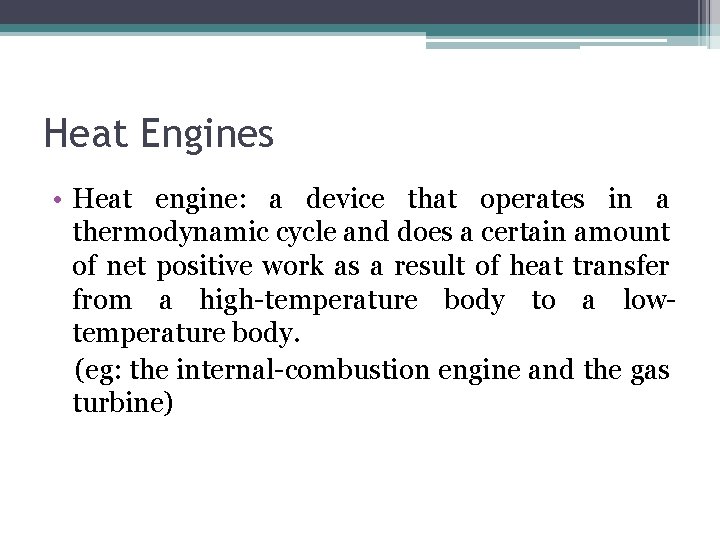
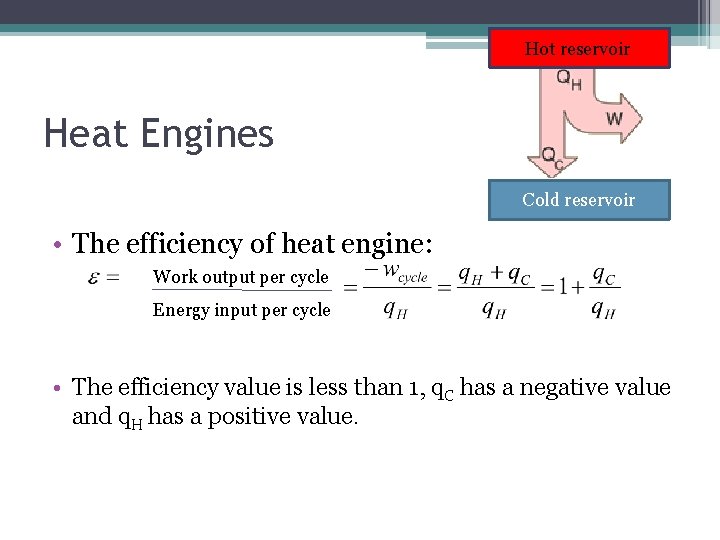
Hot reservoir Heat Engines Cold reservoir • The efficiency of heat engine: Work output per cycle Energy input per cycle • The efficiency value is less than 1, q. C has a negative value and q. H has a positive value.

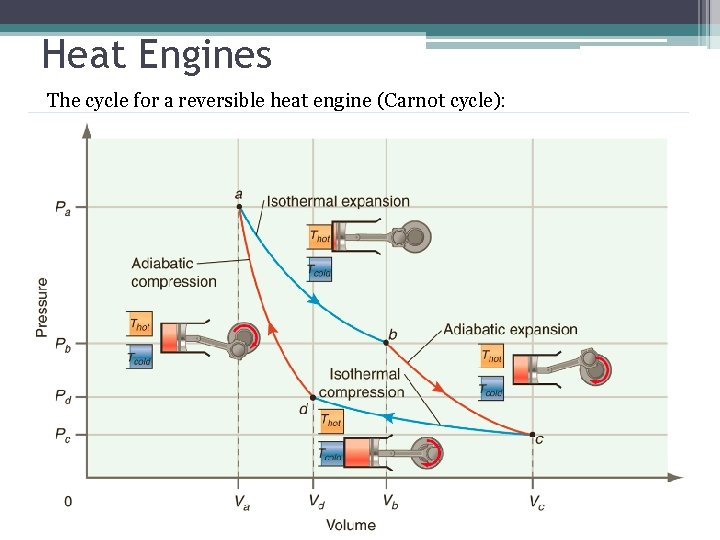
Heat Engines The cycle for a reversible heat engine (Carnot cycle):

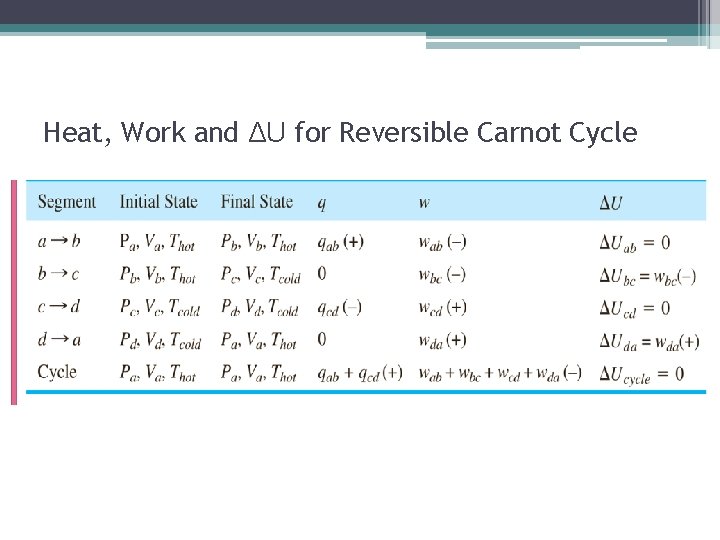
Heat, Work and ΔU for Reversible Carnot Cycle

Work flow in Carnot cycle

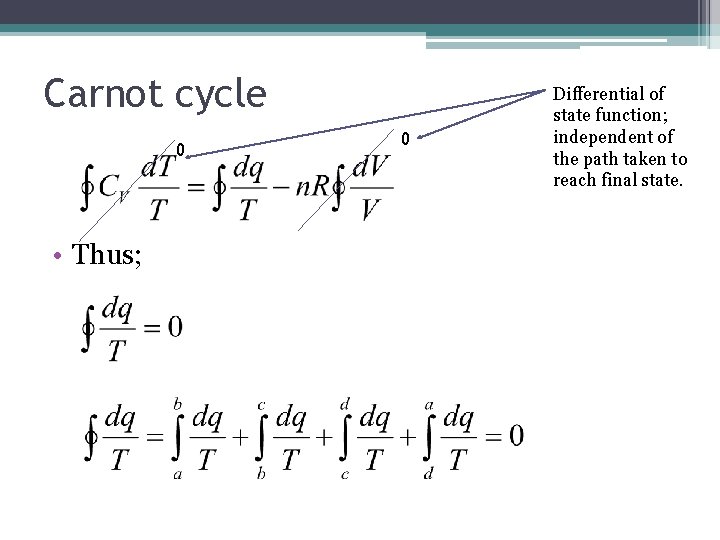
Carnot cycle • For a complete cycle (assuming perfect gas); First Law • Dividing by T and integrating over Carnot cycle;

Carnot cycle 0 • Thus; 0 Differential of state function; independent of the path taken to reach final state.

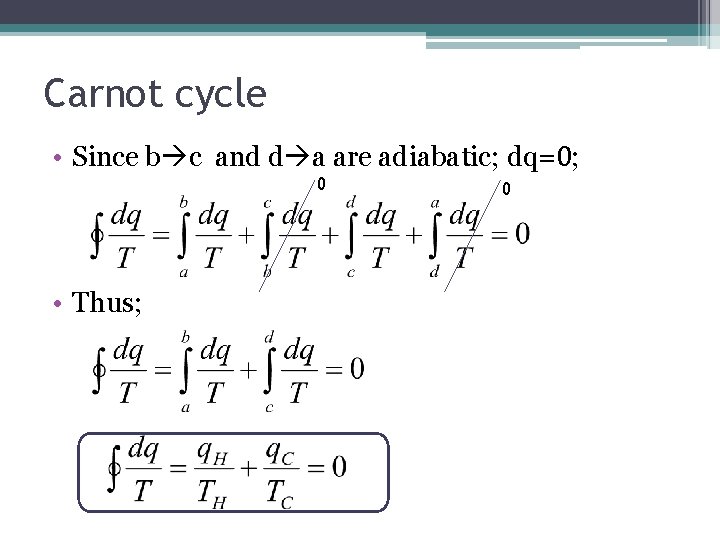
Carnot cycle • Since b c and d a are adiabatic; dq=0; 0 • Thus; 0

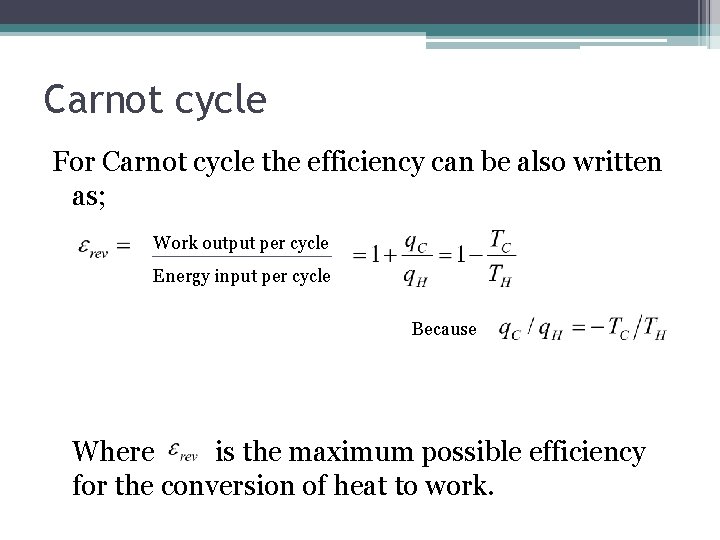
Carnot cycle For Carnot cycle the efficiency can be also written as; Work output per cycle Energy input per cycle Because Where is the maximum possible efficiency for the conversion of heat to work.

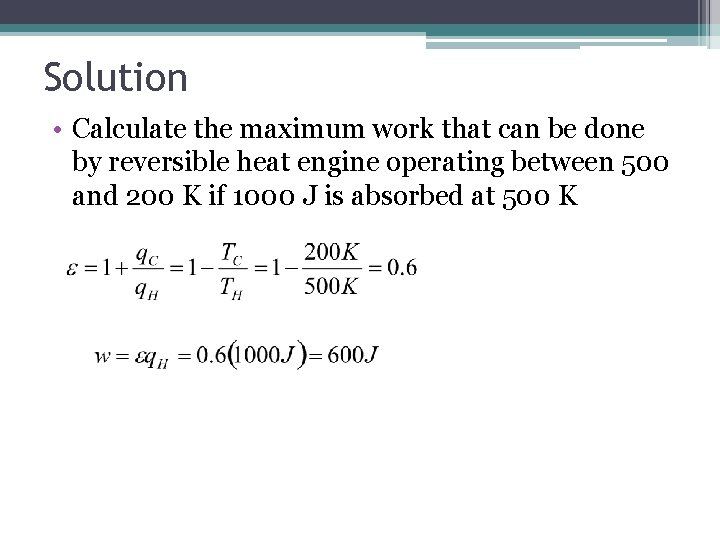
Exercise 1 • Calculate the maximum work that can be done by reversible heat engine operating between 500 and 200 K if 1000 J is absorbed at 500 K

Solution • Calculate the maximum work that can be done by reversible heat engine operating between 500 and 200 K if 1000 J is absorbed at 500 K

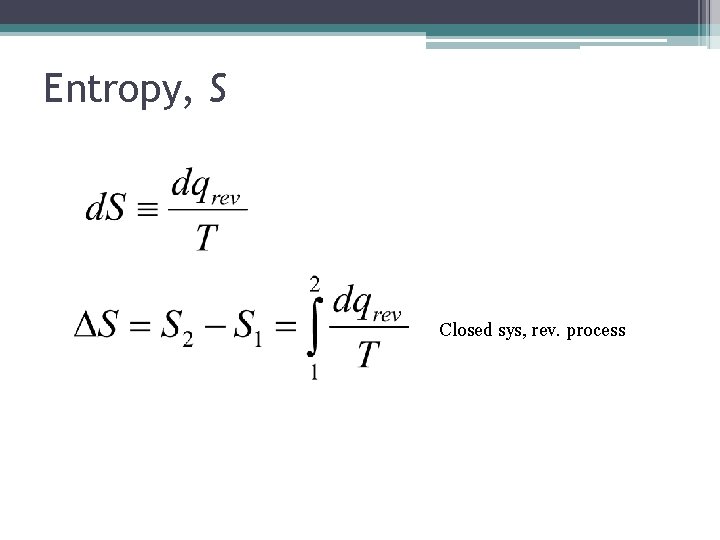
Entropy, S Closed sys, rev. process

Calculation of Entropy Changes 1. Cyclic process; 2. Reversible adiabatic process; 0 Rev. adiab. proc. 3. Reversible phase change at constant T & P at constant P, , thus Rev. phase change at const. T & P

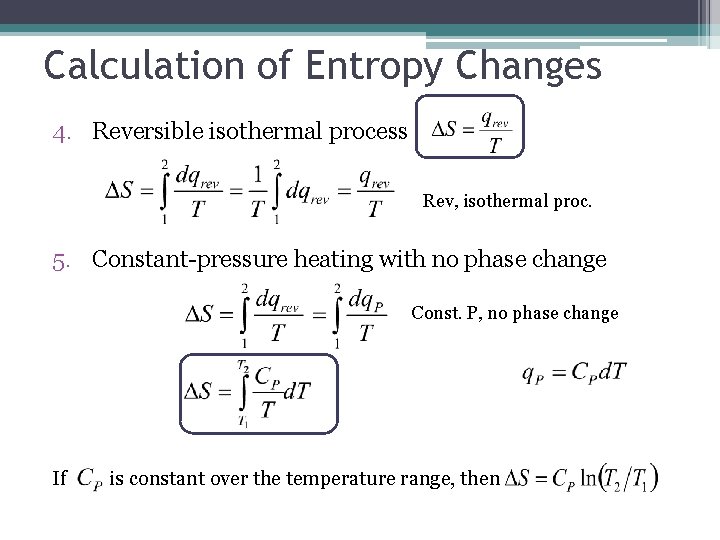
Calculation of Entropy Changes 4. Reversible isothermal process Rev, isothermal proc. 5. Constant-pressure heating with no phase change Const. P, no phase change If is constant over the temperature range, then

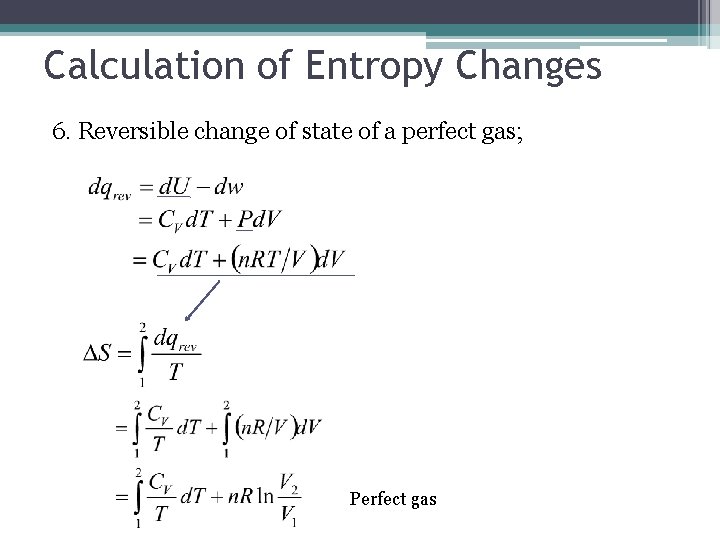
Calculation of Entropy Changes 6. Reversible change of state of a perfect gas; Perfect gas

Calculation of Entropy Changes 7. Irreversible change of state of a perfect gas; Perfect gas 8. Mixing of different inert perfect gases at constant T & P

Exercise 2 One mole of a perfect gas at 300 K is reversibly and isothermally compressed from a volume of 25. 0 L to a volume of 10. 0 L. Because the water bath in the surroundings is very large, T remains essentially constant at 300 K during the process. Calculate ΔS of the system.

Exercise 2 -Solution One mole of a perfect gas at 300 K is reversibly and isothermally compressed from a volume of 25. 0 L to a volume of 10. 0 L. Because the water bath in the surroundings is very large, T remains essentially constant at 300 K during the process. Calculate ΔS. • This is an isothermal process, ΔT=0, thus ΔU=0 (for perfect gas, U depends only on T. ( )

Exercise 3 Calculate ΔS for the melting of 5. 0 g of ice (heat of fusion= 79. 7 cal/g) at 0°C and 1 atm. Estimate ΔS for the reverse process

Exercise 3 - Solution Calculate ΔS for the melting of 5. 0 g of ice (heat of fusion= 79. 7 cal/g) at 0°C and 1 atm. Estimate ΔS for the reverse process. • Identify type of process: ▫ Phase change at constant T & P ▫ At constant P, q= ΔH ▫ Thus, • Calculate ΔS;

Exercise 3 - Solution Calculate ΔS for the melting of 5. 0 g of ice (heat of fusion= 79. 7 cal/g) at 0°C and 1 atm. Estimate ΔS for the reverse process. • ΔS for reverse process (freezing of 5 g liquid water );

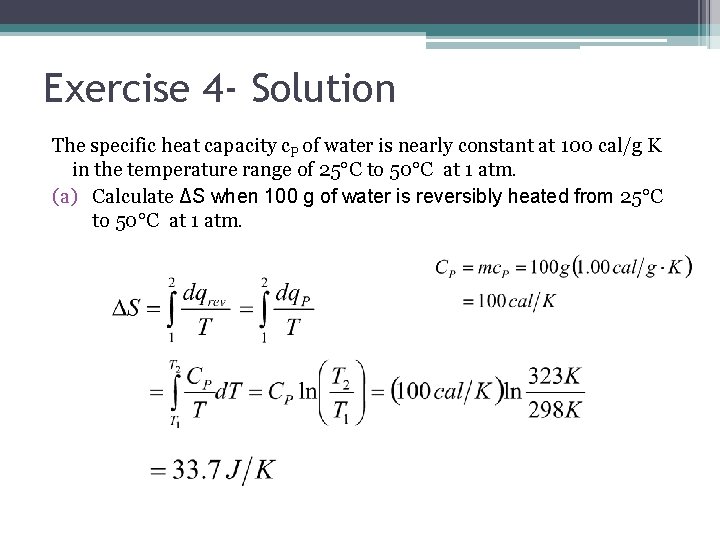
Exercise 4 The specific heat capacity c. P of water is nearly constant at 100 cal/g K in the temperature range of 25°C to 50°C at 1 atm. (a) Calculate ΔS when 100 g of water is reversibly heated from 25°C to 50°C at 1 atm. (b) Without doing a calculation, state whether ΔS for heating 100 g of water from 50°C to 75°C at 1 atm will be greater, equal to or less than ΔS for the 25°C to 50°C heating.

Exercise 4 - Solution The specific heat capacity c. P of water is nearly constant at 100 cal/g K in the temperature range of 25°C to 50°C at 1 atm. (a) Calculate ΔS when 100 g of water is reversibly heated from 25°C to 50°C at 1 atm.

Exercise 4 - Solution The specific heat capacity c. P of water is nearly constant at 100 cal/g K in the temperature range of 25°C to 50°C at 1 atm. (b) Without doing a calculation, state whether ΔS for heating 100 g of water from 50°C to 75°C at 1 atm will be greater, equal to or less than ΔS for the 25°C to 50°C heating. Thus, ↑T, ↓ΔS ΔS for heating 100 g of water from 50°C to 75°C at 1 atm will be smaller than ΔS for the 25°C to 50°C heating.

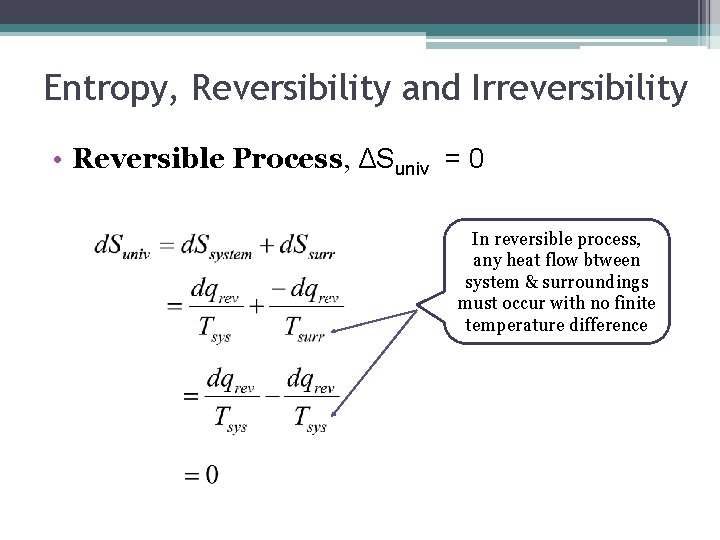
Entropy, Reversibility and Irreversibility • Reversible Process, ΔSuniv = 0 In reversible process, any heat flow btween system & surroundings must occur with no finite temperature difference

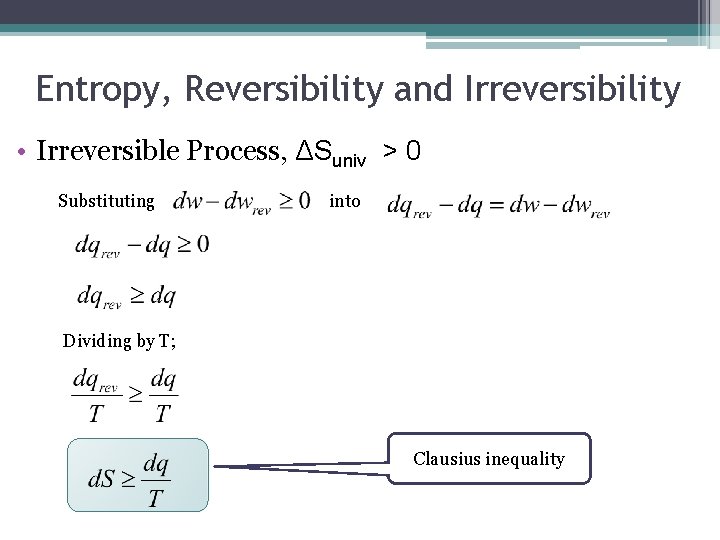
Entropy, Reversibility and Irreversibility • Irreversible Process, ΔSuniv > 0 Recall first law; rearranging More work is done when a change is reversible than when it is irreversible; When energy leaves the system as work, rearranging

Entropy, Reversibility and Irreversibility • Irreversible Process, ΔSuniv > 0 Substituting into Dividing by T; Clausius inequality

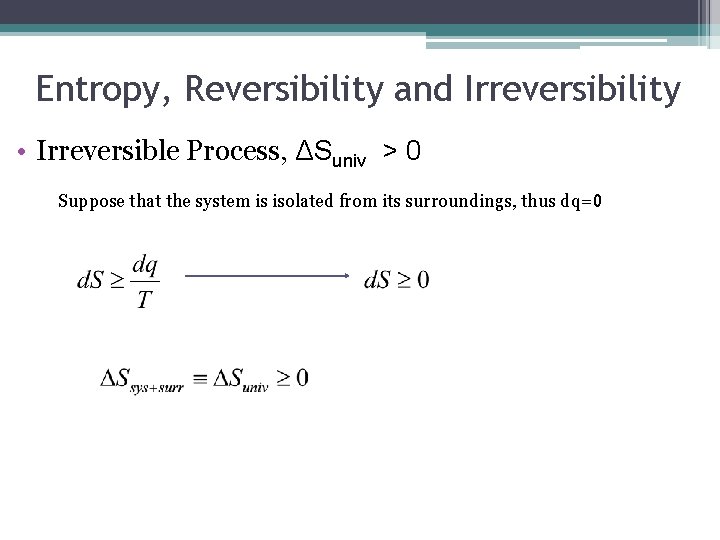
Entropy, Reversibility and Irreversibility • Irreversible Process, ΔSuniv > 0 Suppose that the system is isolated from its surroundings, thus dq=0

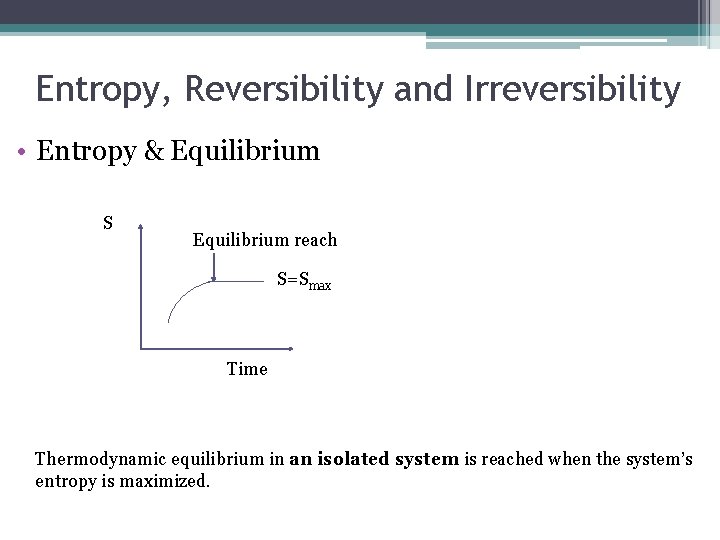
Entropy, Reversibility and Irreversibility • Entropy & Equilibrium S Equilibrium reach S=Smax Time Thermodynamic equilibrium in an isolated system is reached when the system’s entropy is maximized.

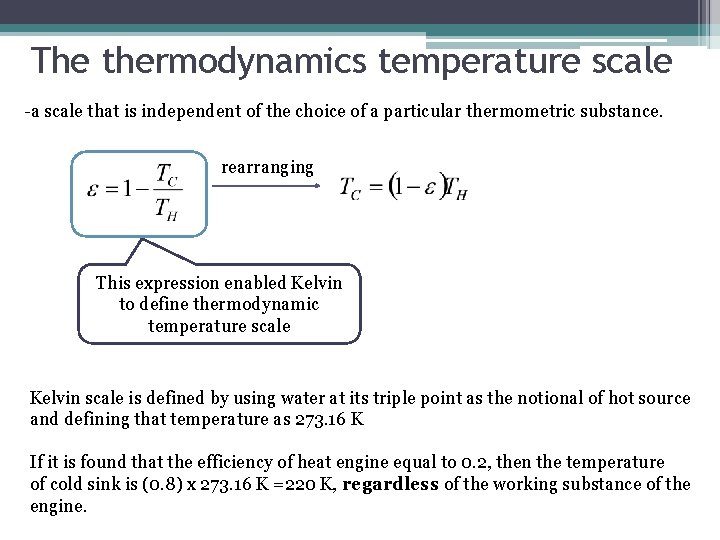
The thermodynamics temperature scale -a scale that is independent of the choice of a particular thermometric substance. rearranging This expression enabled Kelvin to define thermodynamic temperature scale Kelvin scale is defined by using water at its triple point as the notional of hot source and defining that temperature as 273. 16 K If it is found that the efficiency of heat engine equal to 0. 2, then the temperature of cold sink is (0. 8) x 273. 16 K =220 K, regardless of the working substance of the engine.

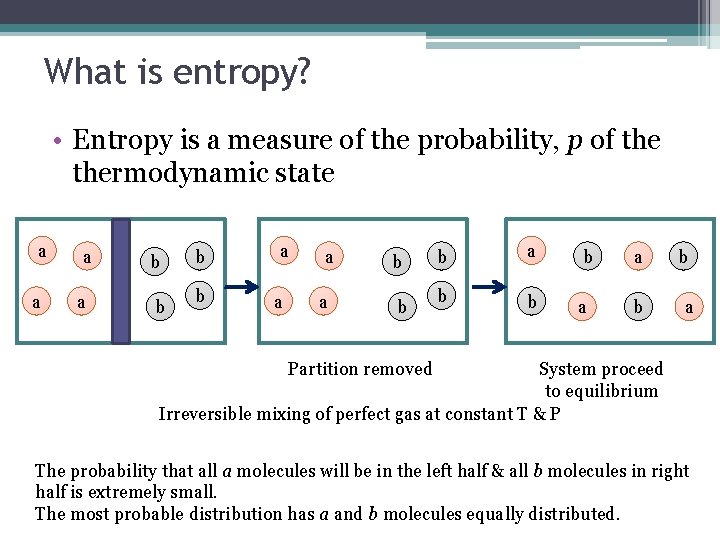
What is entropy? • Entropy is a measure of the probability, p of thermodynamic state a a b b b a b a Partition removed System proceed to equilibrium Irreversible mixing of perfect gas at constant T & P The probability that all a molecules will be in the left half & all b molecules in right half is extremely small. The most probable distribution has a and b molecules equally distributed.

What is entropy? • Entropy is a measure of molecular disorder of a state. Eg: In mixing two gases, the disordered (mixed state) is far more probable than the ordered (unmixed) state.

What is entropy? • Entropy is related to the distribution or spread of energy among the available molecular energy levels. • The greater the number of energy levels, the larger the entropy is. • Increasing the system’s energy (eg: by heating) will increase its entropy because it allows higher energy levels to be significantly occupied • Increasing the volume of a system at constant energy also allows more energy level to be occupied.

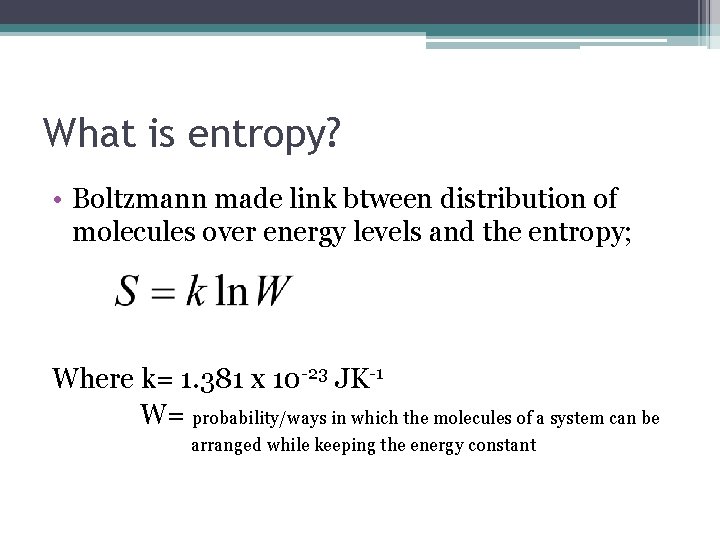
What is entropy? • Boltzmann made link btween distribution of molecules over energy levels and the entropy; Where k= 1. 381 x 10 -23 JK-1 W= probability/ways in which the molecules of a system can be arranged while keeping the energy constant

Exercise • True or false? ▫ ΔSuni for a reversible process in a closed system must be zero ▫ ΔS for a reversible process in a closed system must be zero ▫ For a closed system, equilibrium has been reached when S has been maximized. • What is ΔSuni for each steps of a Carnot cycle?
 360-108-108
360-108-108 Pvad formula
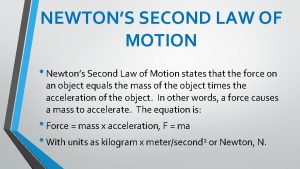
Pvad formula Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law Pt=po(1+r)t
Pt=po(1+r)t The controllers chapter 8
The controllers chapter 8 Ert tool
Ert tool Ert diagram
Ert diagram Ert erp definition
Ert erp definition Ert erp definition
Ert erp definition Ert 1 programa
Ert 1 programa Ert malaysia
Ert malaysia Ert uniform design
Ert uniform design 27 miles per gallon into kilometers per liter
27 miles per gallon into kilometers per liter Boyles law
Boyles law P=k/v
P=k/v Klein
Klein Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Write the exponent of 3 in the prime factorization of 162
Write the exponent of 3 in the prime factorization of 162 Simplify square root of 252
Simplify square root of 252 Greatest common factor
Greatest common factor Hcf 8 9 25
Hcf 8 9 25 Gcf of 24 and 60
Gcf of 24 and 60 Canto 30 parafrasi
Canto 30 parafrasi 108 factors
108 factors Sura 108
Sura 108 Engineering 108
Engineering 108 Corso toscana 108
Corso toscana 108 4-1 work together, p. 97
4-1 work together, p. 97 466560/108
466560/108 Ee 108
Ee 108 Uyga vazifa 308 mashq
Uyga vazifa 308 mashq 108 temples
108 temples 107 108 109
107 108 109 Factor tree of 90
Factor tree of 90 Hasil dari 108 + 132 - 134 adalah
Hasil dari 108 + 132 - 134 adalah Round the factors to estimate the products
Round the factors to estimate the products 101 102 103 104 105 106 107 108 109 110
101 102 103 104 105 106 107 108 109 110 1 mustang way
1 mustang way Engineering 108.com
Engineering 108.com