PHYSICAL CHEMISTRY ERT 108 Semester II 20102011 Huzairy










- Slides: 44

PHYSICAL CHEMISTRY ERT 108 Semester II 2010/2011 Huzairy Hassan School of Bioprocess Engineering Uni. MAP

Standard Thermodynamic Functions of Reaction

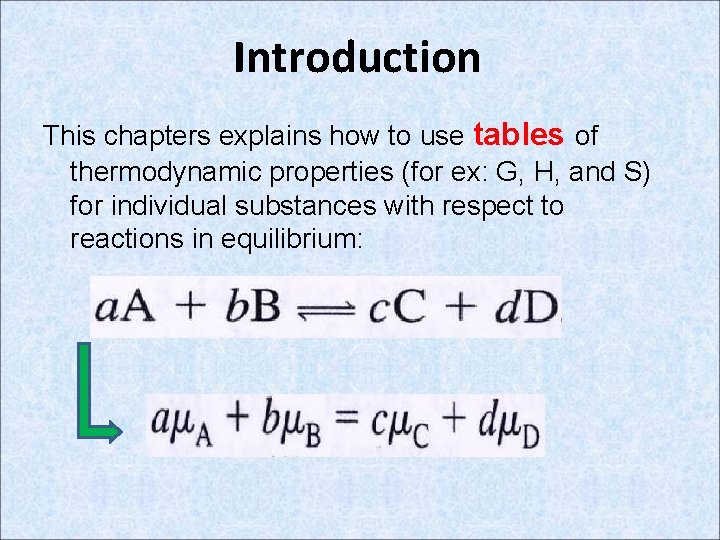
Introduction This chapters explains how to use tables of thermodynamic properties (for ex: G, H, and S) for individual substances with respect to reactions in equilibrium:

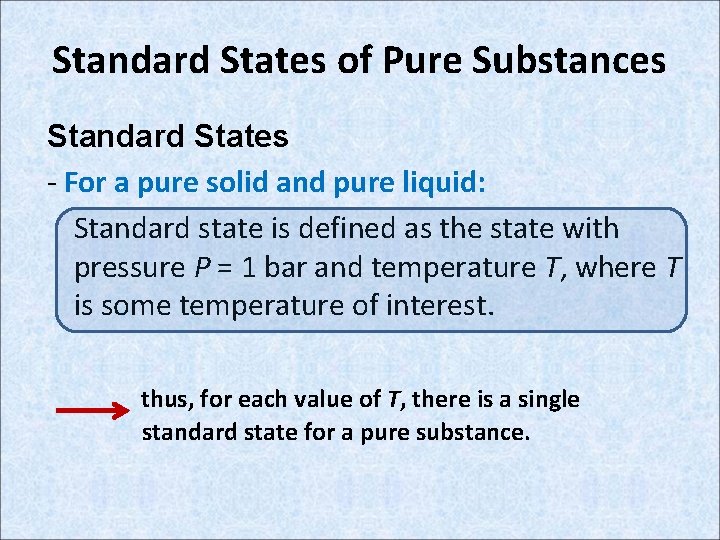
Standard States of Pure Substances Standard States - For a pure solid and pure liquid: Standard state is defined as the state with pressure P = 1 bar and temperature T, where T is some temperature of interest. thus, for each value of T, there is a single standard state for a pure substance.

For example; The molar volume of a pure solid or liquid at 1 bar and 200 K is symbolized by Superscript º (“naught”, “zero”, or “standard”) subscript 200 (temperature)

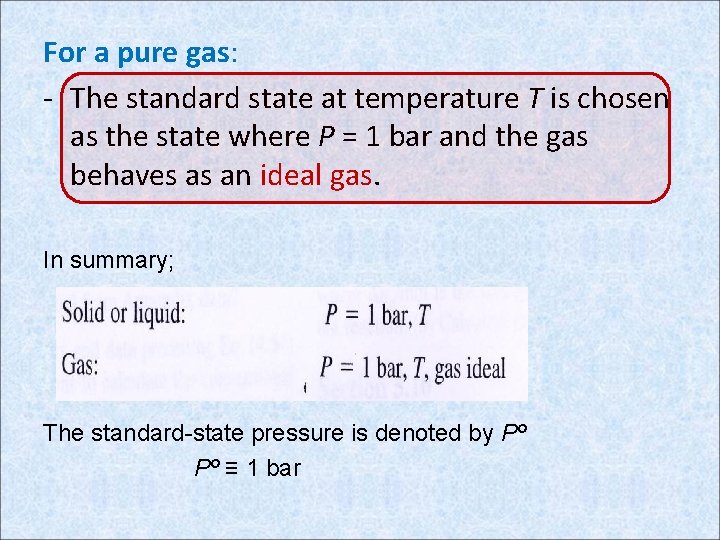
For a pure gas: - The standard state at temperature T is chosen as the state where P = 1 bar and the gas behaves as an ideal gas. In summary; The standard-state pressure is denoted by Pº Pº ≡ 1 bar

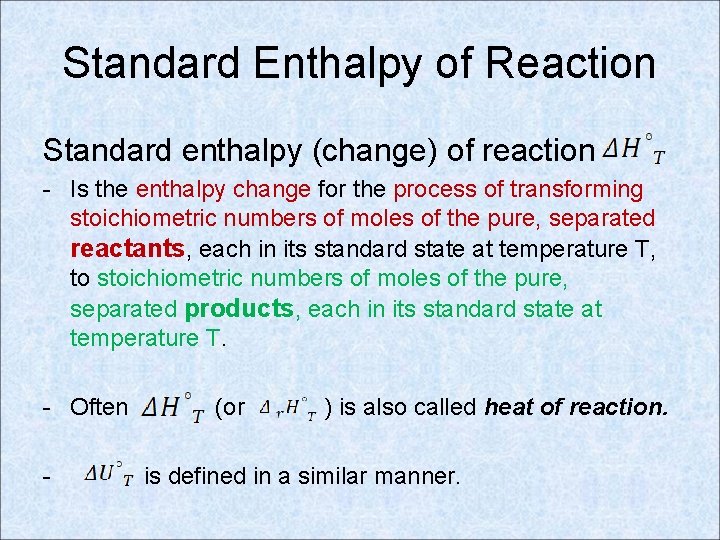
Standard Enthalpy of Reaction Standard enthalpy (change) of reaction - Is the enthalpy change for the process of transforming stoichiometric numbers of moles of the pure, separated reactants, each in its standard state at temperature T, to stoichiometric numbers of moles of the pure, separated products, each in its standard state at temperature T. - Often - (or ) is also called heat of reaction. is defined in a similar manner.

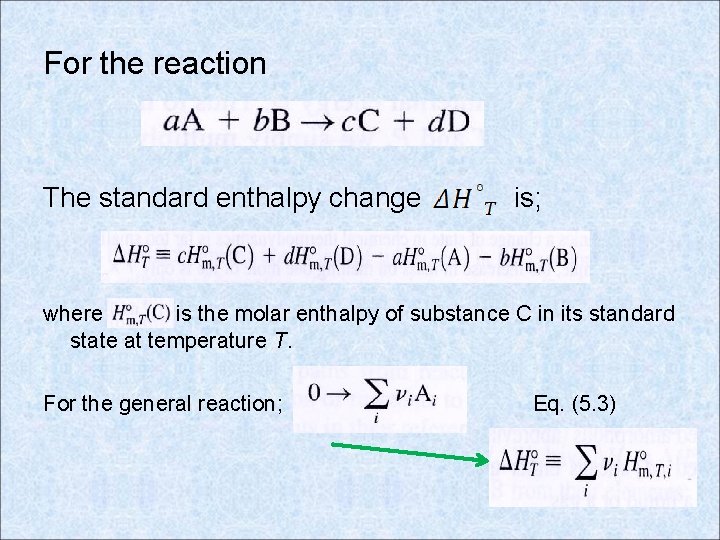
For the reaction The standard enthalpy change is; where is the molar enthalpy of substance C in its standard state at temperature T. For the general reaction; Eq. (5. 3)

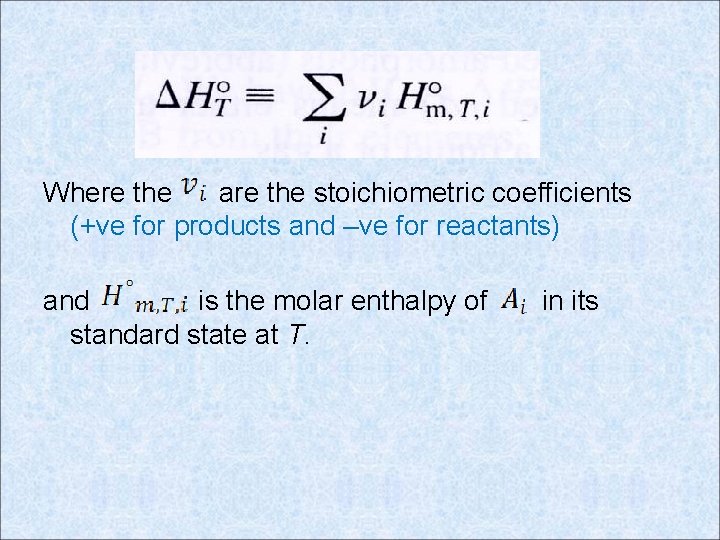
Where the are the stoichiometric coefficients (+ve for products and –ve for reactants) and is the molar enthalpy of standard state at T. in its

For example; for =

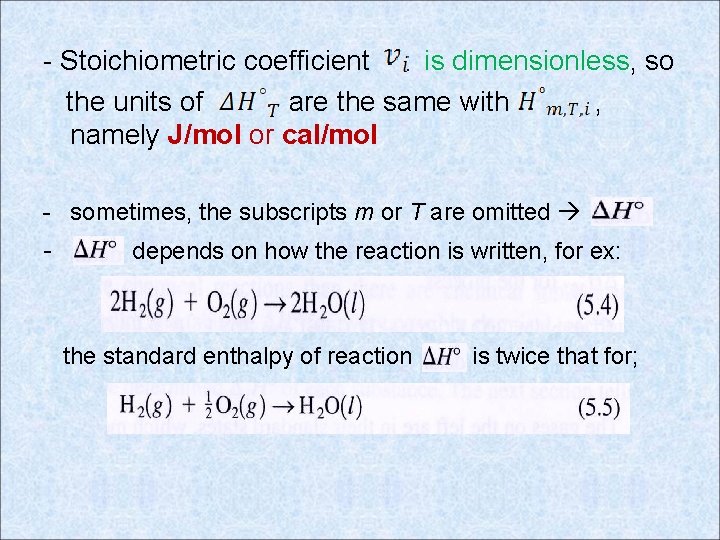
- Stoichiometric coefficient is dimensionless, so the units of are the same with , namely J/mol or cal/mol - sometimes, the subscripts m or T are omitted - depends on how the reaction is written, for ex: the standard enthalpy of reaction is twice that for;

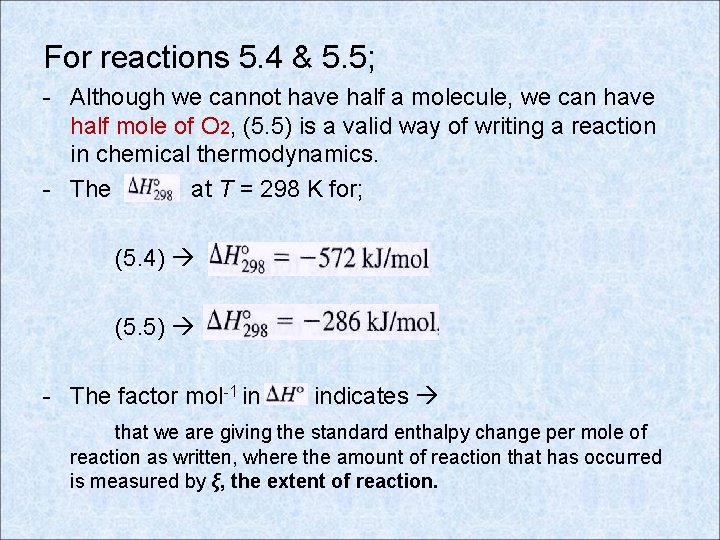
For reactions 5. 4 & 5. 5; - Although we cannot have half a molecule, we can have half mole of O 2, (5. 5) is a valid way of writing a reaction in chemical thermodynamics. - The at T = 298 K for; (5. 4) (5. 5) - The factor mol-1 in indicates that we are giving the standard enthalpy change per mole of reaction as written, where the amount of reaction that has occurred is measured by ξ, the extent of reaction.

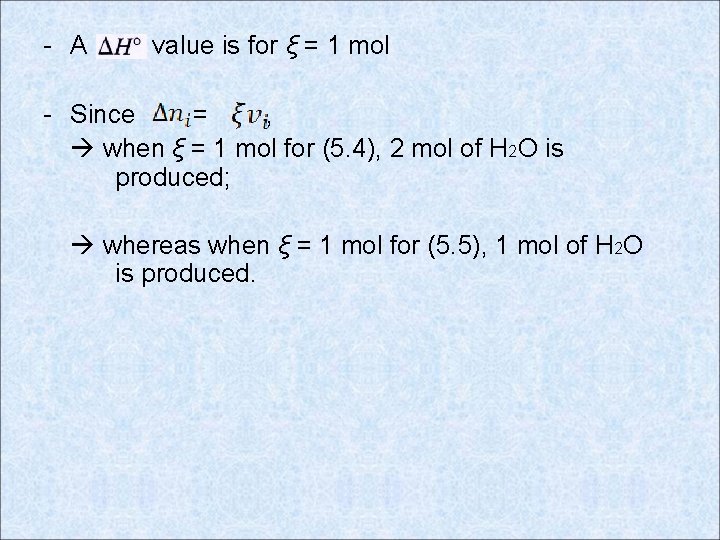
- A value is for ξ = 1 mol - Since = , when ξ = 1 mol for (5. 4), 2 mol of H 2 O is produced; whereas when ξ = 1 mol for (5. 5), 1 mol of H 2 O is produced.

Aims to calculate of a reaction from tabulated thermodynamic data for reactants and products. However, the laws of thermodynamics only allow us to measure changes in entalpy, internal energy and entropies (ΔH, ΔU and ΔS, not the absolute values of U, H, and S, and we cannot tabulate absolute enthalpies of substances. Instead, we can tabulate standard enthalpies of formation In summary

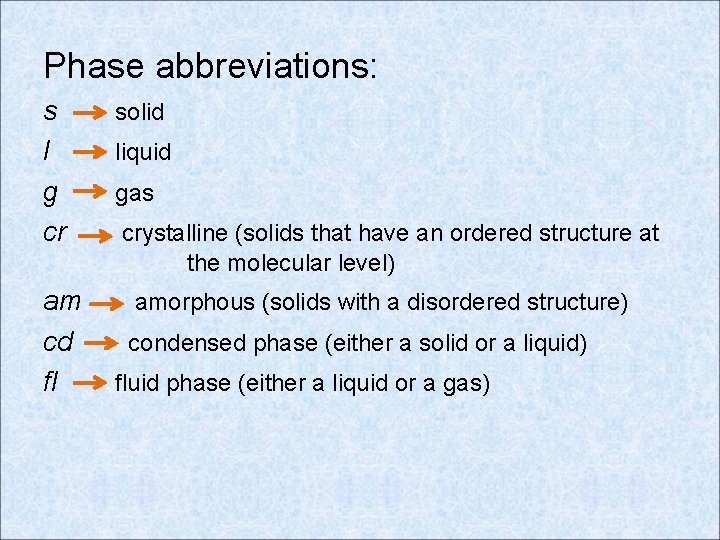
Phase abbreviations: s l g cr am cd fl solid liquid gas crystalline (solids that have an ordered structure at the molecular level) amorphous (solids with a disordered structure) condensed phase (either a solid or a liquid) fluid phase (either a liquid or a gas)

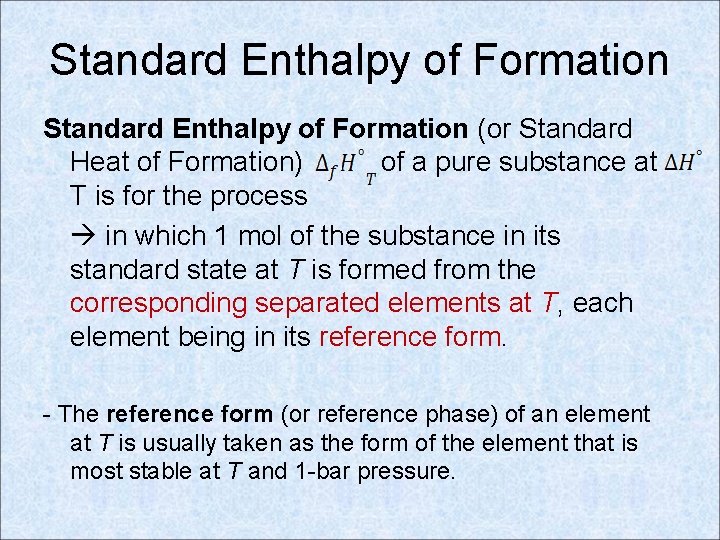
Standard Enthalpy of Formation (or Standard Heat of Formation) of a pure substance at T is for the process in which 1 mol of the substance in its standard state at T is formed from the corresponding separated elements at T, each element being in its reference form. - The reference form (or reference phase) of an element at T is usually taken as the form of the element that is most stable at T and 1 -bar pressure.

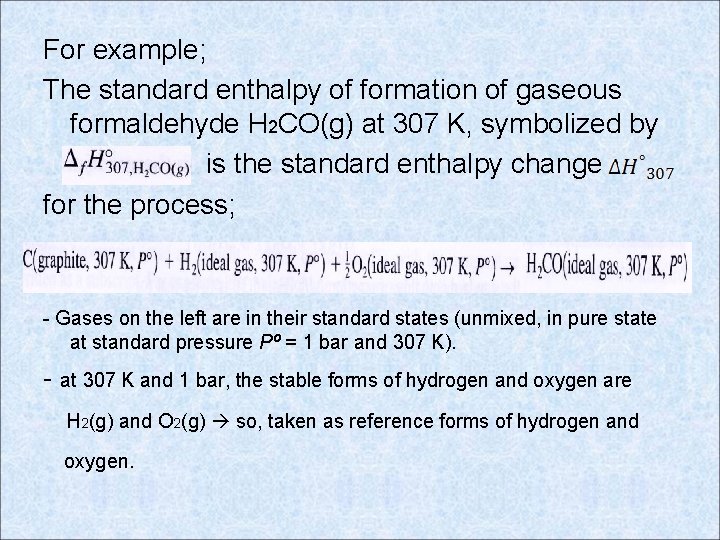
For example; The standard enthalpy of formation of gaseous formaldehyde H 2 CO(g) at 307 K, symbolized by is the standard enthalpy change for the process; - Gases on the left are in their standard states (unmixed, in pure state at standard pressure Pº = 1 bar and 307 K). - at 307 K and 1 bar, the stable forms of hydrogen and oxygen are H 2(g) and O 2(g) so, taken as reference forms of hydrogen and oxygen.


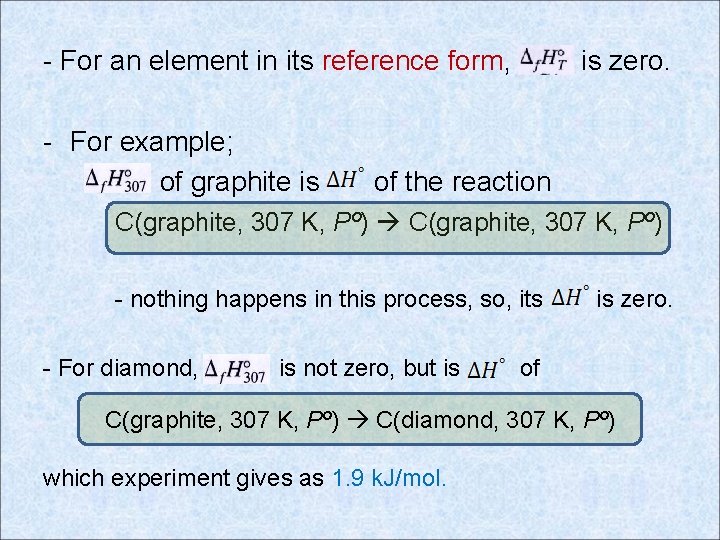
- For an element in its reference form, - For example; of graphite is is zero. of the reaction C(graphite, 307 K, Pº) - nothing happens in this process, so, its - For diamond, is not zero, but is is zero. of C(graphite, 307 K, Pº) C(diamond, 307 K, Pº) which experiment gives as 1. 9 k. J/mol.

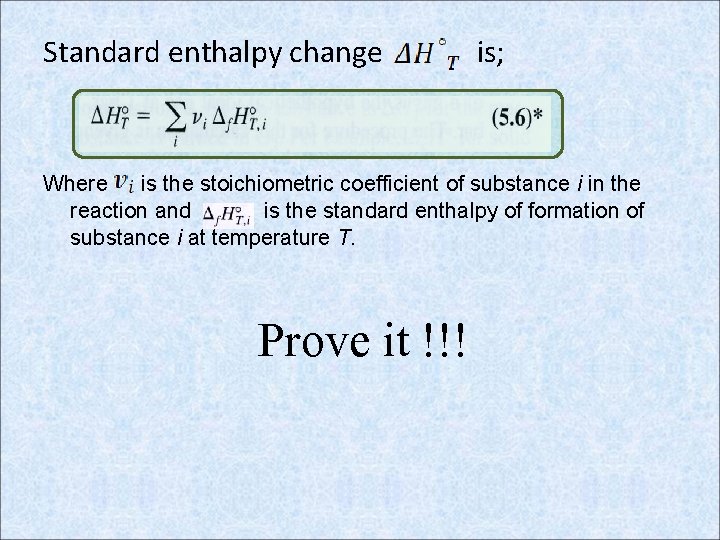
Standard enthalpy change is; Where is the stoichiometric coefficient of substance i in the reaction and is the standard enthalpy of formation of substance i at temperature T. Prove it !!!

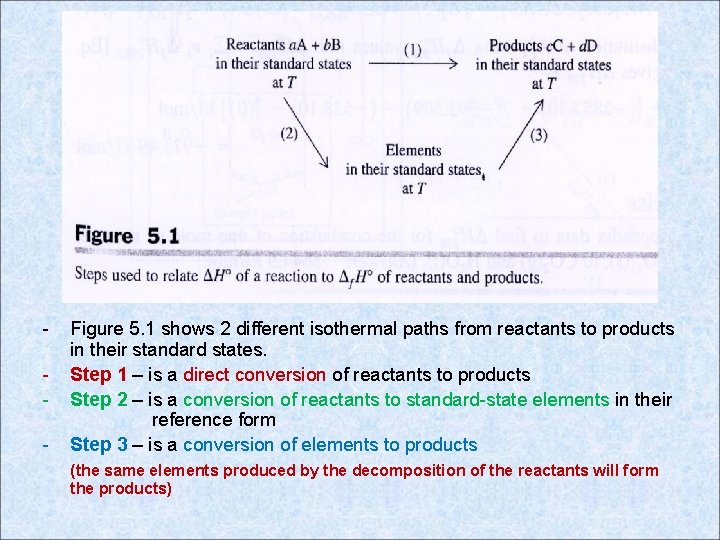
- Figure 5. 1 shows 2 different isothermal paths from reactants to products in their standard states. Step 1 – is a direct conversion of reactants to products Step 2 – is a conversion of reactants to standard-state elements in their reference form Step 3 – is a conversion of elements to products (the same elements produced by the decomposition of the reactants will form the products)

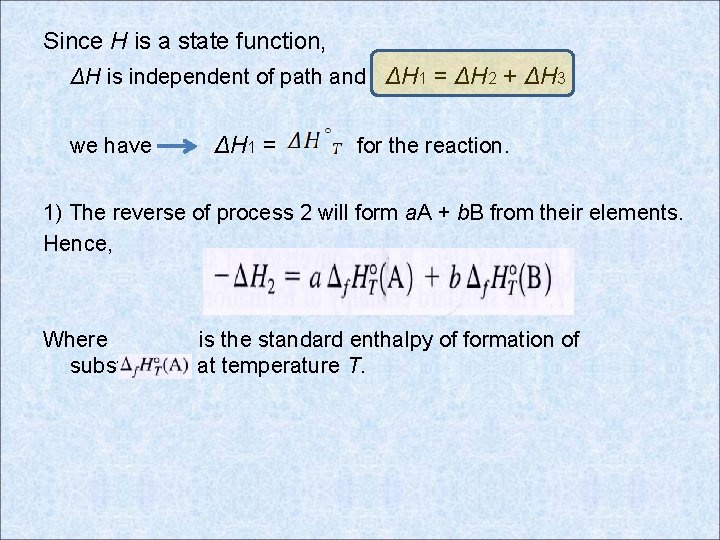
Since H is a state function, ΔH is independent of path and ΔH 1 = ΔH 2 + ΔH 3 we have ΔH 1 = for the reaction. 1) The reverse of process 2 will form a. A + b. B from their elements. Hence, Where is the standard enthalpy of formation of substance A at temperature T.

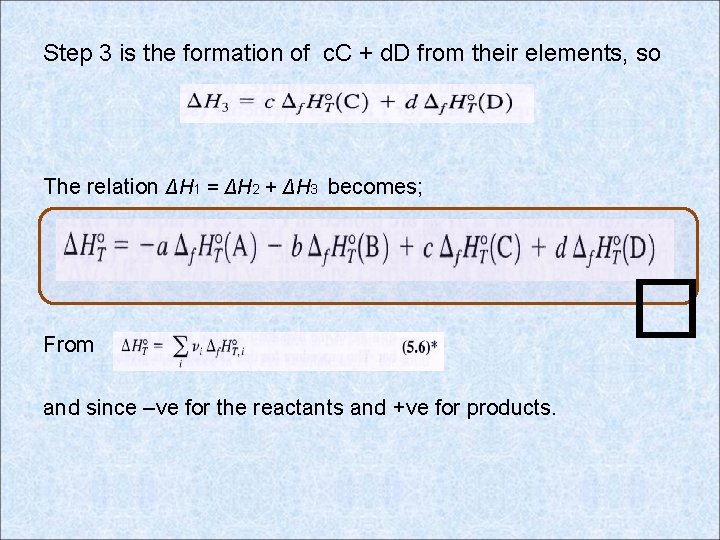
Step 3 is the formation of c. C + d. D from their elements, so The relation ΔH 1 = ΔH 2 + ΔH 3 becomes; From and since –ve for the reactants and +ve for products. �

Determination of Standard Enthalpies of Formation and Reaction Measurement of The quantity is for isothermally converting pure standard-state elements in their reference forms to standard-state substance i. To find steps: we must carry out the following

bar

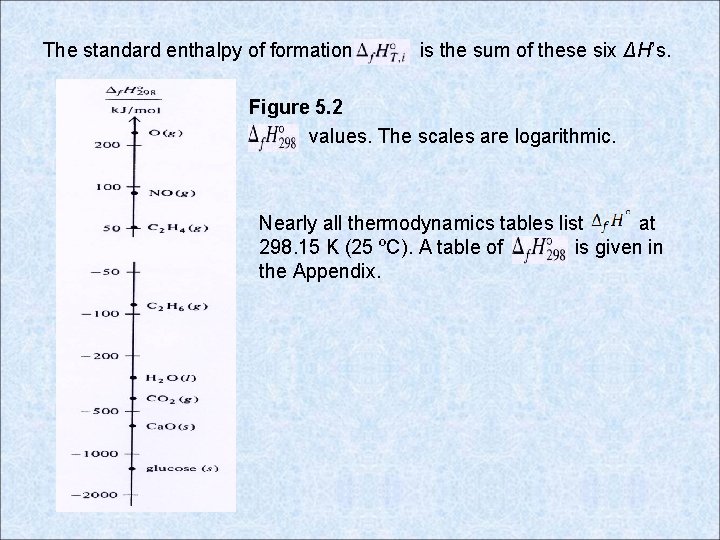
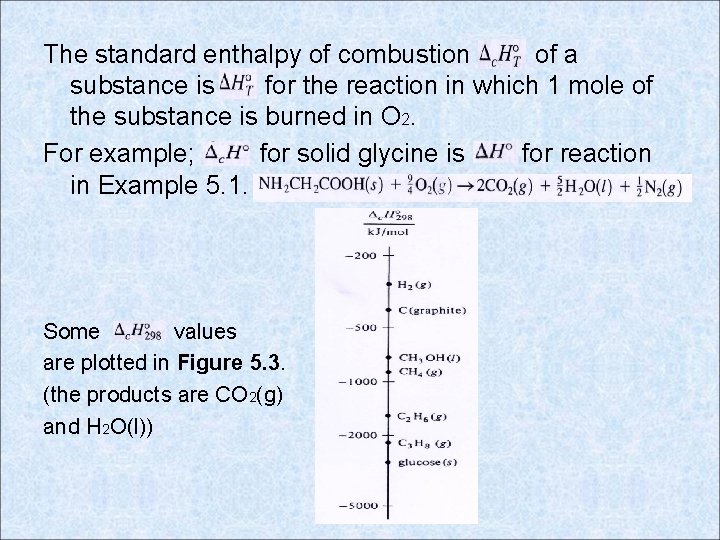
The standard enthalpy of formation is the sum of these six ΔH’s. Figure 5. 2 values. The scales are logarithmic. Nearly all thermodynamics tables list at 298. 15 K (25 ºC). A table of is given in the Appendix.

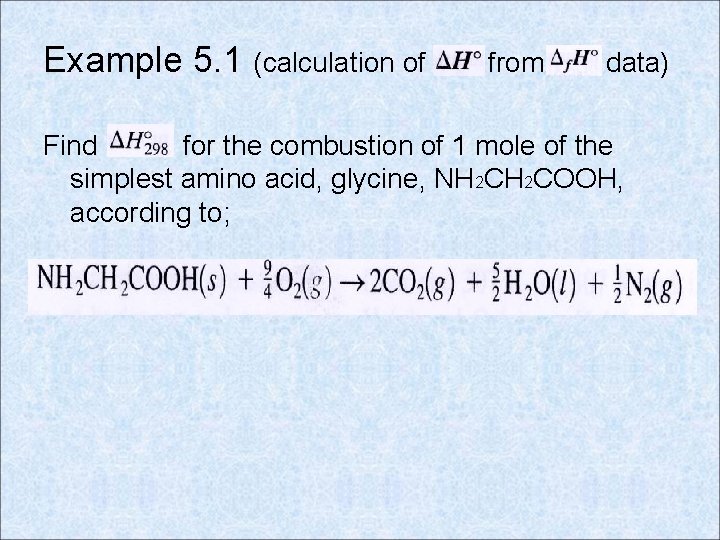
Example 5. 1 (calculation of from data) Find for the combustion of 1 mole of the simplest amino acid, glycine, NH 2 COOH, according to;

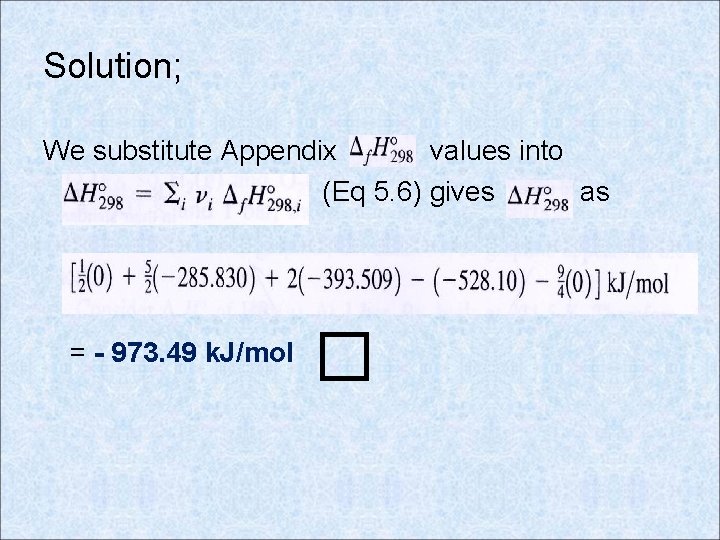
Solution; We substitute Appendix values into (Eq 5. 6) gives as = - 973. 49 k. J/mol �

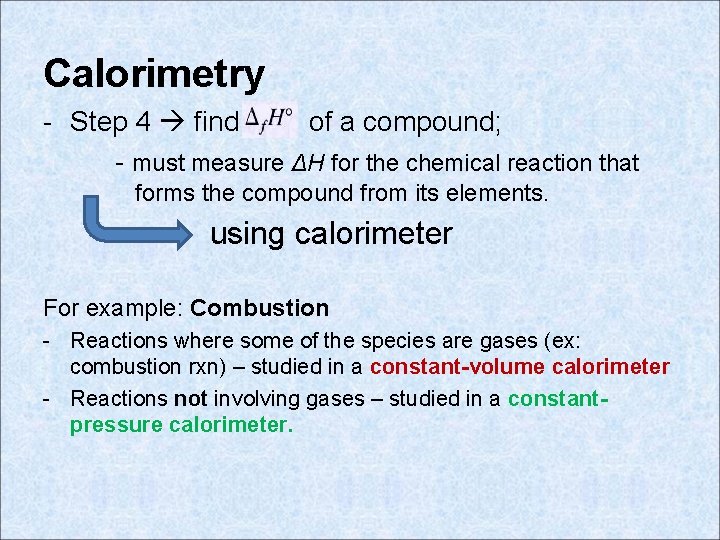
Calorimetry - Step 4 find of a compound; - must measure ΔH for the chemical reaction that forms the compound from its elements. using calorimeter For example: Combustion - Reactions where some of the species are gases (ex: combustion rxn) – studied in a constant-volume calorimeter - Reactions not involving gases – studied in a constantpressure calorimeter.

The standard enthalpy of combustion of a substance is for the reaction in which 1 mole of the substance is burned in O 2. For example; for solid glycine is for reaction in Example 5. 1. Some values are plotted in Figure 5. 3. (the products are CO 2(g) and H 2 O(l))

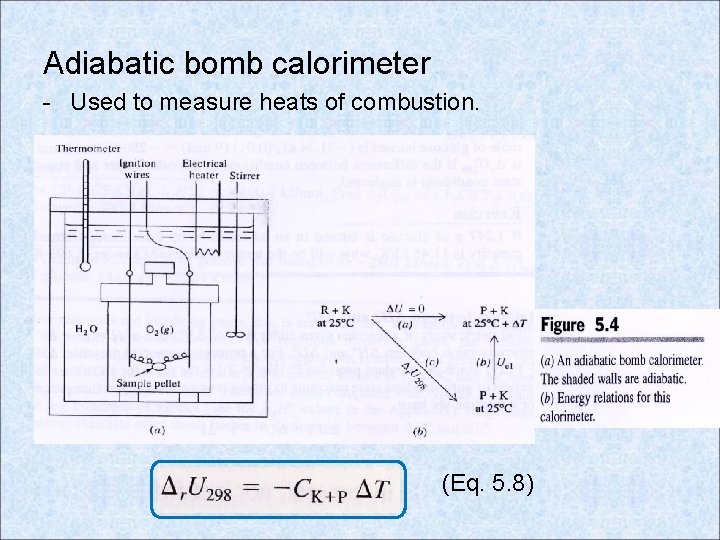
Adiabatic bomb calorimeter - Used to measure heats of combustion. (Eq. 5. 8)

Example 5. 2

Solution:

Relation between ΔHº and ΔUº For a process at constant pressure, ΔH = ΔU + P ΔV ΔHº = ΔUº + Pº ΔVº The changes in standard-state volume and internal energy: The molar volumes of gases at 1 bar are much greater than those of liquids and solids so only consider the gaseous reactants and products.

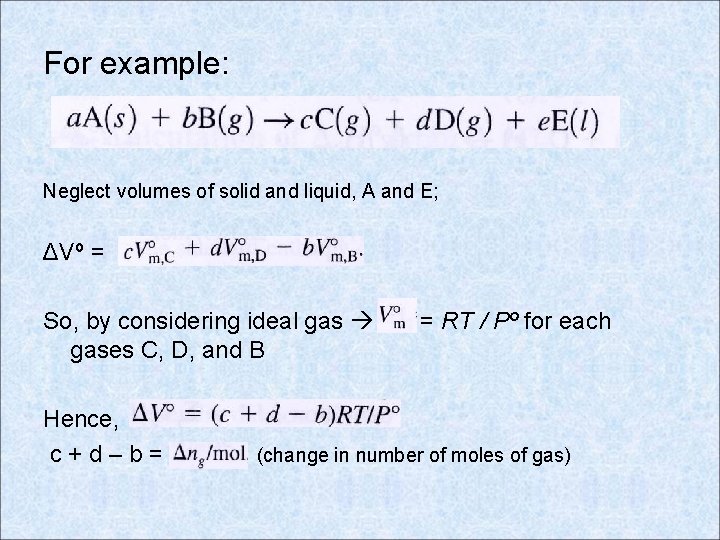
For example: Neglect volumes of solid and liquid, A and E; ΔVº = So, by considering ideal gases C, D, and B Hence, c+d–b= = RT / Pº for each (change in number of moles of gas)

Thus, we have; So, from Becomes

For example; Has So, = 3 – 1 – 5 = -3

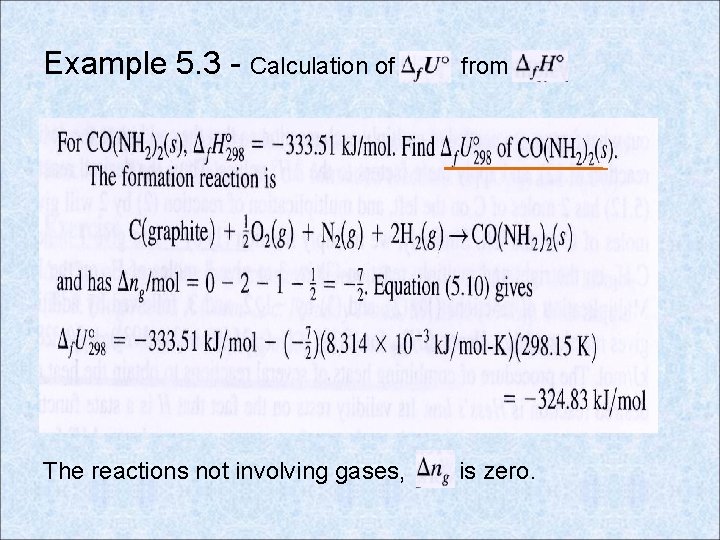
Example 5. 3 - Calculation of from The reactions not involving gases, is zero.

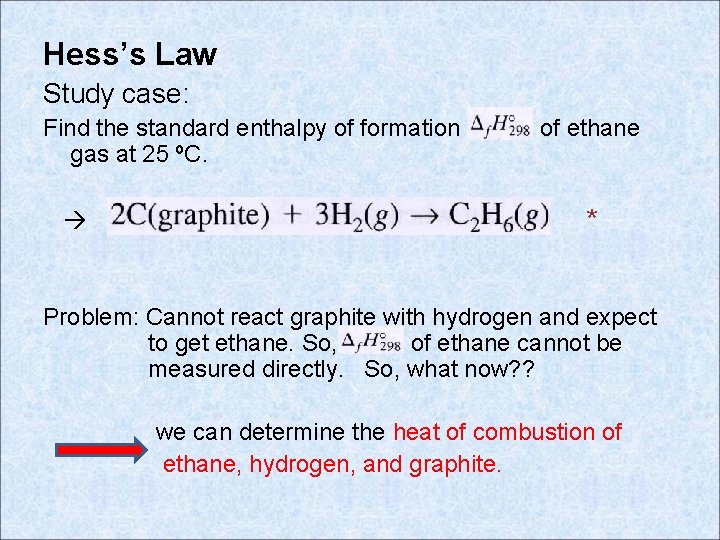
Hess’s Law Study case: Find the standard enthalpy of formation gas at 25 ºC. of ethane * Problem: Cannot react graphite with hydrogen and expect to get ethane. So, of ethane cannot be measured directly. So, what now? ? we can determine the heat of combustion of ethane, hydrogen, and graphite.


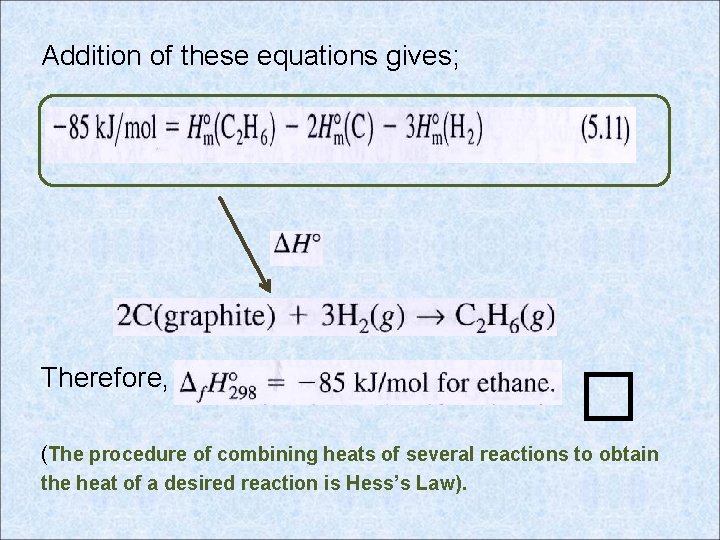
Addition of these equations gives; Therefore, � (The procedure of combining heats of several reactions to obtain the heat of a desired reaction is Hess’s Law).

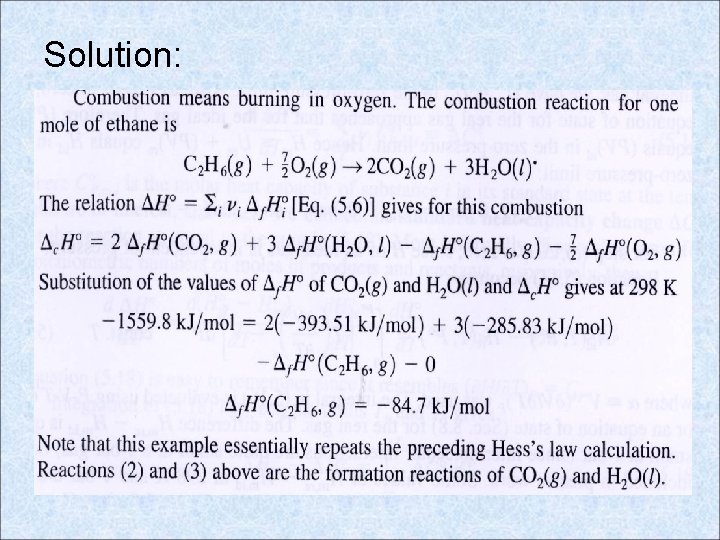
Example 5. 4 – Calculation of from The standard enthalpy of combustion of C 2 H 6(g) to CO 2(g) and H 2 O(l) is -1559. 8 k. J/mol. Use this and Appendix data on CO 2(g) and H 2 O(l) to find

Solution:

Thank you
 360-108-108
360-108-108 Solve for f fva=(c((1 0.108/12)^120-1))/(0.108/12)
Solve for f fva=(c((1 0.108/12)^120-1))/(0.108/12) 108 semester
108 semester Pt = p.ert merupakan rumus dari pertumbuhan penduduk
Pt = p.ert merupakan rumus dari pertumbuhan penduduk Ert controller
Ert controller Ert tool
Ert tool Ert diagram
Ert diagram Ert erp definition
Ert erp definition Ert erp definition
Ert erp definition Ert 1 programa
Ert 1 programa Ert products malaysia
Ert products malaysia Ert uniform design
Ert uniform design Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Chemistry
Chemistry World history semester 1 final exam study guide answers
World history semester 1 final exam study guide answers Chemistry semester exam study guide
Chemistry semester exam study guide Chemistry semester exam review
Chemistry semester exam review Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Prime factorization of 80 using exponents
Prime factorization of 80 using exponents Simplyfying radicals
Simplyfying radicals Greatest common factor
Greatest common factor Hcf of 4 and 9
Hcf of 4 and 9 Gcf of 18 and 36
Gcf of 18 and 36 Parafrasi canto xxxiv inferno
Parafrasi canto xxxiv inferno Factors of 108
Factors of 108 şura ismi
şura ismi Engineering 108
Engineering 108 Corso toscana 108
Corso toscana 108 4-2 work together p. 108 answers
4-2 work together p. 108 answers 466560/108
466560/108 Brute force synchronizer
Brute force synchronizer Ona tili 5 sinf 329 mashq
Ona tili 5 sinf 329 mashq 108 mangalasasana divya desams
108 mangalasasana divya desams 105 106 107
105 106 107 Prime factors of 420
Prime factors of 420 Hasil dari 108+132-134
Hasil dari 108+132-134 Round the factors and estimate the products exit ticket
Round the factors and estimate the products exit ticket 101 102 103 104 105 106 107 108 109 110
101 102 103 104 105 106 107 108 109 110 1 mustang way
1 mustang way Engineering 108.com
Engineering 108.com Prime number chart to 200
Prime number chart to 200 Edmonton mental health clinic 108 street
Edmonton mental health clinic 108 street Maximo comun divisor mcd
Maximo comun divisor mcd 108 lab
108 lab